Larval and/or Adult Exposure to Intraguild Predator Harmonia axyridis Alters Reproductive Allocation Decisions and Offspring Growth in Menochilus sexmaculatus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Pre-Experimental Procedures
2.3. Oviposition Responses by M. sexmaculatus Females
2.4. Offspring Growth
2.5. Statistical Analyses
3. Results
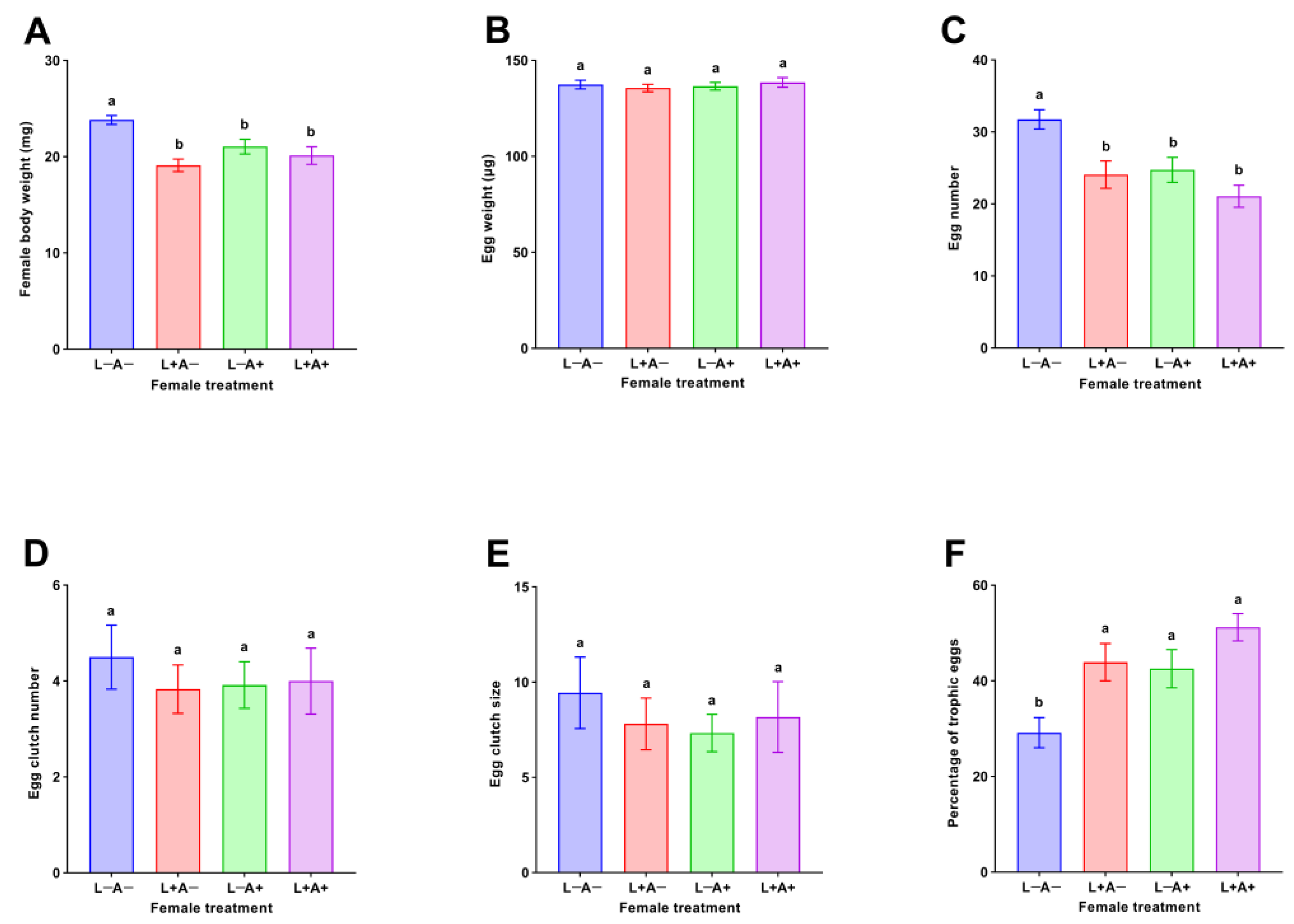
3.1. Oviposition Responses by M. sexmaculatus Females
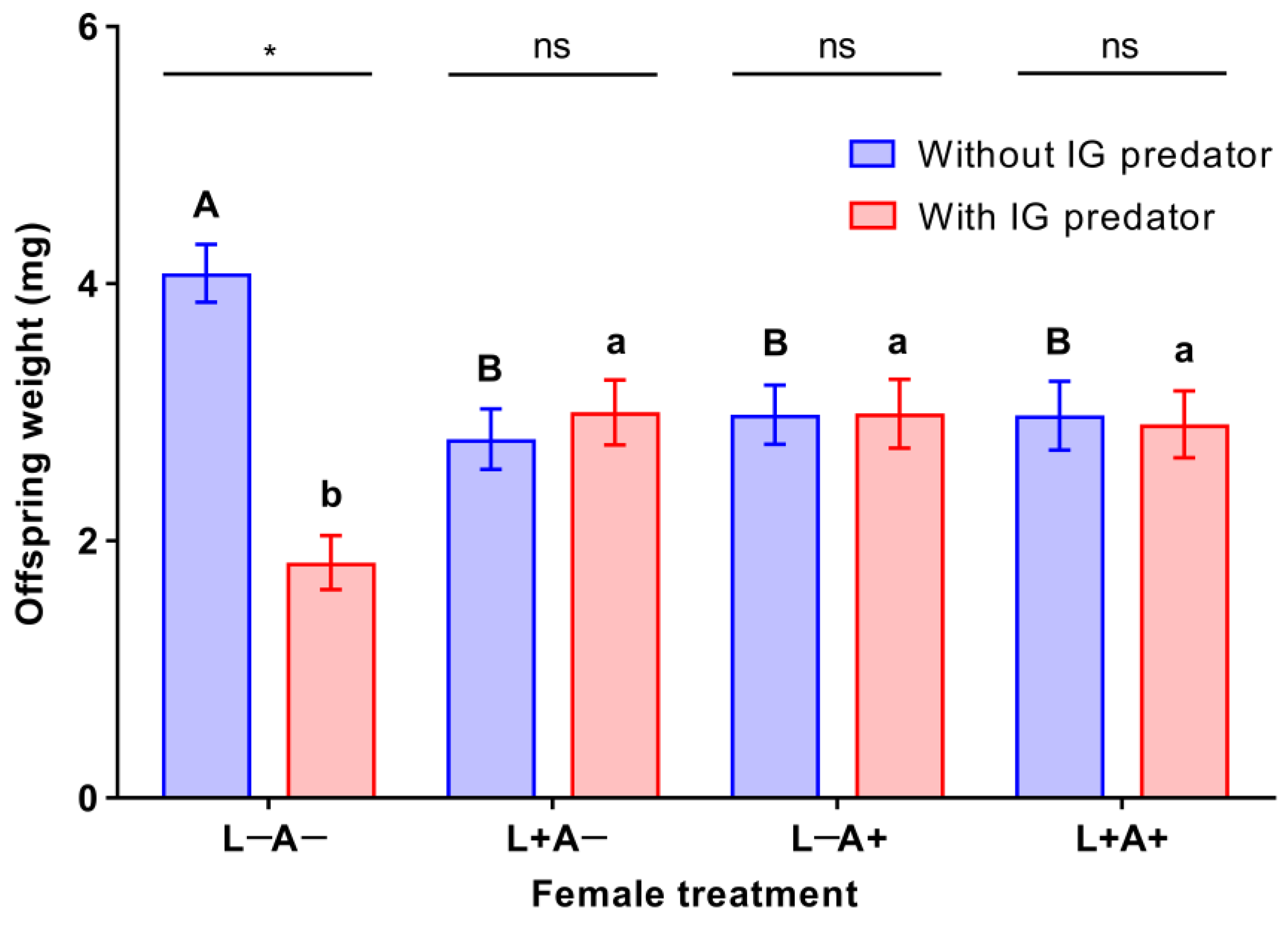
3.2. Offspring Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, S.L. Nonlethal effects in the ecology of predator-prey interactions—What are the ecological effects of anti-predator decision-making? BioScience 1998, 48, 25–34. [Google Scholar] [CrossRef]
- Langerhans, R.B. Evolutionary consequences of predation: Avoidance, escape, reproduction, and diversification. In Predation in Organisms; Elewa, A.M.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 177–220. [Google Scholar]
- Hunter, L.T.B.; Skinner, J.D. Vigilance behaviour in African ungulates: The role of predation pressure. Behaviour 1998, 135, 195–211. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Laforsch, C.; Tollrian, R. Transgenerational induction of defences in animals and plants. Nature 1999, 401, 60–63. [Google Scholar] [CrossRef]
- Benard, M.F. Predator-induced phenotypic plasticity in organisms with complex life histories. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 651–673. [Google Scholar] [CrossRef]
- Eggers, S.; Griesser, M.; Nystrand, M.; Ekman, J. Predation risk induces changes in nest-site selection and clutch size in the Siberian jay. Proc. R. Soc. B 2006, 273, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, M.; Bas, C.; Magalhães, S.; Sabelis, M.W.; De Roos, A.M.; Janssen, A. Predators induce egg retention in prey. Oecologia 2007, 150, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Shine, R.; Downes, S.J. Can pregnant lizards adjust their offspring phenotypes to environmental conditions? Oecologia 1999, 119, 1–8. [Google Scholar] [CrossRef]
- Giesing, E.R.; Suski, C.D.; Warner, R.E.; Bell, A.M. Female sticklebacks transfer information via eggs: Effects of maternal experience with predators on offspring. Proc. R. Soc. B 2011, 278, 1753–1759. [Google Scholar] [CrossRef]
