Simple Summary
Forensic entomology is a globally accepted field of science that incorporates insect knowledge into crime scene investigations. It is crucial to update available concepts, procedures, and techniques of forensic entomology from time to time with new research findings for better use in the field and the laboratory. The current review provides a current account of field and laboratory guidelines for forensic entomologists and crime scene investigators/technicians to conduct entomological procedures related to casework.
Abstract
Forensic entomology is a branch of forensic science that incorporates insects as a part of solving crime. Insect-based evidence recovered at a crime scene can be used to estimate the minimum postmortem interval, determine if a carcass/corpse has been relocated, and contribute to the cause and manner of death. The current review summarises the stepwise usage of forensic entomology methods at a crime scene and in the laboratory, including specimen collection and rearing, identification, xenobiotic detection, documentation, and referencing previous research and casework. It also provides three standards for the collection of insects when attending a crime scene. The Gold standard attributes to a forensic entomologist (FE) who is likely to be well-trained attending a scene. The subsequent standards (Silver and Bronze) have been added because the authors believe that this information is currently missing in the literature. The purpose is so that an attending crime scene agent/proxy with some basic knowledge and some simple tools can recover almost all the insect information required by an FE to make the best estimation of the minimum postmortem interval.
1. Introduction to Review
Although an update, this review is different to other publications which simply advise on the necessary processes and tools required by a fully trained forensic entomologist (FE) to conduct an entomological analysis of a crime scene [1,2]. In contrast to the conclusions of Hall et al., 2012 [1], that insect evidence should be collected and preserved either by an appropriately qualified person or under their instruction, the outcome of this paper is to provide an overview of how inexperienced personnel (with some training), such as crime scene technicians, forensic officers and proxies, attending a crime scene can sample and collect insect material, allowing the FE to make the best estimate of the time since death.
Introduction to Forensic Science
Forensic science is a crucial constituent in crime-solving that agglutinates the investigation with the subsequent judicial activities [3,4]. Today, forensic science has diverged into numerous subfields; some of the more widely known sciences are forensic entomology, forensic toxicology, forensic anthropology, and forensic microbiology [4,5]. These disciplines provide principles and procedures to recover and analyse crime-associated evidence to reconstruct the actual circumstances of a crime scene [3]. This facilitates in the determination of a crime, which eventually results in a prosecution [6].
The physical evidence recovered from a crime scene is primarily used to verify offenders while exonerating any innocent persons, as well as how, when, and where the crime was committed [7]. The recovered physical evidence provides either observation-based clues or may need to be processed by advanced techniques to arrive at further conclusions. As an example, a microscopic analysis of hair could provide information such as the age, gender, and race of an individual present at a crime scene. This may be followed up with a DNA analysis and spectroscopic analysis, providing details relating to an individual’s identification and the presence of explosive and drug residues, respectively [8].
2. Forensic Entomology for Crime Scene Investigations
What is forensic entomology? It is known for using insects and other arthropods to aid in solving criminal and civil crimes [9,10]. Generally, forensic entomology involves three main subcategories: urban, stored product, and medicolegal entomology [8]. Urban entomology investigates the insect-related structural defects of buildings and medical myiasis [11,12]. Stored product entomology is used to assess biosecurity risks associated with food contamination and environmental pollution [13]. The last category, medicolegal entomology, uses insect-based evidence to investigate numerous criminal activities associated with homicides, suspicious fatalities, abuse of vulnerable people, hospital neglect, animal cruelty, contraband trafficking, and wildlife poaching [9,10,14].
The primary function of insects in crime solving is as biological indicators to estimate the time elapsed since death or the minimum postmortem interval (minPMI) [15]. Furthermore, the colonisation time of dipteran larvae in myiasis wounds can provide an indication of the time of neglect for humans, pets, and livestock [16]. The two fundamental principles of utilising insects are determining the minPMI based on their predictable temperature-based development and the sequential colonizing patterns on carcasses [9]. Flies (Order Diptera) are the main protagonists of all the carrion arthropods that are predominantly considered for use in determining the postmortem interval due to their involvement in carcass decomposition as both early and enduring colonisers [17,18]. The role of flies in carcass decomposition interchanges between scavengers of decaying tissues and prey to other predacious invertebrates (e.g., beetles and ants) and vertebrates (e.g., hyenas and vultures) [18,19] (Figure 1a–d).
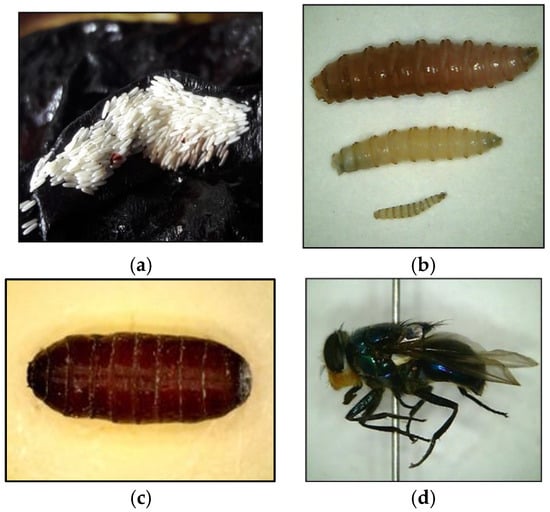
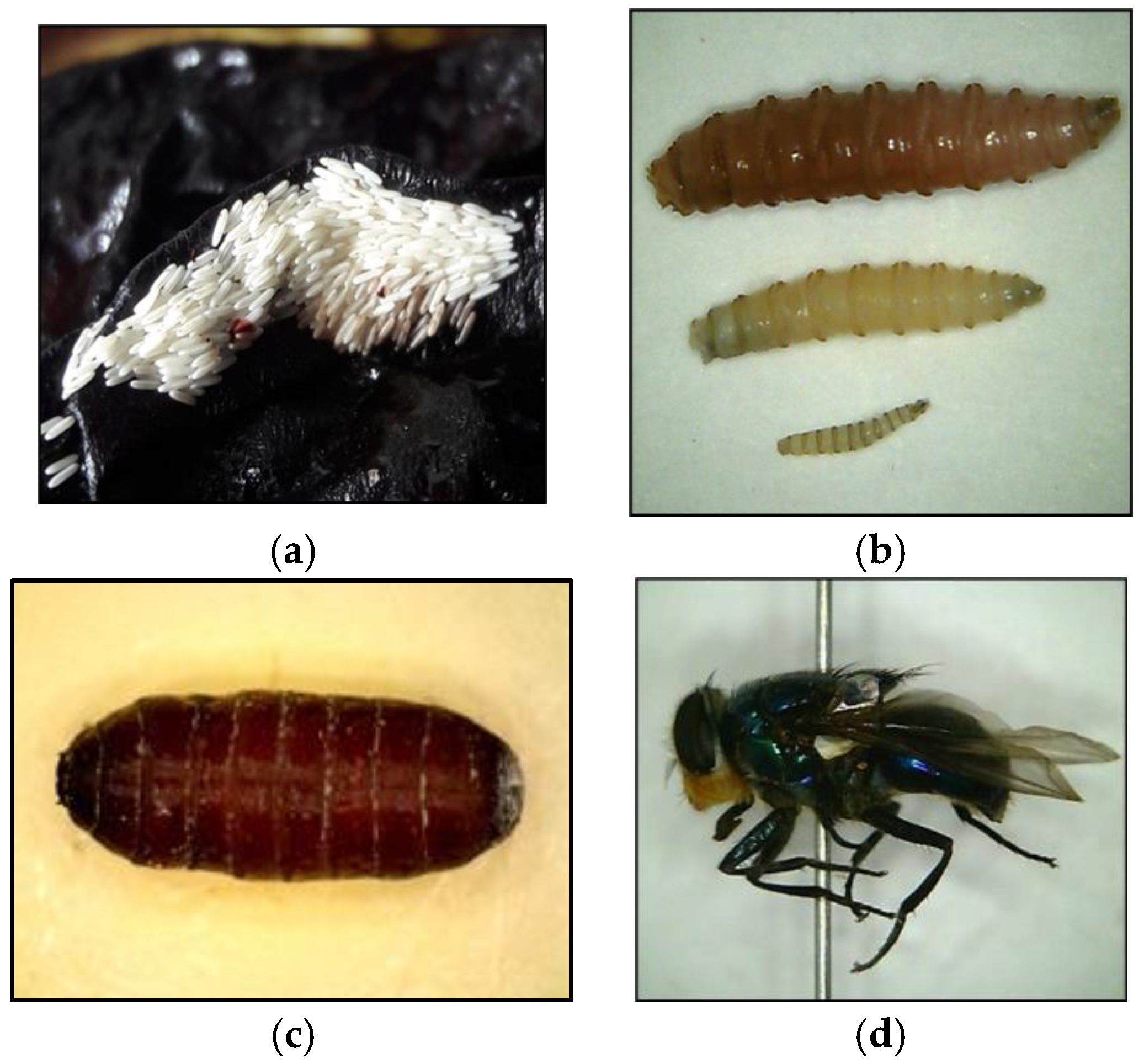
Figure 1.
The different life stages of a fly. The measurements added are from Byrd and Tomberlin, 2020 [9]. (a) Fly egg raft (size range of single egg 1.2–2 mm). (b) Three larval instars (in ascending order; third, second, first instar (size range from 2–23 mm). (c) Fly pupa (size range from 6–23 mm). (d) Adult fly (size range from 6–14 mm).
Typically, the minPMI for early stages of carcass decomposition (i.e., 3–72 h following death) is estimated by pathological methods [20]. However, after this period, fly eggs, larval, and pupal specimens become one of the most reliable indices for the age determination of a carcass [21,22]. This is achieved by three methods: (1) calculating the accumulated degree day/hour units, (2) referring to life history tables, and (3) using species-specific isomegalen/isomorphen curves [23]. In addition, the minPMI in advanced stages of decomposition can be estimated by assessing the succession patterns of flies and other carrion-associated invertebrates, although this is generally less accurate [24]. However, estimating the minPMI based on fly development in advanced stages of decomposition has some drawbacks, for example, the fly larvae and full puparia sampled may not represent the earliest colonisers, which may have already completed their life cycle [25,26]. Generally, the predicted sequence of attracting flies to carrion begins with the Calliphoridae, followed by Sarcophagidae, Muscidae, Sphaeroceridae, Piophilidae, Fannidae, and Phoridae [9,23]. Carcasses are also colonised by beetles (Staphylinidae, Scarabaeidae, Carabidae, Silphidae, Cleridae, and Dermestidae); moths (Pyralidae and Tineidae); and mites (Pterygosomatidae and Omentolaelapidae), which can also be forensically significant [27,28]. Furthermore, the sequence of these invertebrates invading a carcass can vary based on external and internal influences, such as changes in the carcass characteristics (size, nutrient composition, existing decomposition stage, and xenobiotics), abiotic (temperature and humidity), and biotic (terrestrial and aquatic habitats) factors [26].
