Effects of Tebufenozide on Eggs, Larvae and Adults of Chrysoperla carnea (Neuroptera: Chrysopidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Insecticide
2.2. Experiments
2.2.1. Effects of Tebufenozide on C. carnea Eggs
2.2.2. Effects of Tebufenozide on C. carnea Larval Mortality and Development Times
2.2.3. Larval C. carnea Preference for Untreated vs. Treated Prey
2.2.4. Effects of Consumption of Tebufenozide-Treated Prey on C. carnea
2.2.5. Effects of Tebufenozide on the C. carnea Adults Treated via Ingestion
2.2.6. Statistical Analyses
3. Results
3.1. Effects of Tebufenozide on C. carnea Eggs
3.2. Effects of Tebufenozide on C. carnea Larval Mortality and Development Time
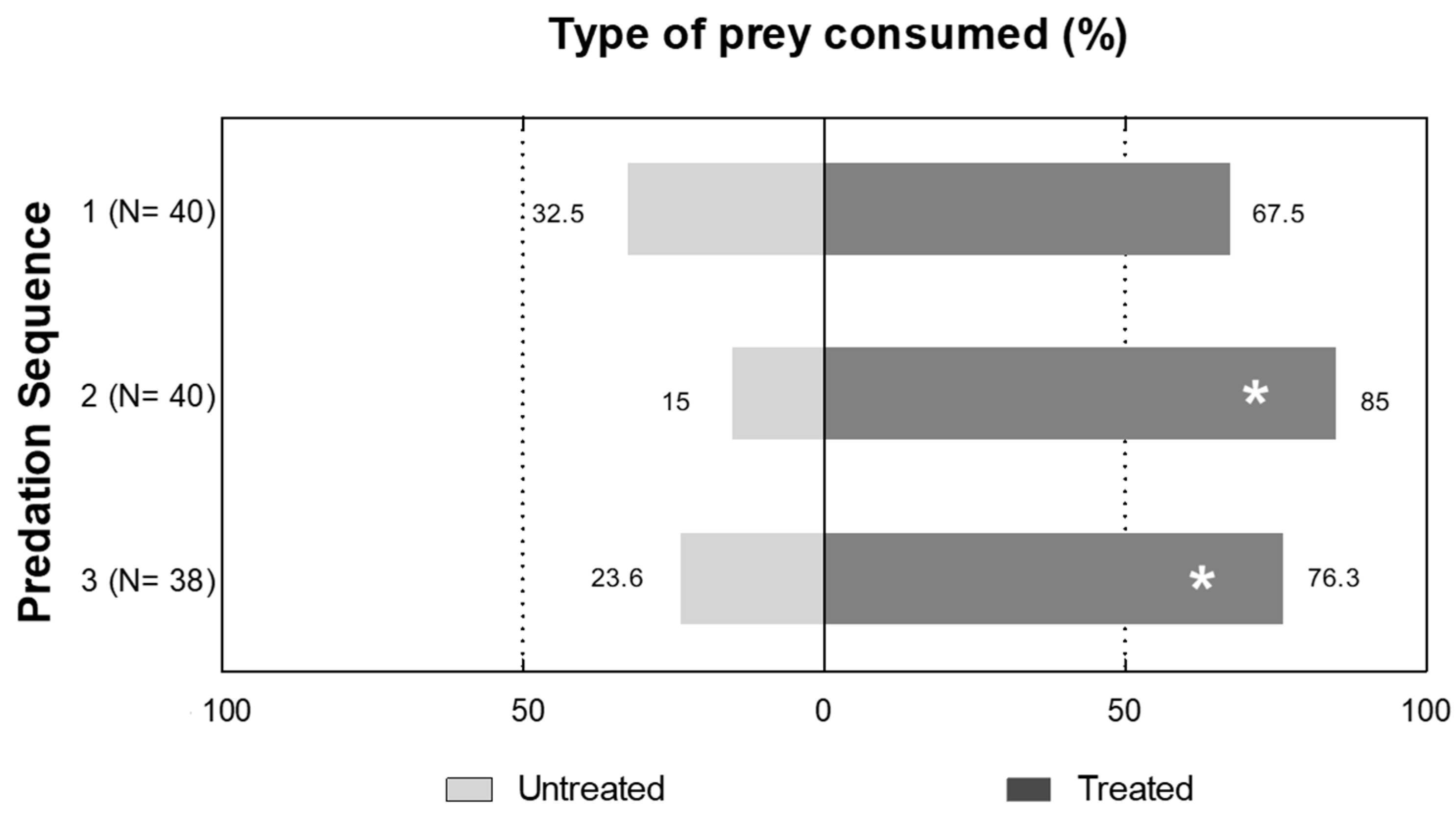
3.3. Preference of C. carnea Prey Selection when Given a Choice between Tebufenozide-Treated S. littoralis Prey and Untreated Prey
3.4. Lethal and Sublethal Effects of Consuming Prey Treated with Tebufenozide on C. carnea Larvae
3.5. Fecundity, Viability and Longevity of Adult C. carnea Treated with Tebufenozide via Ingestion
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flint, M.L.; Van den Bosch, R. Introduction to Integrated Pest Management; Plenum Press: New York, NY, USA, 2012; 240p. [Google Scholar]
- Van Der Blom, J. La introducción artificial de la fauna auxiliar en cultivos agrícolas. Bol. San. Veg. Plagas. 2002, 28, 109–120. [Google Scholar]
- Jonsson, M.; Wratten, S.D.; Landis, D.A.; Gurr, G.M. Recent advances in conservation biological control of arthropods by arthropods. Biol. Control 2008, 45, 172–175. [Google Scholar] [CrossRef]
- Amarasekare, K.G.; Shearer, P.W. Comparing effects of insecticides on two green lacewings species, Chrysoperla johnsoni and Chrysoperla carnea (Neuroptera: Chrysopidae). J. Econ. Entomol. 2013, 106, 1126–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.; Pereira, E.J.; Aguiar, R.W.; Oliveira, E.E. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Dhadialla, T.S.; Carlson, G.R.; Le, D.P. New insecticides with ecdysteroidal and juvenile hormone activity. Annu. Rev. Entomol. 1998, 43, 545–569. [Google Scholar] [CrossRef] [Green Version]
- IRAC (Insecticide Resistance Action Committee). Mode of Action Classification. Available online: http://www.irac-online.org/modes-of-action/ (accessed on 20 November 2020).
- Medina, P.; Smagghe, G.; Budía, F.; Tirry, L.; Viñuela, E. Toxicity and absorption of azadirachtin, diflubenzuron, pyriproxyfen, and tebufenozide after topical application in predatory larvae of Chrysoperla carnea (Neuroptera: Chrysopidae). Environ. Entomol. 2003, 32, 196–203. [Google Scholar] [CrossRef]
- Medina, P.; Budía, F.; Del Estal, P.; Viñuela, E. Effects of three modern insecticides, pyriproxyfen, spinosad, and tebufenozide on survival and reproduction of Chrysoperla carnea (Stephens) adults. Ann. Appl. Biol. 2003, 142, 55–61. [Google Scholar] [CrossRef]