- Segers, F.H.I.D.; Taborsky, B. Juvenile exposure to predator cues induces a larger egg size in fish. Proc. R. Soc. B 2012, 279, 1241–1248. [Google Scholar] [CrossRef]
- Tigreros, N.; Norris, R.H.; Thaler, J.S. Maternal effects across life stages: Larvae experiencing predation risk increase offspring provisioning. Ecol. Entomol. 2019, 44, 738–744. [Google Scholar] [CrossRef]
- Bernardo, J. Maternal effects in animal ecology. Am. Zool. 1996, 36, 83–105. [Google Scholar] [CrossRef]
- Matlock, R.B., Jr. Impact of prey size on prey capture success, development rate, and survivorship in Perillus bioculatus (Heteroptera: Pentatomidae), a predator of the Colorado potato beetle. Environ. Entomol. 2005, 34, 1048–1056. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Fox, C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998, 13, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.W.; Czesak, M.E.; Mousseau, T.A.; Roff, D.A. The evolutionary genetics of an adaptive maternal effect: Egg size plasticity in a seed beetle. Evolution 1999, 53, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mishra, G.; Omkar. Maternal body size and age govern reproduction and offspring phenotype in the zig-zag ladybird beetle (Menochilus sexmaculatus). Can. J. Zool. 2021, 99, 97–105. [Google Scholar] [CrossRef]
- Lucas, É.; Coderre, D.; Brodeur, J. Intraguild predation among aphid predators: Characterization and influence of extraguild prey density. Ecology 1998, 79, 1084–1092. [Google Scholar] [CrossRef]
- Ingels, B.; De Clercq, P. Effect of size, extraguild prey and habitat complexity on intraguild interactions: A case study with the invasive ladybird Harmonia axyridis and the hoverfly Episyrphus balteatus. BioControl 2011, 56, 871–882. [Google Scholar] [CrossRef]
- De Clercq, P.; Peeters, I.; Vergauwe, G.; Thas, O. Interaction between Podisus maculiventris and Harmonia axyridis two predators used in augmentative biological control in greenhouse crops. BioControl 2003, 48, 39–55. [Google Scholar] [CrossRef]
- Pechenik, J.A.; Wendt, D.E.; Jarrett, J.N. Metamorphosis is not a new beginning: Larval experience influences juvenile performance. BioScience 1998, 48, 901–910. [Google Scholar] [CrossRef]