Apart from estimating the minPMI, entomological assessments in some cases can be made to determine the transfer of carcasses from their original crime locations [29]. This is underpinned by the concept that the entomofauna of a carcass resembles the overall insect diversity of the environment where it originally resided, and any secondary movement to an alternative place could change this crime location-specific entomofauna [30,31]. This is generally context driven, depending on the crime scene location and environmental conditions. In addition, disturbance to a carcass may cause any fly larval aggregations to disperse, which are especially visible when densely packed larval masses are present [9]. Additionally, there is a potential use of the characterised internal and external microbial profiles of larval masses infesting carcasses to verify the originality of a crime scene. This could be achieved by assessing the homogeneity of microbial profiles between the larval masses and the crime scene [32].
It has been widely demonstrated that fly tissues can be potentially used for victim identification and determining the cause of death under entomogenetics and entomotoxicology [33,34]. This is crucial when the tissues and fluids of victims are not available to recover from the body for analysis due to the occurrence of tissue degradation by larval feeding, scavenging, and burning [35,36]. An analysis of the contents of parts of the fly alimentary tract using DNA may also reveal the identity of a victim [37]. To identify fly species, DNA can also be extracted from flies at various stages of life, followed by sequencing species-specific nucleotides of their genes, and then referring these to a genetic database [33,34]. Moreover, the age estimation of larvae and pupae based on gene expression is another molecular approach [38].
Previous entomotoxicological studies have shown that fly tissues can be deployed to detect alcohol (e.g., ethanol), drugs (e.g., antidepressants, barbiturates, benzodiazepines, opioids, and phenothiazine), metals (e.g., thioridazine, antimony, barium, cadmium, lead, and mercury), and pesticides (e.g., malathion, parathion) [39,40]. However, the success of these genetic and toxicological determinations using flies depends on the species, development stages, feeding activities, environmental conditions, insect sampling techniques, specimen number, sampling frequency, and insect killing and preserving method [40].
2.1. Forensic Entomologist: An Apprentice to an Expert
The analyses of entomofauna at a crime scene is complex, involving sampling and identifying insects, determining species–habitat relationships, evaluating the development stages and successional patterns, and assessing insect ethology concerning variations in climatic parameters [41]. Becoming a practitioner in forensic entomology requires training in the fundamentals of entomology and ecology. To conduct these tasks, an FE must understand concepts such as insect physiology, taxonomic and phylogenetic relationships, the behavioural adaptation of insects for different ecological setups, and their impacts on human affairs [42,43]. This training should then be narrowed down to specific topics that directly address the diagnostic characteristics and interrelationships of forensically important insect groups. This subset of knowledge related to forensic entomology can be acquired by following an undergraduate or postgraduate level specialised course or a degree program [44]. In most countries, these academic qualifications provide the required accreditation and recognition for an FE to work with their local law enforcement agencies [9,44]. Please note that any academic pursuit must go hand in glove with the understanding and practical aspects of crime scene attendance and protocols, as well as the preparation of expert witness statements and the presentation of such evidence to the court.
These skills and experiences can be developed by conducting mock crime scene trials utilizing human surrogate carcasses (e.g., swine) [43,45]. It is best practice to conduct these field studies with an FE who has experience in actual casework [43,45]. In addition, it is essential to acquire background knowledge of other related forensic subfields such as molecular biology, microbiology, taphonomy, toxicology, pathology, and criminalistics that may be integrated with forensic entomology [44,46].
2.2. Forensic Entomology in Crime Investigation: Complexity and Constraints
Insects play a pivotal role in carcass decomposition [47]. Flies and other arthropods visit a carcass if there are no physical barriers to block their access. When the food source (carcass) is exhausted, the insects leave [48]. The insect colonisation of a carcass primarily relies on the seasonal- and regional-based climate, including temperature, humidity, and photoperiod. It also relies on the intra and interspecies dynamics, including feeding preferences and competition and various habitat scenarios such as terrestrial and aquatic, urban and rural, indoor and outdoor, and burial and exposed [26]. In addition, secondary factors such as the method of death, including burning, poisoning, drowning, dismemberment, concealment, and hanging, as well as carcass features such as age, size, clothing, and trauma also influence the patterns of insect colonisation while impacting on the decomposition process. This complexity associated with insect and carcass interactions consistently challenges an FE to conduct their tasks diligently and accurately. However, knowledge of local entomofauna, prior experience participating in insect-related casework, and the skills in incorporating research into casework are the building blocks required to best estimate the minPMI [49].
Besides these carcass insect complexities, an FE must also deal with many regulatory challenges such as interdisciplinary cooperation with other investigators at crime scenes, including other scientists and forensic field agents, strict deadlines to submit expert witness statements, and courtroom conflicts [50,51].
2.3. Progression and Standardisation in Forensic Entomology
Several early classic works by Mégnin (1894) [52] and Payne (1965) [53] highlighted the importance of insects in contributing to the carrion decomposition process. These studies laid the conceptual framework to utilise insects in solving crime. Mégnin (1894) [52] proposed the first formal definition and testable mechanism of insect succession in carrion, and Payne (1965) [53] emphasised the significant involvement of insects in reducing carrion biomass in the early stages of decomposition. Following these formative works, many studies have been conducted in different regions of the world to introduce identification keys for carrion insects, document carrion-associated insect succession under different temporal and spatial conditions, record fly development periods under field and laboratory conditions, and unite forensic entomology with other related fields such as genetics, toxicology, and microbiology [15,54]. These findings have been incorporated into many crime investigations, published as casework in books and scientific journals, presented in conference proceedings, and sometimes proffered by popular media. All have contributed to the establishment of forensic entomology as a reliable subfield of forensic science [54].
The standardisation of entomological methods developed for solving crime has strengthened the science of forensic entomology and has allowed this science to gain more visibility and acceptability among law enforcement agencies. Several crucial studies have been published recently that critically evaluate many successional and development studies, bridging the gap between research and casework. This has led to the improvement of standards and protocols for the FE to use in real crime situations [23,55,56,57].
Numerous attempts have been made to propose global standards to assist an FE in performing their work logically and systematically under field and laboratory conditions [2,9,56,58]. These standards outline the basic procedures and the equipment required to conduct entomology-associated tasks by an FE at a crime scene and in a laboratory to generate accurate estimates of the minPMI [9,58]. However, these standards are not rigid and can be modified (Table 1) based on the degree of training and experience of the practitioner, availability of resources, and the circumstances of the crime scene. The three standards presented here (Gold, Silver, and Bronze) do not substantially change the basic procedures but provide some alternative ways to limit any misinterpretations [9,49]. At times, an FE cannot attend a crime scene to perform the standard entomology procedures (Gold standards) and it is left to a proxy (e.g., pathologist, crime scene technician or a law enforcement agent) with limited entomological expertise to collect the insect material. The alternative methods (Silver and Bronze standards) are for these proxies. These representatives have different degrees of knowledge, ranging from direct training from an FE and prior experience assisting an FE at a crime scene (Silver standard) to minimal or zero exposure to forensic entomology (Bronze standard). Although they are by no means perfect, these procedures may still be adequate for an FE to construct a reasonable estimate of the minPMI. This evidence may not serve as court evidence but simply contribute to “collar and cuffing evidence”, giving investigators a time frame to include or exclude people associated with the crime. To assist in this endeavour, a quick study of a standard operating procedure concerning the collection of insect evidence or viewing an entomological crime scene application [59] may be appropriate. Such standards should be revised in a timely manner to upgrade them with novel concepts and techniques. In the next section of this review, a crime scene technician or proxy may find the following information useful and set the context for them as to the underlying requirements of the FE for calculating the minPMI.

Table 1.
A guide detailing three standards for forensic entomologists and their proxies to aid in collecting and preserving insect specimens.
3. Crime Scene and Health Pathology Facility
The following procedures fall into two major categories based on the location where specific entomological interpretations are undertaken: crime scene/pathology facility and the entomology laboratory [1,9].
Generally, FEs or proxies collect insect evidence at a crime scene, or later at a health pathology facility, where autopsies are usually conducted. The remains are assigned to these facilities by police, pathologists, and medical examiners/coroners. A request then follows if insect material is evident to determine the minPMI and/or provide a general statement about the entomological context of the crime scene [10,49].
At any of these sites, an FE or proxy must perform several standard tasks (Table 1), namely, the collection of the crime scene parameters (being present or via video and photos), including climatic parameters, insects, and their remnants (e.g., mouth parts, appendages, wings, empty puparia). Following collection, some insects may be preserved and stored, and some placed (40% of eggs larvae and full puparia) on a medium to be reared, followed by transporting these live and dead specimens to an entomology laboratory for further analysis [1,2,9].
An FE or proxy should adhere to the general guidelines set by the law enforcement agency controlling the crime scene [49]. An FE or proxy, similar to all other investigators at a crime scene, must wear the appropriate clothing and personal protection equipment (e.g., closed-toed shoes, long pants, scrubs, (disposable overalls) and masks) to avoid any possible contamination and biohazard risks [9,49]. They must visit the scenes with the necessary equipment and documentation to fulfil three major purposes. Firstly, collecting insects (live and/or dead): labelled plastic containers and vials, a handheld lens, hot water (insulated bottle), forceps, fined tipped artist paint brushes, an insect net, bedding materials (e.g., dry sand, sawdust, vermiculite), and food sources (e.g., beef, pork pieces) for rearing purposes, and ethanol (ethyl alcohol) for killing and preserving insects. Secondly, gather the microclimatic data: a portable weather gauge, an infrared thermometer, and a temperature data lodging device. Thirdly, document necessary information via prepared data entry sheets. An FE should have an a priori knowledge of the standard operating procedures of law enforcement at a particular location and this should determine if other devices such as a camera and a video recorder should be included equipment [9,49].
3.1. Collection of Onsite Data
The collection and preservation of entomological evidence at a crime scene should be carried out as soon as possible after a body is discovered [60]. The delayed recording and sampling of entomofauna could potentially generate misleading information. This is due to the development, succession, and orientation patterns of carrion insects, which continuously alter with climatic changes and other interruptions caused by inadvertent destruction by investigator activities and scavenging of the remains by other vertebrates and invertebrates [61].
Due to a possible high risk of damage and contamination, the insect evidence should be safeguarded by the law enforcement agency protocols at a crime scene. When law enforcement establishes a crime scene perimeter, it is essential to consider the area where insect activity may be evident in and around a carcass [62]. This is dependent on the decomposition stage and the age of the immature insects on the remains [49]. Specifically, eggs and feeding larval stages of flies are restricted to the carcass; however, post-feeding larval and pupal stages can be dispersed underneath the carcass, within the surrounding cadaveric soil, some distance from the remains and buried in the soil. In dwellings, these stages maybe located near physical objectives present in the vicinity of the carcass (e.g., under carpets, cupboards, and other household items) [1,9]. In addition, the dispersion of adult flies inside dwellings could also be influenced by nearby fly attraction sources, such as lights, windows, and mirrors [48]. Although not always possible, the best time to visit an outdoor crime scene to conduct an entomofauna investigation is in the daytime. Firstly, the perspective is clearer of the area surrounding the crime scene, and secondly, insect fauna is mostly diurnal. Undesirably, due to the utilisation of light sources at night around a crime scene, many other extraneous insects may be attracted to the remains compromising any typical insect activity surrounding the carcass [53].