- Ono, E.K.; Zanardi, O.Z.; Santos, K.F.A.; Yamamoto, P.T. Susceptibility of Ceraeochrysa cubana larvae and adults to six insect growth-regulator insecticides. Chemosphere 2017, 168, 49–57. [Google Scholar] [CrossRef]
- Zotti, M.J.; Grutzmacher, A.D.; Lopes, I.H.; Smagghe, G. Comparative effects of insecticides with different mechanisms of action on Chrysoperla externa (Neuroptera: Chrysopidae): Lethal, sublethal and dose–response effects. Insect Sci. 2013, 20, 743–752. [Google Scholar] [CrossRef]
- Canard, M.; Séméria, Y.; New, T.R. Biology of Chrysopidae; Springer: Berlin/Heidelberg, Germany, 1984; Volume 27. [Google Scholar]
- Henry, C.S.; Brook, S.J.; Duelli, P.; Johnson, J.B. Discovering the true Chrysoperla carnea (Insecta: Neuroptera: Chrysopidae) using song analysis, morphology, and ecology. Ann. Entomol. Soc. Am. 2002, 95, 172–191. [Google Scholar] [CrossRef] [Green Version]
- Nordlund, D.A.; Cohen, A.C.; Smith, R.A. Mass-rearing, release techniques, and augmentation. In Lacewings in the Crop Environment; McEwen, P., New, T., Whittington, A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 303–319. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A.; Daane, K.M.; Hagen, K.S. Commercialization of predators: Recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrysoperla). Am. Entomol. 2000, 46, 26–38. [Google Scholar] [CrossRef]
- Pineda, S.; Schneider, M.I.; Smagghe, G.; Martínez, A.M.; Del Estal, P.; Viñuela, E.; Budia, F. Lethal and sublethal effects of Methoxyfenozide and Spinosad on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2007, 100, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Canard, M.; Duelli, P. Predatory behavior of larvae and cannibalism. Biol. Chrysopidae 1984, 27, 92–100. [Google Scholar] [CrossRef]
- Pappas, M.L.; Broufas, G.D.; Koveos, D.S. Effects of various prey species on development, survival and reproduction of the predatory lacewing Dichochrysa prasina (Neuroptera: Chrysopidae). Biol. Control 2007, 43, 163–170. [Google Scholar] [CrossRef]
- Ridgway, R.; Murphy, W.L. Biological control in the field. In Biology of Chrysopidae; Canard, M., Semeria, Y., New, T.R., Eds.; Junk Boston: Boston, MA, USA, 1984; pp. 220–227. [Google Scholar]
- Hassan, S.A. Über die Massenzucht von Chrysoperla carnea. Z. Für Angew. Entomol. 1975, 79, 310–315. [Google Scholar] [CrossRef]
- Poitout, S.; Bues, R. Élevage de chenilles de vingt-huit espèces de lépidopteres Noctuidae et de deux espèces d’Arctiidae sur milieu artificiel simple. Particularités de l’élevage selon les espèces. Ann. De Zool. Ecol. Anim. 1974, 6, 431–441. [Google Scholar]