- Green, D.A.; Extavour, C.G. Insulin signalling underlies both plasticity and divergence of a reproductive trait in Drosophila. Proc. R. Soc. B 2014, 281, 20132673. [Google Scholar] [CrossRef]
- Koch, R.L. The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 2003, 3, 1–16. [Google Scholar] [CrossRef]
- Ranjbar, F.; Michaud, J.P.; Jalali, M.A.; Ziaaddini, M. Intraguild predation between two lady beetle predators is more sensitive to density than species of extraguild prey. BioControl 2020, 65, 713–725. [Google Scholar] [CrossRef]
- Polis, G.A.; Holt, R.D. Intraguild predation: The dynamics of complex trophic interactions. Trends Ecol. Evol. 1992, 7, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Seiter, M.; Schausberger, P. Maternal intraguild predation risk affects offspring anti-predator behavior and learning in mites. Sci. Rep. 2015, 5, 15046. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, B.K.; Yasuda, H. Competitive ability of ladybird predators of aphids: A review of Cheilomenes sexmaculata (Fabr.) (Coleoptera: Coccinellidae) with a worldwide checklist of preys. J. Aphidol. 2001, 14, 1–20. [Google Scholar]
- Sugiura, K.; Takada, H. Suitability of seven aphid species as prey of Cheilomenes sexmaculata (Fabricius) (Coleoptera: Coccinellidae). Jpn. J. Appl. Entomol. Zool. 1998, 42, 7–14. [Google Scholar] [CrossRef]
- Chaudhary, D.D.; Kumar, B.; Mishra, G.; Omkar. Functional response in Coccinellid beetles (Coleoptera: Coccinellidae) is modified by prey-density experience. Can. Entomol. 2022, 154, e11. [Google Scholar] [CrossRef]
- Mishra, G.; Singh, N.; Shahid, M.; Omkar. The effects of three sympatric ladybird species on oviposition by Menochilus sexmaculatus (Coleoptera: Coccinellidae). Chemoecology 2013, 23, 103–111. [Google Scholar] [CrossRef]
- Xu, G.; Liu, J.; Wei, Y.; Cai, P. Preliminary study on the biological characteristics of Phenacoccus solenopsis Tinsley. World J. For. 2017, 6, 41–46. [Google Scholar]
- Yu, X.L.; Zhou, Y.T.; Feng, Y.; Qiu, B.L. Direct effect of cannibalism and intraguild predation in Menochilus sexmaculatus (Coleoptera: Coccinellidae). J. Entomol. Sci. 2023. under review. [Google Scholar]