It is essential to request from the forensic investigator in charge any related information concerning the management of the scene. This should include photographs and video footage from any initial visits of investigators to the scene, and any evidence of physical alterations made to the immediate environment after the remains were discovered (e.g., opening windows, switching on/off heaters or air conditioners, and placing light sources to conduct investigations at night). A potential bias associated with a crime scene is informing the FE or proxy about the date of the last sighting of the deceased. Such information should ideally only be obtained after estimations of the minPMI have been ascertained by the FE [51].
Following the arrival at a crime scene and consulting with law enforcement, the FE or proxy should be able to view the remains and observe the patterns of insects on and around the carcass. Following this assessment, the FE or proxy can realise what tools are required to work the scene. Sometimes, large larval aggregations may be present in and around the natural orifices of carcasses, such as in and around the mouth, nostrils, ears, and the genital region [63]. These body regions provide protection and suitable soft tissues for larval feeding in the early stages of development [63,64]. These early colonizing sites can also occur on other parts of the body based on factors such as surface humidity, insulation, body orientation, clothing, and the wrapping of bodies and wounds [64]. For instance, when bodies are hung above the ground, decomposition fluids and larvae fall on the ground directly beneath the body, known as the ‘drip zone’ [65]. In addition, when bodies are enclosed in suitcases, fly egg clusters may be visible on the exposed zips of these suitcases [66]. These observations of early fly oviposition and larval aggregations may provide useful insights on antemortem determinations, such as the cause and manner of death [64].
The study of carrion decomposition is also an integral part of the basic training to become an FE, and typically involves the determination of the decomposition stage of a cadaver [61]. The insect colonizing patterns associated with different decomposition stages can provide clues to determine the minPMI [67]. Generally, on land, carcass decomposition comprises five main stages: fresh, bloated, active decay, advanced decay, and dry/remain stages [68]. In contrast, in water, this is generally six stages: submerged fresh, early floating, floating decay, bloated deterioration, floating remains, and sunken remains [69]. When assuming an existing decomposition stage, the estimated time to reach that stage should then be calculated in conjunction with specific factors, which directly modify the chemical composition of the carcass and in turn reflect the insect colonisation patterns. These factors include the climatic parameters (temperature, humidity, and photoperiod), the fly access (concealment and inside dwellings), and how the decedent died (e.g., hanging, burning, and drowning). Typically, hanging bodies require sampling from both the corpse and from the drip zone, if it has established on the soil directly below the corpse. A light burn might enhance the body for colonisation as the skin splits, but a more severe burn repels insects [9]. When a carcass is submerged in a body of water, it reduces terrestrial faunal attraction while favouring access by aquatic fauna. In addition, size and age of the carcass impacts the constitution and amount of flesh and body liquids affecting insect colonisation and decomposition [26]. Additionally, it is vital to record any other sources nearby that may impact insect attraction to the carcass (garbage bins, toilets, waste management sites, and another carcass). This could significantly influence the insect abundance and successional patterns [9].
3.2. Collection of Microclimatic Data at a Crime Scene
Insects are poikilothermic, their body temperatures fluctuate along with the ambient temperatures to be in homeostasis [70]. Therefore, entomological assessments at crime scenes should be conducted concerning ambient temperatures, as it affects the development and succession patterns of carrion insects [71,72]. Specifically, temperature impacts insect development by regulating their movement, metamorphosis, reproduction, and diapause [73]. In contrast, succession patterns change with temperature fluctuations as it affects the microbial activities associated with the carcass decomposition [74].
The best available source to obtain environmental temperature readings at a crime location is the crime scene itself matched to the temperatures from the nearest meteorological station [2]. Investigators can request daily or hourly temperature data for as many days as required prior to discovering the remains [2,9,75]. However, when cadavers are inside a dwelling, these meteorological station data are only helpful if the indoor space is open to the outdoor by windows, doors, and vents. In such situations, the outside temperature data must be paired with the indoor temperatures when constructing the thermal simulation model for the crime scene environment [76]. In situations where the indoor temperature is controlled (heaters, fans, and air conditioners), the temperature produced by these devices should be reviewed as part of the temperature data. These temperatures must also be correlated with the actual temperature readings at the site [9]. At the crime scene, hourly or minimum/maximum temperatures can be measured by a temperature data logging device placed near the carcass, protected with a Stevenson screen [49]. It is suggested that meteorological data be optimised by conducting a site-specific error calculation via a regression analysis to estimate the minPMI [77,78].
Apart from the environmental temperatures, body, water, soil, and larval mass temperatures should also be recorded based on the context of the crime. A contactless infrared thermometer can measure the body, and larval mass temperatures, and the soil and water body temperatures can be recorded by a temperature probe [9,49]. The decision of the temperature data most relevant for minPMI estimations must be made based on their degree of heat transfer for larval development. For example, it is necessary to consider aggregated larval mass (maggot mass) temperature for estimating the minPMI. As a rule, large larval masses can be up to 20 °C above ambient temperature, so that when larvae are identified as being part of a larval mass, this heat source is then the most relevant to late second instar and third instar larval growth, not the ambient temperature [23].
In addition, it is vital to record extreme weather events, such as heavy rain within the period of interest, as these events could also affect the arrival, development, and succession patterns of carrion insects [79]. Humidity and photoperiod can also be recorded along with temperature. Moon phase is also important, as a waxing gibbous moon may affect adult blow fly movement. Such data are vital when recreating crime scene conditions, or a research study in a growth chamber to determine the development rate of larvae [80].
3.3. Recovery of Insects for Storage or Rearing Purposes
The specimens collected at a crime scene should be a clear cross-section of the different development stages (eggs, larvae, full and empty puparia, and adults) of various insect successional groups (flies, beetles, mites, and moths) wherever located on the carcass and its surrounding environment [2]. The number of individuals recovered at each stage of development must be adequate for conducting a range of entomological assessments in the laboratory (i.e., species identification, PMI estimation, and xenobiotic determination). However, these sampling numbers depend on their availability and could range from a single egg or larva to several egg rafts and larval masses [81].
Insects collected at a crime scene should be divided into two batches, one batch preserved and the other reared to adult. This is generally performed at the scene, but if appropriate equipment is unavailable, then they must be cooled (not frozen) to slow down development and dispatched rapidly to a laboratory [9]. Preserved specimens are used for identifying the species, determining their age at the time of sampling based on their instar stage, the larval length and width measurement, and extracting gut contents for genetic and toxicological analysis [82].
3.3.1. Preservation of Insects
As mentioned above, insects recovered from a carcass should be preserved at their collection point [9]. When kept alive in containers to preserve for a later time and not cooled, their age status will alter, may exacerbate some cannibalistic behaviour, and reduce the number of collected larvae for further analyses [83]. In such circumstances, the development rate is compromised, and such samples should not be used to estimate the minPMI [83]. However, if a complete record of the thermal history of a sample is provided, an FE should be able to backtrack the age of the insects at the collection time and extrapolate useful information concerning the minPMI for the investigators.
Fly eggs and adult stages can be difficult for estimating the PMI, as limited knowledge is available pertaining to only a few species [9,84]. However, sometimes, the only samples collected are these life history stages, and a practiced FE may be able to identify the morphological changes that occur during egg embryogenesis [78], as well as using some of the methods described by Tyndale-Biscoe (1984) [85] with regard to the age of adults, especially those captured inside a dwelling. Once eggs are located, they can be collected after differentiating them from soil and litter particles using a handheld lens and a fined-tipped artist paint brush [86]. In contrast, the adult flies at the scene can be trapped using an insect net. Following capture, they can simply be removed by dipping or spraying the fly with 70% alcohol and then transferring the dead specimen to a vial. If alcohol is unavailable, then the adult fly can be captured in the net and carefully placed into an insect killing jar prior to preservation [9]. Alternatively, insect sticky paper can be used during daylight to passively collect adult flies [87]. Following their collection, individual flies can be extracted from the sticky paper using a fine-tipped paintbrush and vegetable oil.
When collecting delicate first and second instar larvae, a fine-tipped paintbrush can be used. Older pre- and post-third instar larvae and pupal stages can be removed by forceps or a spoon (i.e., designed to fit inside a collection vial) [9,49]. Unlike forceps, which are limited to collecting individual larva, a spoon can easily collect a large cross-section of larvae across a surface or inside a larval mass [49]. Collected larvae should then be placed in hot water (not boiling) for approximately 1 min before being placed in vials containing 80% ethanol [88]. In order to make sure that the morphological features of the insect in metamorphosis are preserved, full puparia should be pierced with a pin before being placed in hot water, followed by immersion into 70–80% ethanol [89,90]. The required hot water can be carried to the scene in a thermos flask/cup or an insulated bottle and can sometimes be obtained from a café or take-away establishment nearby. Prior to storing these vials, an appropriate label should be placed on the outside (written in indelible ink) indicating the time, date, sampling location, person collecting, and the part of body where sample was collected. A second label with the same information but written in pencil (pencil will not dissolve in alcohol) should be inserted into the vial [2,9].
Beetles collected at a crime scene should be handpicked using forceps and hot water, dipped to kill them before being placed in vials containing 70% ethanol. Beetles may be observed by thoroughly examining the litter surrounding the carcass as well as sieving the soil under the carcass to a depth of approximately 20 cm [91]. It is worthwhile to mention here that Silver and Bronze personnel should collect all insects associated with the corpse and allow the FE to make the necessary decisions on their relevance.
When dealing with a crime scene located in an aquatic environment, the larval stages of freshwater insects such as mayflies (Order: Ephemeroptera), stone flies (Order: Plecoptera), and caddis flies (Order: Trichoptera) should be collected from submerged and floating bodies into plastic vials (70% ethanol) using forceps. Once again, it is recommended that Silver and Bronze personnel collect all aquatic fauna that they observe associated with the corpse. In contrast, it is recommended to preserve approximately 40% of sampled specimens from stream-bottom remains in properly labelled vials containing 95% ethanol to minimise their decay during storage [71,92].
The remaining live aquatic insects collected from a carcass should be transported to the laboratory for rearing and identification. These aquatic insects should be placed in plastic containers with water from where they were sampled (e.g., a lake, sea, pond, or stream). During transport, the specimens should be kept cool by placing the container in ice and the container lid should be opened occasionally to aerate the water [69].
3.3.2. Preparation of Insects at the Crime Scene for Rearing Purposes
The live insects for rearing purposes collected at the crime scene should be placed on a dish (Petri dish, foil) with a suitable food source (e.g., meat). This dish is then placed on 2–3 cm of bedding material (sawdust, vermiculite, builders’ sand) inside a container with a mesh lid [9]. When preparing full puparia for transporting to the laboratory, they can be directly transferred into containers half filled with bedding material with a standard lid. No feeding source or ventilation is required if all insect material is transported inside a cooler or kept refrigerated.