- Vargas-Osuna, E. La Reproducción de Spodoptera littoralis (Lepidoptera: Noctuidae) y sus Alteraciones por el Virus de la Poliedrosis Nuclear (VPN) (Baculoviridae: Baculovirus). Doctoral Thesis, Universidad de Córdoba, Córdoba, Spain, 1985. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Ortiz Moreno, J.F.; Vargas Osuna, E. Toxicidad de las Delta-Endotoxinas de Bacillus thuringiensis Sobre Larvas de Helicoverpa armigera y Compatibilidad con sus Enemigos Naturales. Doctoral Thesis, Universidad de Córdoba, Córdoba, Spain, 2009. [Google Scholar]
- Sachdev, B.; Zarin, M.; Khan, Z.; Malhotra, P.; Kumar Seth, R. Effect of gamma radiation on Phenoloxidase pathway, antioxidant defense mechanism in Helicoverpa armigera (Lepidoptera: Noctuidae) and its implication in inherited sterility towards pest suppression. Int. J. Radiat. Biol. 2014, 90, 7–19. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [Green Version]
- Nation, J.L. Insect Physiology and Biochemistry; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Pasini, R.A.; Grützmacher, A.D.; Pazini, J.B.; Silva De Armas, F.; Bueno, F.A.; Pires, S.N. Side effects of insecticides used in wheat crop on eggs and pupae of Chrysoperla externa and Eriopis connexa. Phytoparasitica 2018, 46, 115–125. [Google Scholar] [CrossRef]
- Suarez-Lopez, Y.A.; Hatem, A.E.; Aldebis, H.K.; Vargas-Osuna, E. Lethal and sublethal effects of lufenuron on the predator Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). Crop Prot. 2020, 135, 105217. [Google Scholar] [CrossRef]
- Rimoldi, F.; Schneider, M.I.; Ronco, A.E. Susceptibility of Chrysoperla externa eggs (Neuroptera: Chrysopidae) to conventional and biorational insecticides. Environ. Entomol. 2008, 37, 1252–1257. [Google Scholar] [CrossRef]
- Godoy, M.S.; Carvalho, G.A.; Moraes, J.C.; Cosme, L.V.; Goussain, M.M.; Carvalho, C.F.; Morais, A.A. Seletividade de seis inseticidas utilizados em citros a pupas e adultos de Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). Neotrop. Entomol. 2004, 33, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Medina, P.; Smagghe, G.; Budia, F.; Del Estal, P.; Tirry, L.; Viñuela, E. Significance of penetration, excretion and transovarial uptake to toxicity of three insect growth regulators in predatory lacewing adults. Arch. Insect Biochem. Physiol. 2002, 51, 91–101. [Google Scholar] [CrossRef]
- Yu, S.J. Selectivity of insecticides to the spined soldader bug (Heteroptera: Pentatomidae) and its lepidopterus prey. J. Econ. Entomol. 1988, 18, 119–222. [Google Scholar] [CrossRef]
- Mandour, N. Influence of spinosad on immature and adult stages of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). BioControl 2008, 54, 93–102. [Google Scholar] [CrossRef]