- Tigreros, N.; Norris, R.H.; Wang, E.H.; Thaler, J.S. Maternally induced intraclutch cannibalism: An adaptive response to predation risk? Ecol. Lett. 2017, 20, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.; Zaib, M.S.; Zakria, M.; Hani, U.; Zaka, S.M.; Ane, M.N. Cheilomenes sexmaculata (Coccinellidae: Coleoptera) as a potential biocontrol agent for aphids based on age-stage, two-sex life table. PLoS ONE 2020, 15, e0228367. [Google Scholar] [CrossRef] [PubMed]
- Osawa, N.; Yoshinaga, A. The presence of micropyles in the shells of developing and undeveloped eggs of the ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2009, 106, 607–610. [Google Scholar] [CrossRef]
- Osawa, N. Provision of small sterile eggs is a circumstance-dependent maternal investment in sibling cannibalism in the ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2022, 119, 133–139. [Google Scholar] [CrossRef]
- Walzer, A.; Schausberger, P. Threat-sensitive anti-intraguild predation behaviour: Maternal strategies to reduce offspring predation risk in mites. Anim. Behav. 2011, 81, 177–184. [Google Scholar] [CrossRef]
- Magalhães, S.; Tudorache, C.; Montserrat, M.; van Maanen, R.; Sabelis, M.W.; Janssen, A. Diet of intraguild predators affects antipredator behavior in intraguild prey. Behav. Ecol. 2005, 16, 364–370. [Google Scholar] [CrossRef]
- Dixon, A.F.G.; Guo, Y. Egg and cluster size in ladybird beetles (Coleoptera: Coccinellidae): The direct and indirect effects of aphid abundance. Eur. J. Entomol. 1993, 90, 457–463. [Google Scholar]
- Cohet, Y.; David, J. Control of the adult reproductive potential by preimaginal thermal conditions: A study in Drosophila melanogaster. Oecologia 1978, 36, 295–306. [Google Scholar] [CrossRef]
- Boulétreau-Merle, J.; Allemand, R.; Cohet, Y.; David, J.R. Reproductive strategy in Drosophila melanogaster: Significance of a genetic divergence between temperate and tropical populations. Oecologia 1982, 53, 323–329. [Google Scholar] [CrossRef]
- Stewart, L.A.; Hemptinne, J.-L.; Dixon, A.F.G. Reproductive tactics of ladybird beetles: Relationships between egg size, ovariole number and developmental time. Funct. Ecol. 1991, 5, 380–385. [Google Scholar] [CrossRef]
- Perry, J.C.; Roitberg, B.D. Trophic egg laying: Hypotheses and tests. Oikos 2006, 112, 706–714. [Google Scholar] [CrossRef]
- Osawa, N. Sibling cannibalism in the ladybird beetle Harmonia axyridis: Fitness consequences for mother and offspring. Res. Popul. Ecol. 1992, 34, 45–55. [Google Scholar] [CrossRef]