3.4. Laboratory
The following sections pertain to the work that an FE must conduct on either the material collected by themselves, or the material collected by their proxy. To identify, measure larval, pupal, and adult external body characters and rear immatures to adult stage. An entomology laboratory should be equipped with the following basic apparatus: stereo microscopes, vernier callipers, growth chambers or temperature-regulated rooms, preservative chemicals (ethanol), gloves, forceps, scalpels, and Petri dishes. Other equipment may include DNA/RNA reagent kits, PCR apparatus and genetic sequencers to identify insects [93]. Please note that carrion insects, brought into an entomology laboratory, either alive or dead, should be handled with care to enable a range of analyses to be conducted [9].
3.5. Identification
The identification of specimens to species level should be completed first. Without this knowledge, other analyses will be compromised [94]. Generally, insect identifications are made using morphological and/or molecular methods. Other techniques such as hyperspectral or CT imaging, tomography, and chemical techniques may also be used; however, each has associated pros and cons according to expense and availability [95,96]. Nonetheless, whichever technique is used, it should provide the best identification outcome. In fact, the most informative and best identification is to send the collected specimens relevant to the minPMI to a dipteran taxonomist. The following sections discuss the two main techniques used to identify insects: the morphological identification and molecular identification.
The morphological identification of carrion insects to species level can be achieved using taxonomic keys and standard or electron microscopic images [97,98]. Dichotomous taxonomic keys provide a stepwise assessment using morphological characters that guide the user to identify the species [94]. The level of accuracy and confidence in using taxonomic keys for species determination develops with a skill base in entomology and long-term usage to resolve different morphological characters of specimens through microscopic examination [94]. Comprehensive keys for fly identification are numerous and have been generated by James (1947) [99] and Zumpt (1965) [100] and later upgraded by Smith (1986) [101] and Wood (1989) [102]. Recently, De Carvalho et al. (2008) [103], Szpila et al. (2015) [104], Whitworth (2019) [105], and Wallman (2001) [106] proposed identification keys for flies, focusing on specific species, development stages, and their geographical locations. In contrast, fewer taxonomic keys exist for forensically important beetles [107,108].
Ultrastructure studies of eggs, larvae, full and empty puparia, and adult stages of different fly species by scanning electron microscopes (SEM) aid in precise species identification. Typical egg-associated ultrastructures are micropylar plate, median area, respiratory plastron, hatching lines, and chorion sculpturing [109,110]. The suite of larval characters includes the posterior spiracles, respiratory slits, peritreme ornamentation, anterior spiracles, cephaloskeleton, and spine arrangement [110,111]. When identifying full puparia, characteristics such as bubble membranes and respiratory horns are used, along with spine arrangements and posterior spiracles that are correlated with their preceding larval structures [96,108,109,110]. The typical characteristics for the species distinction of adult stages include setae pattern arrangement on the thorax and wing venation [112].
In addition, some SEM-based ultrastructures of forensically important coleopteran have been published for several families such as the Nitidulide and Carabidae. These ultrastructures include the antennomeres, head capsulae, and mouthparts [113,114].
The incorporation of molecular methods, specifically DNA barcoding for identifying crime-associated insects, is an alternative if the user does not have appropriate taxonomic training but has the necessary laboratory skills or can outsource to a molecular biology laboratory to perform the molecular techniques [115]. Additionally, in situations where fewer specimens or fragments of specimens with no diagnostic characters are recovered and where there is an unavailability of taxonomic keys to refer to for the collected development stage, molecular methods are the best recourse for species identification [115,116,117].
DNA barcoding for insect identification is directed to detect a unique short sequence of nucleotides of the genome of each species to discriminate from other related species [118]. It is widely accepted that Cytochrome C oxidase subunit 1 (CO1), the last enzyme of the mitochondrial respiratory chain, is a suitable marker for the DNA barcoding of insects due to its slow mutation rate and highly conserved nature across species [119].
Generally, molecular identification of crime-collected specimens are conducted under five essential steps: preparation of samples, DNA extraction, PCR amplification, sequencing, and sequence library identification [118]. Adult, larval, and pupal stages of flies stored in 70% ethanol can be used for DNA extraction purposes. The specific primers for PCR amplification can be obtained by referring to previous publications [35,36]. However, limited genetic studies conducted on forensic flies in different locations of the world means that there is a scarcity of the sequence data of those species in these regions [117].
Previous studies that have provided the DNA sequences for carrion dipterans include Stevens and Wall (2001) [120] for Calliphorid species in the United Kingdom, Wallman et al. (2005) [121] for Calliphorid species in Australia, Harvey et al. (2003) [122] for Calliphora dubia (Macquart), Chrysomya rufifacies (Maquart), and Lucilia cuprina (Meigen), Harvey et al. (2003) [123] for Calliphorid species in Southern Africa and Australia, and the global scale study on Calliphorid species by Harvey et al. (2008) [124].
3.6. Examining Larvae and Full Puparia for Age Determination
Development studies provide time frames required for immature flies that can be used as indications to estimate the minPMI [23]. The instar of fly larvae can be determined by a microscopic observation of the number of respiratory slits available at their posterior spiracles [100]. Typically, a first instar blow fly larva has a single slit, whereas second and third instar larvae have two and three slits, respectively. In contrast, pre- and post-feeding stages of third instar larvae are discriminated by their body colour change (translucent to opaque), a food distended crop, body compaction, and wandering behaviour away from the carcass [23].
In addition, the length and width of larvae can also be considered as an indication of age [125]. The length and width of larvae can be measured by a stage micrometre and a vernier calliper. The correlation of actual length and width data of a sampled individual with reference values given in previous publications aid in determining the age of the specimen, hence the PMI. However, temperature variations, the type of food substrates, and the presence of xenobiotics in it reflect on any of these age determinations. Magni et al. (2012) [117] referred to the length of oven-dried larvae of Lucilia illustris (Meigen) with desiccated larval specimens collected at a crime scene for their age determination. Some of the essential studies with length change reference values are available for Ch. megacephala [126,127,128], Ch. rufifacies [129,130], Ch. bezziana [131], L. sericata [132], and L. cuprina [133].
Furthermore, in previous studies, length and instar stage variation of larvae under different constant temperature regimes were demonstrated using isomegalen and isomorphen curves, respectively. These curves are available for species such as, Calliphora vicina (Robineae-Desvoidy), L. sericata, Ch. albiceps, Protophormia terraenovae (Robineae-Desvoidy), Ch. megacephala, and Liopygia argyrostoma (Robineae-Desvoidy) [128,132,133].
Pupal morphogenesis can be considered for minPMI estimations as the full puparia coexist with carcasses over a prolonged period [89,134]. The extent of the external organ development of pupae can be examined via a stereo microscope after removing the puparium using a surgical knife and forceps. A photograph showing the status of the external organ development of a sampled pupa can be coupled with a reference photograph series given for the same species in a previous publication for the age determination. However, it is essential to consider the temperature variations of the environment that the full puparia were exposed to prior to sampling, as low temperatures can delay pupal morphogenesis [130]. In this regard, photographic timelines for external organ developments of pupae (intra-puparium development) are given for Megaselia spiracularis (Schmitz) [135], Megaselia scalaris (Loew) [136], Calliphora vicina (Rob-Desvoidy) [90], Hermetia illucens (L.) [137], Ch. Megacephala [138], Ch. rufifacies, and Dohrniphora cornuta (Bigot) [139].
Insect aging of larvae and full puparia can also be performed in the laboratory using methods such as hyperspectral [125] or CT imaging [140], tomography [141], and biomolecular [142] and chemical [143] techniques. Such techniques require advanced instrumentation and a high level of expertise to conduct these analyses.
3.7. Rearing
The rearing of eggs, larval, and pupal specimens until the adult stage can often facilitate a more accurate species identification [144]. Adult-level identification has a greater certainty in correct species determination, and they are well-described in taxonomic keys [145]. When rearing dipteran species in an insectary, they should be confined in insect cages and provided ad libitum with food, water, and oviposition substrates [17,23].
Immature stages are best reared in growth chambers whereby temperature, humidity, and photoperiod can be controlled. Such development studies are used to determine the time duration required to ascertain the development stage of the sampled species and their larval length and width changes. It is recommended to use swine muscle as larval food for these growth chamber studies as the domestic pig (Sus scrofa L.) is primarily considered as the proxy of humans when conducting development studies [146]. Additionally, when dealing with a wildlife-associated crime, the identical tissue type should be the feeding substrate utilised in growth chamber studies [147].
3.8. Xenobiotic Detection
The utilisation of insects for xenobiotic detection relies on the prolonged tissue retention of drugs, pesticides, and metals, leading to high sensitivity for analytical detection techniques [39,148].
The fly larval, pupal, and adult stages collected at a crime scene can be used as a source to extract and detect chemicals. Previous studies showed that larvae collected from internal organs such as liver, or from the head and muscles of a carcass retain high concentrations of chemicals [40].
The larvae sourced from these regions should be kept alive and starved for several hours prior to analysis to ensure the absorption of any chemicals into their metabolic system. It is recommended to store killed larvae under dry conditions at −20 °C. Such steps will ensure a higher retention and concentration of chemicals because storing in ethanol may diminish trace volumes of drugs in larvae [40,149].
At the laboratory, sampled fly development stages can be prepared for analysis via three methods: (1) macerated and homogenised, (2) digested via a strong acid or enzyme, and (3) pulverised by grinding. The available analytical methods for these xenobiotic detections are immunoassay, high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and gas chromatography-mass spectrometry (GC-MS) [30].
4. Documentation
Documentation at a crime scene is vital to conserve the integrity of the evidence and for the continuity of the chain of custody. Previous studies showed that the layout of the protocol sheets used by an FE varies because it is based on the requirements of investigation agencies and legal systems in that region [150]. This documentation concerns two parts [151]. Firstly, the crime scene processes (information gathered by either the FE or proxy) and secondly the laboratory processes (information processed by the FE only). Once these processes are complete, the FE should have enough information to produce an expert witness statement [152] (Table 2).

Table 2.
Specific information that needs to be included into protocol sheets. Many review papers also list such protocols and should be consulted [1].
Generally, an FE will visit the crime scene once; therefore, it is necessary to record all the information while present at a crime scene [9]. Occasionally, the FE may have to return to the scene to gain some context surrounding the scene as well as sample more of the insect material because the original visit was at night. To retain more time for sampling while keeping documentation to a minimum, it is best practice to have a protocol sheet containing specific questions with as many possible multiple-choice answers. These sheets should be designed to gather all essential information related to a crime scene that explains the crime characteristics, microclimatic data, quantity and quality of sampling specimens, and the details of the sampler. Additionally, the laboratory data sheet should be prepared to document all the analyses conducted in the laboratory, which an expert can easily refer to for verification purposes [60].