- Garzon, A.; Medina, P.; Amor, F.; Vinuela, E.; Budia, F. Toxicity and sublethal effects of six insecticides to last instar larvae and adults of the biocontrol agents Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Adalia bipunctata (L.) (Coleoptera: Coccinellidae). Chemosphere 2015, 132, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, H.; Anjum, S.; Asif, A. Toxicity of some insecticides against cotton jassid Amrasca devastans (Dist.) and its predator Chrysoperla carnea (Steph.). J. Agric. Res. 2017, 55, 311–321. [Google Scholar]
- Maia, J.B.; Carvalho, G.A.; Medina, P.; Garzon, A.; Gontijo, P.; Da, C.; Viñuela, E. Lethal and sublethal effects of pesticides on Chrysoperla carnea larvae (Neuroptera: Chrysopidae) and the influence of rainfastness in their degradation pattern over time. Ecotoxicology 2016, 25, 845–855. [Google Scholar] [CrossRef]
- Bueno, A.F.; Freitas, S. Effect of the insecticides abamectin and lufenuron on eggs and larvae of Chrysoperla externa under laboratory conditions. BioControl 2004, 49, 277–283. [Google Scholar] [CrossRef]
- Smagghe, G.; Zotti, M.; Retnakaran, A. Targeting female reproduction in insects with biorational insecticides for pest management: A critical review with suggestions for future research, Current Opinion. Insect Sci. 2018, 31, 65–69. [Google Scholar] [CrossRef]
- Trisyono, A.; Chippendale, G.M. Effect of the nonsteroidal ecdysone agonists methoxyfenozide and tebufenozide on the European corn borer. J. Econ. Entomol. 1997, 90, 1486–1492. [Google Scholar] [CrossRef]
- Rugno, G.R.; Zanardi, O.Z.; Cuervo, J.B.; De Morais, M.R.; Yamamoto, P.T. Impact of insect growth regulators on the predator Ceraeochrysa cincta (Schneider) (Neuroptera: Chrysopidae). Ecotoxicology 2016, 25, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Mena Castillo, J.D. Compatibilidad del Hongo Entomopatógeno Beauveria bassiana (Balsamo) Vuillemin y el Depredador Chrysoperla carnea (Stephens) Como Agentes de Control de Plagas. Master’s Thesis, Universidad de Córdoba, Córdoba, Spain, 2019. [Google Scholar]
- Pickett, J.A.; Glinwood, R.T. Chemical ecology. In Aphids as Crop Pest; van Emden, H.F., Harrington, R., Eds.; CAB International: Wallingford, CT, USA, 2007; pp. 235–260. [Google Scholar] [CrossRef]
- Navarro-Roldán, M.A.; Gemeno, C. Sublethal effects of neonicotinoid insecticide on calling behavior and pheromone production of tortricid moths. J. Chem. Ecol. 2017, 43, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Gesing, M.A.; Segeler, M.; Müller, C. Sublethal insecticide exposure of an herbivore alters the response of its predator. Environ. Pollut. 2019, 247, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Huerta, A.; Medina, P.; Budia, F.; Viñuela, E. Toxicity via ingestion of four insecticides and the dye floxin-B on larvae and adults of Chrysoperla carnea (Neuroptera: Chrysopidae). Boletín De Sanid. Vegetal. Plagas 2004, 30, 721–732. Available online: https://www.mapa.gob.es/ministerio/pags/Biblioteca/Revistas/pdf_plagas%2FBSVP-30-04-721-732.pdf (accessed on 1 January 2020).