- Osawa, N. Sex-dependent effects of sibling cannibalism on life history traits of the ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae). Biol. J. Linn. Soc. 2002, 76, 349–360. [Google Scholar] [CrossRef]
- Benton, T.G.; St Clair, J.J.H.; Plaistow, S.J. Maternal effects mediated by maternal age: From life histories to population dynamics. J. Anim. Ecol. 2008, 77, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, T.G.G.; Müller, W.; von Engelhardt, N.; Carere, C.; Eising, C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005, 29, 329–352. [Google Scholar] [CrossRef]
- Bestion, E.; Teyssier, A.; Aubret, F.; Clobert, J.; Cote, J. Maternal exposure to predator scents: Offspring phenotypic adjustment and dispersal. Proc. R. Soc. B 2014, 281, 20140701. [Google Scholar] [CrossRef]
- Coslovsky, M.; Richner, H. Predation risk affects offspring growth via maternal effects. Funct. Ecol. 2011, 25, 878–888. [Google Scholar] [CrossRef]
- Storm, J.J.; Lima, S.L. Mothers forewarn offspring about predators: A transgenerational maternal effect on behavior. Am. Nat. 2010, 175, 382–390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Tang, R.; Liu, T.; Qiu, B. Larval and/or Adult Exposure to Intraguild Predator Harmonia axyridis Alters Reproductive Allocation Decisions and Offspring Growth in Menochilus sexmaculatus. Insects 2023, 14, 496. https://doi.org/10.3390/insects14060496
Yu X, Tang R, Liu T, Qiu B. Larval and/or Adult Exposure to Intraguild Predator Harmonia axyridis Alters Reproductive Allocation Decisions and Offspring Growth in Menochilus sexmaculatus. Insects. 2023; 14(6):496. https://doi.org/10.3390/insects14060496
Chicago/Turabian StyleYu, Xinglin, Rui Tang, Tongxian Liu, and Baoli Qiu. 2023. "Larval and/or Adult Exposure to Intraguild Predator Harmonia axyridis Alters Reproductive Allocation Decisions and Offspring Growth in Menochilus sexmaculatus" Insects 14, no. 6: 496. https://doi.org/10.3390/insects14060496
APA StyleYu, X., Tang, R., Liu, T., & Qiu, B. (2023). Larval and/or Adult Exposure to Intraguild Predator Harmonia axyridis Alters Reproductive Allocation Decisions and Offspring Growth in Menochilus sexmaculatus. Insects, 14(6), 496. https://doi.org/10.3390/insects14060496