4.1. Referring to the Literature
The accuracy of establishing a successful entomological interpretation of the circumstances at a crime scene depends on the empirical analysis developed by an FE throughout their career [44]. One of the best ways of acquiring and upgrading the knowledge and skills associated with insects for solving crime is referring to previously published research and casework [153]. These publications essentially guide a practitioner to incorporate the available knowledge into procedures and provide a comprehensive guideline for researchers and academics to develop training models and conduct future research [153].
4.2. Fly Development Studies
Development studies outline the variations in the fly life history [23]. The data from these development studies can be directly incorporated to estimate the minPMI, as these publications essentially contain life history tables and isomegalen/isomorphen curves. The timeline data given in life history tables and the lower development threshold temperatures can be used to calculate the ADD/ADH [25,154], and hence, the minPMI. However, when selecting a previous development study to corroborate a minPMI estimation of a particular fly species, the FE should determine if the temperature regimes, photoperiod and humidity, and larval feeding tissue type, along with the location where the source colonies originated, are appropriate to use [155].
Besides providing the baseline data required to calculate the minPMI, these studies highlight the laboratory protocols that must be maintained when conducting trials related to crime scenes. These protocols need to detail the colony generation, rearing and maintaining the genetic diversity of the colony, simulation of environmental conditions in an insectary/growth chamber, and larval sampling and data gathering [17]. Numerous authors have conducted development studies on numerous blow fly species (Table 3).

Table 3.
Development studies conducted on some forensically important fly species from different global regions. This table presents an overview only and is a starting point for any person interested in forensic entomology.
4.3. Insect Successional Studies
Insect successional studies provide a descriptive account of insect colonisation patterns concerning the decomposition changes in carcasses. These studies are either observational or experimental, and both are directed to gather information such as insect checklists, environment temperature, and rainfall data of the study period, and the characteristics and duration of the decomposition stage [188].
Generally, observational-based successional studies are conducted to represent seasonal and ecosystem changes (terrestrial and aquatic), whereas experimental studies consider different death and carcass scenarios [189]. The data given in succession studies can be incorporated to help interpret the insect ecology and behaviour at a crime scene when geographical location and temperatures can be matched. In this regard, Matuszewski et al. (2019) [188] summarised global accounts of successional studies conducted using different carcass types under varied ecosystems.
4.4. Casework
Occasionally, a case is published which is the combination of facts and opinions of an FE who investigated a particular crime scene and may be relevant to the current case under investigation. Reference to casework can provide guidance to produce a robust witness statement but can also provide insight into areas where further research may benefit better interpretations. Some attempts have been made to describe how a forensic entomology report should be compiled with the latest methods by Kotzé et al. (2021) [190], detailing 16 subheadings which might be considered depending on the circumstances of a case.
5. Conclusions
This review essentially updates much of the current knowledge pertaining to forensic entomology, either described in the literature or experienced by the authors involved in casework. One of the major concerns of FEs is the education and practical training of proxies who attend crime scenes and collect insects and other biological materials. Many proxies may have basic skills in collecting and preserving insects across Gold, Silver, and Bronze standards and they can now pick and choose what they deem appropriate in respect to these activities. Reading this paper will help assist in their decisions. It cannot be emphasised enough that these activities need to be of a high quality if these evidence and associated information are to be of any benefit to the FE, and of course the case, especially for their ultimate consideration by the judiciary.
Author Contributions
Conceptualisation: I.R.D., P.A.M. and T.B.B.; writing—original draft preparation: T.B.B. and I.R.D.; writing—review and editing: I.R.D., T.B.B. and P.A.M.; supervision: I.R.D. and P.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Martin Hall for his input and comments on an earlier draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hall, M.; Whitaker, A.; Richards, C. Forensic Entomology. In The Forensic Ecology Handbook: From Crime Scene to Court, 1st ed.; Marques-Grant, N., Roberts, J., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 2012; pp. 111–140. ISBN 978-1-119-97419-2. [Google Scholar]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M. Best practice in forensic entomology—Standards and guidelines. Int. J. Legal Med. 2007, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- James, S.H.; Nordby, J.J. Forensic Science: An Introduction to Scientific and Investigative Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 0-8493-1246-9. [Google Scholar]
- Saks, M.J.; Koehler, J.J. The coming paradigm shift in forensic identification science. Science 2005, 309, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Saferstein, R. Criminalistics: An Introduction to Forensic Science, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001.
- Brettell, T.A.; Butler, J.M.; Saferstein, R. Forensic science. Anal. Chem. 2005, 77, 3839–3860. [Google Scholar] [CrossRef]
- Horswell, J. Crime Scene Investigation. In The Practice of Crime Scene Investigation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Katz, E.; Halámek, J. Forensic science: A multidisciplinary approach. J. Forensic Leg. Investig. Sci. 2016, 1, 1–4. [Google Scholar]
- Byrd, J.H.; Tomberlin, J.K. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-8153-5020-0. [Google Scholar]
- Dadour, I.R.; Morris, B. Forensic Entomology: A Synopsis, Guide, and Update. In Essentials of Autopsy Practice, 1st ed.; Rutty, G.N., Ed.; Springer: London, UK, 2014; pp. 105–130. [Google Scholar] [CrossRef]
- Robinson, W.H. Urban Insects and Arachnids: A Handbook of Urban Entomology, 1st ed.; Cambridge University Press: Cambridge, UK, 2005; ISBN 9780521812535. [Google Scholar]
- Bugelli, V.; Tarozzic, I.; Galante, N.; Bortolini, S.; Franceschetti, L. Review on forensic importance of myiasis: Focus on medicolegal issues on post-mortem interval estimation and neglect evaluation. Legal Med. 2023, 63, 102263. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Athanassiou, C.G. Improving stored product insect pest management: From theory to practice. Insects 2019, 10, 332. [Google Scholar] [CrossRef]
- Haines, A.M.; Webb, S.L.; Wallace, J.R. Conservation Forensics: The Intersection of Wildlife Crime, Forensics, and Conservation. In Wildlife Biodiversity Conservation, 1st ed.; Underkoffler, S.C., Adams, H.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 125–146. [Google Scholar] [CrossRef]
- Matuszewski, S. Post-mortem interval estimation based on insect evidence: Current challenges. Insects 2021, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Bambaradeniya, Y.T.B.; Karunaratne, W.A.I.P.; Rakinawasam, S.V.; Tomberlin, J.K.; Goonerathne, I.; Kotakadeniya, R.B. Myiasis incidences reported in and around central province of Sri Lanka. Int. J. Dermatol. 2019, 58, 336–342. [Google Scholar] [CrossRef]
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- Forbes, S.L.; Carter, D.O. Processes and Mechanisms of Death and Decomposition of Vertebrate Carrion. In Carrion Ecology, Evolution, and Their Applications, 1st ed.; Benbow, M.E., Tomberlin, J.K., Torone, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 13–30. [Google Scholar] [CrossRef]
- Payne, J.A.; King, E.W.; Beinhart, G. Arthropod succession and decomposition of buried pigs. Nature 1968, 219, 1180–1181. [Google Scholar] [CrossRef]
- Noriki, S.; Iino, S.; Kinoshita, K.; Fukazawa, Y.; Inai, K.; Sakai, T.; Kimura, H. Pathological analysis of cadavers for educational dissection by using postmortem imaging. Pathol. Int. 2019, 69, 580–600. [Google Scholar] [CrossRef] [PubMed]
- Bajerlein, D.; Taberski, D.; Matuszewski, S. Estimation of postmortem interval (PMI) based on empty puparia of Phormia regina (Meigen) (Diptera: Calliphoridae) and third larval stage of Necrodes littoralis (L.) (Coleoptera: Silphidae)–Advantages of using different PMI indicators. J. Forensic Leg. Med. 2018, 55, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Pittner, S.; Bugelli, V.; Weitgasser, K.; Zissler, A.; Sanit, S.; Lutz, L.; Amendt, J. A field study to evaluate PMI estimation methods for advanced decomposition stages. Int. J. Legal Med. 2020, 134, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Bambaradeniya, Y.T.B.; Magni, P.A.; Dadour, I.R. Current Status of Five Warm Season Diptera Species in Estimating the Post-Mortem Interval. Ann. Entomol. Soc. Am. 2023, 116, 19–50. [Google Scholar] [CrossRef]
- Kreitlow, K.L.T. Insect Succession in a Natural Environment. In Forensic Entomology, 2nd ed.; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 251–269. [Google Scholar]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef]
- Kulshrestha, P.; Satpathy, D.K. Use of beetles in forensic entomology. Forensic Sci. Int. 2001, 120, 15–17. [Google Scholar] [CrossRef]