- Singh, S.R.; Walters, K.F.A.; Port, G.R.; Northin, P. Consumption rates and predatory activity of adult and fourth instar larvae of the seven spot ladybird, Coccinella septempunctata (L.), following contact with dimethoate residue and contaminated prey in laboratory arenas. Biol. Contr. 2004, 30, 127–133. [Google Scholar] [CrossRef]
- Giolo, F.P.; Medina, P.; Grützmacher, A.D.; Viñuela, E. Effects of pesticides commonly used in peach orchards in Brazil on predatory lacewing Chrysoperla carnea under laboratory conditions. BioControl 2009, 54, 625–635. [Google Scholar] [CrossRef]
- Smagghe, G.; Degheele, D. Action of the nonsteroidal ecdysteroid mimic, RH-5992 on insects of different orders. Pestic. Sci. 1994, 42, 85–92. [Google Scholar] [CrossRef]
- Poletti, M.; Maia, A.; Omoto, C. Toxicity of neonicotinoid insecticides to Neoseiulus californicus and Phytoseiulus macropilis (Acari: Phytoseiidae) and their impact on functional response to Tetranychus urticae (Acari: Tetranychidae). Biol. Control 2007, 40, 30–36. [Google Scholar] [CrossRef]
- He, Y.J.; Zhao, Y.; Zheng, N.; Desneux, K. Lethal effect of imidacloprid on the coccinellid predator Serangium japonicumand sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicology 2012, 21, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.; Hunt, T.E.; Giesler, L.J.; Siegfried, B.D. Thiamethoxam toxicity and effects on consumption behavior in Orius insidiosus (Hemiptera: Anthocoridae) on soybean. Environ. Entomol. 2017, 46, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.; Smagghe, G.; Pineda, S.; Vinuela, E. The ecological impact of four IGR insecticides in adults of Hyposoter didymator (Hym., Ichneumonidae): Pharmacokinetics approach. Ecotoxicology 2008, 17, 181. [Google Scholar] [CrossRef] [PubMed]
- Kanna, S.S.; Chandrasekaran, S. Safety of emamectin 5 SG on Trichogramma chilonis and Chrysoperla carnea under laboratory conditions. J. Ecotoxicol. Environ. Monit. 2006, 16, 509–514. [Google Scholar] [CrossRef]
| Dose (mL/L) | Eggs 1 Day after Oviposition | Eggs 2 Days after Oviposition | ||
|---|---|---|---|---|
| n | % Viability ± SE | n | % Viability ± SE | |
| 0 | 130 | 88.58 ± 5.27 | 140 | 90.68 ± 2.16 |
| 0.75 | 147 | 88.58 ± 3.04 | 207 | 88.43 ± 2.73 |
| Doses (mL/L) | N | Mortality | n | Larval Development Time | n | Pupal Duration | |
|---|---|---|---|---|---|---|---|