- Frost, C.L.; Braig, H.R.; Amendt, J.; Perotti, M.A. Indoor Arthropods of Forensic Importance: Insects Associated with Indoor Decomposition and Mites as Indoor Markers. In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 93–108. [Google Scholar] [CrossRef]
- Vanin, S. Advances in Forensic Entomology in Missing Persons Investigations. In Handbook of Missing Persons, 1st ed.; Morewitz, S.J., Colls, C.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 309–317. [Google Scholar] [CrossRef]
- Prasad, S.; Aneesh, E.M. Tools and techniques in forensic entomology—A critical review. Int. J. Trop. Insect Sci. 2020, 42, 2785–2794. [Google Scholar] [CrossRef]
- Charabidze, D.; Gosselin, M.; Hedouin, V. Use of necrophagous insects as evidence of cadaver relocation: Myth or reality? Peer J. 2017, 5, e3506. [Google Scholar] [CrossRef]
- Weatherbee, C.R.; Pechal, J.L.; Eric Benbow, M. The dynamic maggot mass microbiome. Ann. Entomol. Soc. Am. 2017, 110, 45–53. [Google Scholar] [CrossRef]
- Di Luise, E.; Magni, P.; Staiti, N.; Spitaleri, S.; Romano, C. Genotyping of human nuclear DNA recovered from the gut of fly larvae. Forensic Sci. Int. 2008, 1, 591–592. [Google Scholar] [CrossRef]
- Wells, J.D.; Stevens, J.R. Application of DNA-based methods in forensic entomology. Annu. Rev. Entomol. 2008, 53, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. The metagenomics worldwide research. Curr. Genet. 2017, 63, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Roumpeka, D.D.; Wallace, R.J.; Escalettes, F.; Fotheringham, I.; Watson, M. A review of bioinformatics tools for bioprospecting from metagenomic sequence data. Front. Genet. 2017, 8, 23. [Google Scholar] [CrossRef]
- Carvalho, F.; Dadour, I.R.; Groth, D.M.; Harvey, M.L. Isolation and detection of ingested DNA from the immature stages of Calliphora dubia (Diptera: Calliphoridae) A forensically important blowfly. Forensic Sci. Med. Pathol. 2005, 1, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Baqué, M.; Amendt, J.; Verhoff, M.A.; Zehner, R. Descriptive analyses of differentially expressed genes during larval development of Calliphora vicina (Diptera: Calliphoridae). Int. J. Legal Med. 2015, 129, 891–902. [Google Scholar] [CrossRef]
- Kintz, P.; Tracqui, A.; Ludes, B.; Waller, J.; Boukhabza, A.; Mangin, P.; Chaumont, A.J. Fly larvae and their relevance in forensic toxicology. Am. J. Forensic Med. Pathol. 1990, 11, 63–65. [Google Scholar] [CrossRef]
- Gosselin, M.; Wille, S.M.; Fernandez, M.D.M.R.; Di Fazio, V.; Samyn, N.; De Boeck, G.; Bourel, B. Entomotoxicology, experimental set-up and interpretation for forensic toxicologists. Forensic Sci. Int. 2011, 208, 1–9. [Google Scholar] [CrossRef]
- Stewart, M.A. The teaching of entomology. J. Econ. Entomol. 1929, 22, 777–781. [Google Scholar] [CrossRef]
- Morris, B.; Harvey, M.L.; Dadour, I.R. International Collaborations and Training. In International Dimensions & Frontiers in Forensic Entomology, 1st ed.; Tomberlin, J., Benbow, E., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Volume 30, pp. 399–416. [Google Scholar] [CrossRef]
- Schoenly, K.G.; Haskell, N.H.; Mills, D.K.; Bieme-Ndi, C.; Larsen, K.; Lee, Y. Recreating death’s acre in the school yard: Using pig carcasses as model corpses to teach concepts of forensic entomology & ecological succession. Am. Biol. Teach. 2006, 68, 402–410. [Google Scholar]
- Magni, P.; Guercini, S.; Leighton, A.; Dadour, I. Forensic entomologists: An evaluation of their status. J. Insect Sci. 2013, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Schoenly, K.G.; Michaud, J.P.; Moreau, G. Design and Analysis of Field Studies in Carrion Ecology. In Carrion Ecology, Evolution, and Their Applications, 1st ed.; Benbow, M.E., Tomberlin, J.K., Torone, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 129–148. ISBN 978-1-4665-7547-9. [Google Scholar]
- Benbow, M.E.; Pechal, J.L. Forensic Entomology and the Microbiome. In Forensic Entomology: The Utility of Arthropods in Legal Investigations, 3rd ed.; Byrd, J.H., Tomberlin, J.K., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 499–517. [Google Scholar]
- Michaud, J.P.; Schoenly, K.G.; Moreau, G. Rewriting ecological succession history: Did carrion ecologists get there first? Q. Rev. Biol. 2015, 90, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Benecke, M.; Barksdale, L. Distinction of bloodstain patterns from fly artifacts. Forensic Sci. Int. 2003, 137, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Dadour, I.R.; Cook, D.F.; Fissioli, J.N.; Bailey, W.J. Forensic entomology: Application, education and research in Western Australia. Forensic Sci. Int. 2001, 120, 48–52. [Google Scholar] [CrossRef]
- Archer, M.S.; Wallman, J.F. The development of forensic entomology in Australia and New Zealand: An overview of casework practice, quality control and standards. Aust. J. Forensic Sci. 2017, 49, 125–133. [Google Scholar] [CrossRef]
- Lutz, L.; Zehner, R.; Verhoff, M.A.; Bratzke, H.; Amendt, J. It is all about the insects: A retrospective on 20 years of forensic entomology highlights the importance of insects in legal investigations. Int. J. Legal Med. 2021, 135, 2637–2651. [Google Scholar] [CrossRef]
- Mégnin, P. La faune des Cadavres. Application de l’entomologie a la Médicine Légal (Fauna of Cadavers. Application of Enomology in Legal Medicine); Encyclopdie scientifique des Aides-Mémoire; Les Belles Lettres: Paris, France, 1894; p. 214. [Google Scholar]
- Payne, J.A. A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology 1965, 46, 592–602. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; Vanlaerhoven, S. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421. [Google Scholar] [CrossRef]
- Keh, B. Scope and applications of forensic entomology. Annu. Rev. Entomol. 1985, 30, 137–154. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Byrd, J.H.; Wallace, J.R.; Benbow, M.E. Assessment of decomposition studies indicates need for standardized and repeatable research methods in forensic entomology. J. Forensic Res. 2012, 3, 1000147. [Google Scholar] [CrossRef]
- Moreau, G. The pitfalls in the path of probabilistic inference in forensic entomology: A review. Insects 2021, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, E.; Dourel, L. Forensic entomology: Implementing quality assurance for expertise work. Int. J. legal Med. 2013, 127, 1031–1037. [Google Scholar] [CrossRef]
- Magni, P.A.; Dadour, I. SmartInsects–Smartphone & Forensic Entomology. 2013 (Version 1.0). Mobile Application Software: IOS. Retrieved from App Store. Available online: https://apps.apple.com/us/app/smartinsects/id985074731 (accessed on 15 December 2022).
- Campobasso, C.P.; Introna, F. The forensic entomologist in the context of the forensic pathologist’s role. Forensic Sci. Int. 2001, 120, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Krettek, R.; Niess, C.; Zehner, R.; Bratzke, H. Forensic entomology in Germany. Forensic Sci. Int. 2000, 113, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Dolinski, C.; Shapiro-Ilan, D.; Lewis, E.E. Insect Cadaver Applications: Pros and Cons. In Nematode Pathogenesis of Insects and Other Pests, 1st ed.; Campos-Herrera, R., Ed.; Springer: Cham, Switzerland, 2015; pp. 207–229. [Google Scholar] [CrossRef]
- LeBlanc, H.N.; Logan, J.G. Exploiting Insect Olfaction in Forensic Entomology. In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 205–221. [Google Scholar] [CrossRef]
- Charabidze, D.; Depeme, A.; Devigne, C.; Hedouin, V. Do necrophagous blowflies (Diptera: Calliphoridae) lay their eggs in wounds?: Experimental data and implications for forensic entomology. Forensic Sci. Int. 2015, 253, 71–75. [Google Scholar] [CrossRef]
- Kinnaird, E.L. Decomposition and Insect Succession on Hanging Carcasses in Western Australia. Master by Research, The University of Western Australia, Perth, 3 November 2016. Available online: https://research-repository.uwa.edu.au/en/publications/decomposition-and-insect-succession-on-hanging-carcasses-in-weste (accessed on 15 December 2022).
- Bhadra, P.; Hart, A.J.; Hall, M.J.R. Factors affecting accessibility to blowflies of bodies disposed in suitcases. Forensic Sci. Int. 2014, 239, 62–72. [Google Scholar] [CrossRef]
- Wescott, D.J. Recent advances in forensic anthropology: Decomposition research. Forensic Sci. Res. 2018, 3, 278–293. [Google Scholar] [CrossRef]
- Finley, S.J.; Benbow, M.E.; Javan, G.T. Microbial communities associated with human decomposition and their potential use as postmortem clocks. Int. J. Legal Med. 2015, 129, 623–632. [Google Scholar] [CrossRef]
- Wallace, J.R. Aquatic Vertebrate Carrion Decomposition. In Carrion Ecology, Evolution, and Their Applications, 1st ed.; Benbow, M.E., Tomberlin, J.K., Torone, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 247–271. [Google Scholar]
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Technol. 2000, 21, 103–111. [Google Scholar] [CrossRef]
- Sharanowski, B.J.; Walker, E.G.; Anderson, G.S. Insect succession and decomposition patterns on shaded and sunlit carrion in Saskatchewan in three different seasons. Forensic Sci. Int. 2008, 179, 219–240. [Google Scholar] [CrossRef]
- Bambaradeniya, Y.T.B.; Karunarathne, W.A.I.P.; Goonerathne, I.; Kotakadeniya, R.B.; Tomberlin, J.K. Use of development data to estimate colonization time of the myiasis-causing fly, Chrysomya bezziana (Diptera: Calliphoridae) collected from human wounds. Sri Lanka J. Forensic Med. Sci. Law 2016, 7, 19–26. [Google Scholar] [CrossRef]
- Iancu, L.; Dean, D.E.; Purcarea, C. Temperature influence on prevailing necrophagous Diptera and bacterial taxa with forensic implications for postmortem interval estimation: A review. J. Med. Entomol. 2018, 55, 1369–1379. [Google Scholar] [PubMed]
- Guo, J.; Fu, X.; Liao, H.; Hu, Z.; Long, L.; Yan, W.; Cai, J. Potential use of bacterial community succession for estimating post-mortem interval as revealed by high-throughput sequencing. Sci. Rep. 2016, 6, 24197. [Google Scholar] [CrossRef] [PubMed]
- Hofer, I.M.; Hart, A.J.; Martín-Vega, D.; Hall, M.J. Estimating crime scene temperatures from nearby meteorological station data. Forensic Sci. Int. 2020, 306, 110028. [Google Scholar] [CrossRef] [PubMed]
- Charabidze, D.; Hedouin, V. Temperature: The weak point of forensic entomology. Int. J. Legal Med. 2019, 133, 633–639. [Google Scholar] [CrossRef]
- Johnson, A.P.; Wallman, J.F.; Archer, M.S. Experimental and casework validation of ambient temperature corrections in forensic entomology. J. Forensic Sci. 2012, 57, 215–221. [Google Scholar] [CrossRef]
- Hofer, I.M.; Hart, A.J.; Martín-Vega, D.; Hall, M.J. Optimising crime scene temperature collection for forensic entomology casework. Forensic Sci. Int. 2017, 270, 129–138. [Google Scholar] [CrossRef]
- Turchetto, M.; Vanin, S. Climate Change and Forensic Entomology. In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 327–351. [Google Scholar] [CrossRef]
- Michaud, J.P.; Schoenly, K.G.; Moreau, G. Sampling flies or sampling flaws? Experimental design and inference strength in forensic entomology. J. Med. Entomol. 2012, 49, 1–10. [Google Scholar] [CrossRef]
- Anderson, G.S.; Cervenka, V.J.; Haglund, W.; Sorg, M. Insects Associated with the Body: Their Use and Analyses. In Advances in Forensic Taphonomy: Method, Theory, and Archaeological Perspectives, 1st ed.; Haglund, W.D., Sorg, M., Eds.; CRC Press: Boca Raton, FL, USA, 2002; p. 173200. ISBN 13: 978-14200-5835-2. [Google Scholar]
- Touroo, R.; Fitch, A. Crime Scene Findings and the Identification, Collection, and Preservation of Evidence. In Veterinary Forensic Pathology, 1st ed.; Brooks, J.S., Ed.; Springer: Cham, Switzerland, 2018; pp. 9–25. [Google Scholar] [CrossRef]
- Mohr, R.M.; Tomberlin, J.K. Development and validation of a new technique for estimating a minimum postmortem interval using adult blow fly (Diptera: Calliphoridae) carcass attendance. Int. J. Legal Med. 2015, 129, 851–859. [Google Scholar] [CrossRef]
- Martín-Vega, D.; Hall, M.J.R. Estimating the age of Calliphora vicina eggs (Diptera: Calliphoridae): Determination of embryonic morphological landmarks and preservation of egg samples. Int. J. Legal Med. 2016, 130, 845–854. [Google Scholar] [CrossRef]
- Tyndale-Biscoe, M. Age-grading methods in adult insects: A review. Bull. Entomol. Res. 1984, 74, 341–377. [Google Scholar] [CrossRef]
- Firoozfar, F.; Moosa-Kazemi, H.; Baniardalani, M.; Abolhassani, M.; Khoobdel, M.; Rafinejd, J. Mass rearing of Lucilia sericata Meigen (Diptera: Calliphoridae). Asian Pac. J. Trop. Biomed. 2011, 1, 54–56. [Google Scholar] [CrossRef]
- Bilaniuk, V.; Beresford, D.V. Sampling adult blow flies (Diptera: Calliphoridae) at pig carcasses with sticky traps: Effects of trap colour, height, and inclination. J. Can. Soc. Forensic Sci. 2010, 43, 181–190. [Google Scholar] [CrossRef]
- Adams, Z.J.; Hall, M.J. Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci. Int. 2003, 138, 50–61. [Google Scholar] [CrossRef]
- Davies, K.; Harvey, M.L. Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J. Forensic Sci. 2013, 58, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Thorne, A.; Harvey, M. Calliphora vicina (Diptera: Calliphoridae) pupae: A timeline of external morphological development and a new age and PMI estimation tool. Int. J. Legal Med. 2015, 129, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, S.; von Hoermann, C.; Degasperi, G.; Brandt, K.; Steiger, S.; Ayasse, M. Temporal variability of the rove beetle (Coleoptera: Staphylinidae) community on small vertebrate carrion and its potential use for forensic entomology. Forensic Sci. Int. 2021, 323, 110792. [Google Scholar] [CrossRef]
- Dalal, J.; Sharma, S.; Bhardwaj, T.; Dhattarwal, S.K. Assessment of post-mortem submersion interval using total aquatic decomposition scores of drowned human cadavers. J. Forensic. Sci. 2023, 68, 549–557. [Google Scholar] [CrossRef]
- Rivers, D.B.; Dahlem, G.A. The Science of Forensic Entomology, 2nd ed.; John Wiley & Sons: Toronto, ON, Canada, 2023; ISBN 97811199403664. [Google Scholar]
- Stamper, T.; Weidner, L.; Nigoghosian, G.; Johnson, N.; Wang, C.; Levesque-Bristol, C. Towards understanding how to instruct students in dichotomous identification keys in a mixed STEM forensic science education environment. JFSE 2020, 2, 1. [Google Scholar]
- Drijfhout, F.P. Cuticular Hydrocarbons: A New Tool in Forensic Entomology? In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Pechal, J.L.; Moore, H.; Drijfhout, F.; Benbow, M.E. Hydrocarbon profiles throughout adult Calliphoridae aging: A promising tool for forensic entomology. Forensic Sci. Int. 2014, 245, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.; Sukontason, K.L.; Boonchu, N.; Chaiwong, T.; Piangjai, S. Ultrastructure of eggshell of Chrysomya nigripes Aubertin (Diptera: Calliphoridae). Parasitol. Res. 2004, 93, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, Y.; Magaña, C.; Martínez-Sánchez, A.; Rojo, S. Diptera of forensic importance in the Iberian Peninsula: Larval identification key. Med. Vet. Entomol. 2010, 24, 293–308. [Google Scholar] [CrossRef] [PubMed]
- James, M.T. The Flies That Cause Myiasis in Man; US Department of Agriculture, Government Printing Office: Washington, DC, USA, 1947. [Google Scholar]
- Zumpt, F. Myiasis in Man and Animals in the Old World: A Textbook for Physicians, Veterinarians and Zoologists; Butterworth & Co., Ltd.: London, UK, 1965. [Google Scholar]
- Smith, K.V.G. A Manual of Forensic Entomology, 1st ed.; Department of Entomology, British Museum: London, UK, 1986; ISBN 9780565009908. [Google Scholar]
- Wood, D.M.; Borkent, A. Phylogeny and Classification of the Nematocera. In Manual of Nearctic Diptera, 1st ed.; McAlpine, J.F., Wood, D.M., Eds.; Research Branch Agriculture Canada Monograph No. 32; Canadian Government Publishing Centre: Hull, QC, Canada, 1989; pp. 1333–1370. [Google Scholar]
- Carvalho, C.J.B.D.; Mello-Patiu, C.A.D. Chave de identificação para as espécies comuns de Diptera da América do Sul de interesse forense. Rev. Bras. Entomol. 2008, 52, 390–406. [Google Scholar] [CrossRef]
- Szpila, K.; Richet, R.; Pape, T. Third instar larvae of flesh flies (Diptera: Sarcophagidae) of forensic importance—Critical review of characters and key for European species. Parasitol. Res. 2015, 114, 2279–2289. [Google Scholar] [CrossRef]
- Whitworth, T. Keys to the Genera and Species of Blow Flies (Diptera: Calliphoridae) of America, North of Mexico. In Forensic Entomology: The Utility of Arthropods in Legal Investigations, 3rd ed.; Byrd, J.H., Tomberlin, J.K., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 413–443. [Google Scholar]
- Wallman, J.F. A key to the adults of species of blowflies in southern Australia known or suspected to breed in carrion. Med. Vet. Entomol. 2001, 15, 433–437. [Google Scholar] [CrossRef]
- Hanley, G.A.; Cuthrell, D.L. The Carrion Beetles of North Dakota, Including Species Descriptions and Identification Keys for the Entire North American Silphid Fauna; Minot State University: Minot, ND, USA, 2010. [Google Scholar]
- Brunke, A.; Newton, A.; Klimaszewski, J.; Majka, C.; Marshall, S. Staphylinidae of eastern Canada and adjacent United States. Key to subfamilies: Staphylininae: Tribes and subtribes, and species of Staphylinina. Can. J. Arthropod Identif. 2011, 12, 1–110. [Google Scholar] [CrossRef]
- Mendonça, P.M.; dos Santos-Mallet, J.R.; de Mello, R.P.; Gomes, L.; de Carvalho Queiroz, M.M. Identification of fly eggs using scanning electron microscopy for forensic investigations. Micron 2008, 39, 802–807. [Google Scholar] [CrossRef]
- Ubero-Pascal, N.; Arnaldos, I.; López-Esclapez, R.; García, M.D. Microscopy and forensic entomology. Microsc. Sci. Technol. Appl. Educ. Microsc. Book Ser. 2010, 4, 1548–1556. [Google Scholar]
- Grzywacz, A. Third instar larva morphology of Hydrotaea cyrtoneurina (Zetterstedt, 1845) (Diptera: Muscidae)-a species of forensic interest. Pol. J. Entomol. 2013, 82, 303–315. [Google Scholar] [CrossRef]
- Hore, G.; Maity, A.; Naskar, A.; Ansar, W.; Ghosh, S.; Saha, G.K.; Banerjee, D. Scanning electron microscopic studies on antenna of Hemipyrellia ligurriens (Wiedemann, 1830) (Diptera: Calliphoridae)—A blow fly species of forensic importance. Acta Trop. 2017, 172, 20–28. [Google Scholar] [CrossRef]
- Giglio, A.; Ferrero, E.A.; Perrotta, E.; Tripepi, S.; Brandmayr, T.Z. Ultrastructure and comparative morphology of mouth-part sensilla in ground beetle larvae (Insecta, Coleoptera, Carabidae). Zool. Anz. 2003, 242, 277–292. [Google Scholar] [CrossRef]
- Ortloff, A.; Zanetti, N.; Centeno, N.; Silva, R.; Bustamante, F.; Olave, A. Ultramorphological characteristics of mature larvae of Nitidula carnaria (Schaller 1783) (Coleoptera: Nitidulidae), a beetle species of forensic importance. Forensic Sci. Int. 2014, 239, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Hung, T.H.; Shiao, S.F. Molecular identification of forensically important blow fly species (Diptera: Calliphoridae) in Taiwan. J. Med. Entomol. 2004, 41, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Batovska, J.; Blacket, M.J.; Brown, K.; Lynch, S.E. Molecular identification of mosquitoes (Diptera: Culicidae) in southeastern Australia. Ecol. Evol. 2016, 6, 3001–3011. [Google Scholar] [CrossRef]
- Magni, P.A.; Harvey, M.L.; Saravo, L.; Dadour, I.R. Entomological evidence: Lessons to be learnt from a cold case review. Forensic Sci. Int. 2012, 223, 31–34. [Google Scholar] [CrossRef]
- Rolo, E.S.A. DNA Barcoding and Forensic Entomology: A Molecular Approach for Diptera Species’ Identification. Ph.D. Thesis, University of Lisbon, Lisboa, Portugal, 2010. Available online: https://hdl.handle.net/10451/2024 (accessed on 15 December 2022).