| % | Corrected 1 | Mean ± SE (Days) | Mean ± SE (Days) | ||||
| 0 | 60 | 3.3 | - | 46 | 5.95 a ± 0.234 | 41 | 9.80 a ± 0.136 |
| 0.012 | 60 | 1.7 | - | 53 | 4.63 bc ± 0.175 | 47 | 9.45 bc ± 0.113 |
| 0.06 | 60 | 1.7 | - | 58 | 4.63 c ± 0.181 | 51 | 9.27 c ± 0.063 |
| 0.30 | 60 | 1.7 | - | 47 | 4.54 c ± 0.158 | 46 | 9.41 bc ± 0.073 |
| 0.15 | 60 | 6.7 | 3.4 | 49 | 5.30 b ± 0.227 | 43 | 9.51 bc ± 0.090 |
| 0.75 | 60 | 16.7 * | 13.4 | 36 | 4.68 c ± 0.188 | 31 | 9.68 ab ± 0.149 |
| Doses (mL/L) | N | Mortality | n | Larval Development Time | n | Pupal Duration | |
|---|---|---|---|---|---|---|---|
| % * | Corrected 1 | Mean ± SE (Days) | Mean ± SE (Days) | ||||
| 0 | 57 | 7.01 | - | 53 | 8.00 a ± 0.47 | 40 | 9.58 a ± 0.24 |
| 0.15 | 59 | 11.86 | 4.78 | 52 | 6.94 ab ± 0.47 | 42 | 9.42 a ± 0.23 |
| 0.75 | 54 | 9.25 | 2.17 | 49 | 6.16 b ± 0.48 | 42 | 9.57 a ± 0.23 |
| Doses (mL/L) | N | Fecundity (Number of Eggs) | % Viability | Female Longevity (Days) | Male Longevity (Days) |
|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||
| 0 | 15 | 112.53 ± 19.10 | 95.17 ± 0.88 | 16.53 ± 1.96 | 25.80 ± 1.96 |
| 0.15 | 16 | 92.56 ± 18.49 | 91.58 ± 0.88 | 17.56 ± 1.89 | 25.06 ± 1.90 |
| 0.75 | 13 | 142.46 ± 20.51 | 96.02 ± 0.95 | 18.69 ± 2.10 | 27.15 ± 2.11 |
| Doses (mL/L) | Fecundity(Number of Eggs) | % Eggs Hatchability | Female Longevity (Days) | Male Longevity (Days) | |
|---|---|---|---|---|---|
| N | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| 0 | 10 | 356.8 ± 49.75 | 78.1 ± 6.38 | 38.4 ± 7.05 | 40.7 ± 17.18 |
| 0.75 | 10 | 313.3 ± 36.94 | 77.5 ± 5.47 | 35.7 ± 10.04 | 43.6 ± 17.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez-Lopez, Y.A.; Hatem, A.E.; Aldebis, H.K.; Vargas-Osuna, E. Effects of Tebufenozide on Eggs, Larvae and Adults of Chrysoperla carnea (Neuroptera: Chrysopidae). Insects 2023, 14, 521. https://doi.org/10.3390/insects14060521
Suarez-Lopez YA, Hatem AE, Aldebis HK, Vargas-Osuna E. Effects of Tebufenozide on Eggs, Larvae and Adults of Chrysoperla carnea (Neuroptera: Chrysopidae). Insects. 2023; 14(6):521. https://doi.org/10.3390/insects14060521
Chicago/Turabian StyleSuarez-Lopez, Yurany Andrea, Adel E. Hatem, Hani K. Aldebis, and Enrique Vargas-Osuna. 2023. "Effects of Tebufenozide on Eggs, Larvae and Adults of Chrysoperla carnea (Neuroptera: Chrysopidae)" Insects 14, no. 6: 521. https://doi.org/10.3390/insects14060521