- Boehme, P.; Amendt, J.; Disney, R.H.L.; Zehner, R. Molecular identification of carrion-breeding scuttle flies (Diptera: Phoridae) using COI barcodes. Int. J. Legal Med. 2010, 124, 577–581. [Google Scholar] [CrossRef]
- Stevens, J.; Wall, R. Genetic relationships between blowflies (Calliphoridae) of forensic importance. Forensic Sci. Int. 2001, 120, 116–123. [Google Scholar] [CrossRef]
- Wallman, J.F.; Leys, R.; Hogendoorn, K. Molecular systematics of Australian carrion-breeding blowflies (Diptera: Calliphoridae) based on mitochondrial DNA. Invertebr. Syst. 2005, 19, 1–15. [Google Scholar] [CrossRef]
- Harvey, M.L.; Dadour, I.R.; Gaudieri, S. Mitochondrial DNA cytochrome oxidase I gene: Potential for distinction between immature stages of some forensically important fly species (Diptera) in western Australia. Forensic Sci. Int. 2003, 131, 134–139. [Google Scholar] [CrossRef]
- Harvey, M.L.; Mansell, M.W.; Villet, M.H.; Dadour, I.R. Molecular identification of some forensically important blowflies of southern Africa and Australia. Med. Vet. Entomol. 2003, 17, 363–369. [Google Scholar] [CrossRef]
- Harvey, M.L.; Gaudieri, S.; Villet, M.H.; Dadour, I.R. A global study of forensically significant calliphorids: Implications for identification. Forensic Sci. Int. 2008, 177, 66–76. [Google Scholar] [CrossRef]
- Day, D.M.; Wallman, J.F. Width as an alternative measurement to length for post-mortem interval estimations using Calliphora augur (Diptera: Calliphoridae) larvae. Forensic Sci. Int. 2006, 159, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yuan, X.; Zhu, F.; Lei, C. Development time and size-related traits in the oriental blowfly, Chrysomya megacephala along a latitudinal gradient from China. J. Therm. Bio. 2010, 35, 366–371. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yang, L.; Tao, L.; Wang, J. Development of Chrysomya megacephala at constant temperatures within its colony range in Yangtze River Delta region of China. Forensic Sci. Res. 2018, 3, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bambaradeniya, Y.T.B.; Karunaratne, W.I.P.; Tomberlin, J.K.; Goonerathne, I.; Kotakadeniya, R.B.; Magni, P.A. Effect of temperature and tissue type on the development of the forensic fly Chrysomya megacephala (Diptera: Calliphoridae). J. Med. Entomol. 2019, 56, 1571–1581. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Y.; Sun, Y.; Zhang, Y.; Wang, M.; Wang, J. Development of Chrysomya rufifacies (Diptera: Calliphoridae) at constant temperatures within its colony range in Yangtze River Delta Region of China. J. Med. Entomol. 2019, 56, 1215–1224. [Google Scholar] [CrossRef]
- Bambaradeniya, Y.T.B.; Karunaratne, W.A.I.P.; Tomberlin, J.K.; Magni, P.A. Effect of type of tissue on the development of Chrysomya rufifacies (Diptera: Calliphoridae) in Sri Lanka. J. Med. Entomol. 2021, 58, 1673–1679. [Google Scholar] [CrossRef]
- Bambaradeniya, Y.T.B.; Karunaratne, W.A.I.P.; Tomberlin, J.K.; Goonerathne, I.; Kotakadeniya, R.B. Effect of temperature and tissue type on the development of myiasis causing fly; Chrysomya bezziana (Diptera: Calliphoridae). J. Med. Entomol. 2019, 56, 625–631. [Google Scholar] [CrossRef]
- Grassberger, M.; Reiter, C. Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen-and isomorphen-diagram. Forensic Sci. Int. 2001, 120, 32–36. [Google Scholar] [CrossRef]
- Bambaradeniya, Y.T.B.; Karunaratne, W.I.P.; Tomberlin, J.K.; Goonerathne, I.; Kotakadeniya, R.B. Temperature and tissue type impact development of Lucilia cuprina (Diptera: Calliphoridae) in Sri Lanka. J. Med. Entomol. 2018, 55, 285–291. [Google Scholar] [CrossRef]
- Ma, T.; Huang, J.; Wang, J.F. Study on the pupal morphogenesis of Chrysomya rufifacies (Macquart) (Diptera: Calliphoridae) for postmortem interval estimation. Forensic Sci. Int. 2015, 253, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.X.; Liu, G.C. Pupal age estimation of forensically important Megaselia spiracularis Schmitz (Diptera: Phoridae). Forensic Sci. Int. 2013, 231, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.X.; Liu, G.C. Pupal age estimation of forensically important Megaselia scalaris (Loew) (Diptera: Phoridae). Forensic Sci. Int. 2014, 236, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Wang, Y. A comparative study of the decomposition of pig carcasses in a methyl methacrylate box and open-air conditions. J. Forensic Legal Med. 2016, 42, 92–95. [Google Scholar] [CrossRef]
- Nur Aliah, N.A.; Heo, C.C.; Noor Shafini, M.; Mohd Hafizi, M. Age estimation of forensically important blowfly, Chrysomya megacephala (Diptera: Calliphoridae) pupae using micro-computed tomography imaging. Trop. Biomed. 2019, 36, 640–653. [Google Scholar]
- Feng, D.X.; Wu, J.; Sun, D.P. Intrapuparial age estimation of forensically important Dohrniphora cornuta (Diptera: Phoridae). J. Med. Entomol. 2021, 58, 616–624. [Google Scholar] [CrossRef]
- Voss, S.C.; Magni, P.; Dadour, I.; Nansen, C. Reflectance-based determination of age and species of blowfly puparia. Int. J. Legal Med. 2017, 131, 263–274. [Google Scholar] [CrossRef]
- Brown, K.; Harvey, M. Optical coherence tomography: Age estimation of Calliphora vicina pupae in vivo? Forensic Sci. Int. 2014, 242, 157–161. [Google Scholar] [CrossRef]
- Moore, H.E.; Adam, C.D.; Drijfhout, F.P. Potential Use of Hydrocarbons for Aging Lucilia sericata Blowfly Larvae to Establish the Postmortem Interval. J. Forensic Sci. 2013, 58, 404–412. [Google Scholar] [CrossRef]
- Frederickx, C.; Dekeirsschieter, J.; Brostaux, Y.; Wathelet, J.P.; Verheggen, F.J.; Haubruge, E. Volatile organic compounds released by blowfly larvae and pupae: New perspectives in forensic entomology. Forensic Sci. Int. 2012, 219, 215–220. [Google Scholar] [CrossRef]
- Yanmanee, S.; Husemann, M.; Benbow, M.E.; Suwannapong, G. Larval development rates of Chrysomya rufifacies Macquart, 1842 (Diptera: Calliphoridae) within its native range in South-East Asia. Forensic Sci. Int. 2016, 266, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, S.S. The Identification of Diptera of the Grave and Their Succession Patterns during Winter and Summer in Central South Africa, with Reference to Forensic Applications. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2016. Available online: http://hdl.handle.net/11660/4033 (accessed on 2 May 2023).
- Swiger, S.L.; Hogsette, J.A.; Butler, J.F. Laboratory colonization of the blow flies, Chrysomya megacephala (Diptera: Calliphoridae) and Chrysomya rufifacies (Diptera: Calliphoridae). J. Econ. Entomol. 2014, 107, 1780–1784. [Google Scholar] [CrossRef]
- Archer, M.S.; Jones, S.D.; Wallman, J.F. Delayed reception of live blowfly (Calliphora vicina and Chrysomya rufifacies) larval samples: Implications for minimum postmortem interval estimates. Forensic Sci. Res. 2018, 3, 27–39. [Google Scholar] [CrossRef]
- Pounder, D.J. Forensic entomo-toxicology. J. Forensic Sci. Soc. 1991, 31, 469–472. [Google Scholar] [CrossRef]
- Introna, F.; Campobasso, C.P.; Goff, M.L. Entomotoxicology. Forensic Sci. Int. 2001, 120, 42–47. [Google Scholar] [CrossRef]
- Scott, A.M. Crime Scene Documentation. In Wiley Encyclopedia of Forensic Science, 1st ed.; Jamieson, A., Moenssens, A., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Byrd, J.; Sutton, L. Forensic entomology for the investigator. Wiley Interdiscip. Rev. Forensic Sci. 2020, 2, 1370. [Google Scholar] [CrossRef]
- Miller, M.T. Crime Scene Investigation. In Forensic Science: An Introduction to Scientific and Investigative Techniques, 1st ed.; James, S.H., Nordby, J.J., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 143–164. [Google Scholar]
- Hall, M.J.R. The relationship between research and casework in forensic entomology. Insects 2021, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, W.L. Forensic Entomology. Med. Vet. Entomol. 2019, 5, 51–60. [Google Scholar] [CrossRef]
- Weidner, L.M.; Meeds, A.W.; Noblesse, A.P.; Hans, K.R. A review of Forensic Entomology literature in the southwestern United States. Wiley Interdiscip. Rev. Forensic Sci. 2021, 3, e1421. [Google Scholar] [CrossRef]
- Niederegger, S.; Pastuschek, J.; Mall, G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci. Int. 2010, 199, 72–78. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.L.; Wang, J.F.; Wang, M.; Yang, L.J.; Tao, L.Y.; Hou, Z.L. Development of the green bottle fly Lucilia illustris at constant temperatures. Forensic Sci. Int. 2016, 267, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Tantawi, T.I. Different developmental strategies in two boreal blow flies (Diptera: Calliphoridae). J. Med. Entomol. 1993, 30, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Ames, C.; Turner, B. Low temperature episodes in development of blowflies: Implications for postmortem interval estimation. Med. Vet. Entomol. 2003, 17, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Ireland, S.; Turner, B. The effects of larval crowding and food type on the size and development of the blowfly, Calliphora vomitoria. Forensic Sci. Int. 2006, 159, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Niederegger, S.; Wartenberg, N.; Spiess, R.; Mall, G. Influence of food substrates on the development of the blowflies Calliphora vicina and Calliphora vomitoria (Diptera, Calliphoridae). Parasitol. Res. 2013, 112, 2847–2853. [Google Scholar] [CrossRef]
- Wood, T.; Pyper, K.; Casali, F. Effects of cocaine and heroin, and their combination, on the development rate of Calliphora vomitoria (Diptera: Calliphoridae). Sci. Justice 2022, 62, 471–475. [Google Scholar] [CrossRef]
- Donovan, S.E.; Hall, M.J.R.; Turner, B.D.; Moncrieff, C.B. Larval growth rates of the blowfly, Calliphora vicina, over a range of temperatures. Med. Vet. Entomol. 2006, 20, 106–114. [Google Scholar] [CrossRef]
- Hwang, C.C.; Turner, B.D. Small-scaled geographical variation in life-history traits of the blowfly Calliphora vicina between rural and urban populations. Entomol. Exp. Appl. 2009, 132, 218–224. [Google Scholar] [CrossRef]
- Aak, A.; Birkemoe, T.; Leinaas, H.P. Phenology and life history of the blowfly Calliphora vicina in stockfish production areas. Entomol. Exp. Appl. 2011, 139, 35–46. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Khamesipour, A.; Akbarzadeh, K.; Akhavan, A.A.; Rassi, Y.; Oshaghi, M.A.; Rafinejad, J. Experimental colonization and life table of the Calliphora vicina (Robineau-Desvoidy) (Diptera: Calliphoridae). J. Entomol. Zool. Stud. 2014, 2, 45–48. [Google Scholar]
- Baqué, M.; Filmann, N.; Verhoff, M.A.; Amendt, J. Establishment of developmental charts for the larvae of the blow fly Calliphora vicina using quantile regression. Forensic Sci. Int. 2015, 248, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.H.; Allen, J.C. The development of the black blow fly, Phormia regina (Meigen). Forensic Sci. Int. 2001, 120, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Nabity, P.D.; Higley, L.G.; Heng-Moss, T.M. Light-induced variability in development of forensically important blow fly Phormia regina (Diptera: Calliphoridae). J. Med. Entomol. 2007, 44, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.L. Development Modeling of Lucilia sericata and Phormia regina (Diptera: Calliphoridae). Ph.D. Thesis, University of Nebraska-Lincoin, Lincoln, NE, USA, 2014. [Google Scholar]
- Roe, A.L.; Higley, L.G. Stage Transitions in Lucilia sericata and Phormia regina (Diptera: Calliphoridae) and Implications for Forensic Science. Insects 2023, 14, 315. [Google Scholar] [CrossRef]
- Sukhapanth, N.; Upatham, E.S.; Ketavan, C. Effects of feed and media on egg production, growth and survivorship of flies (Diptera: Calliphoridae, Muscidae and Sarcophagidae). J. Sci. Soc. Thailand 1988, 14, 41–50. [Google Scholar] [CrossRef]
- Amoudi, M.A.; Diab, F.M.; Abou-Fannah, S.S. Development rate and mortality of immature Parasarcophaga (Liopygia) ruficornis (Diptera: Sarcophagidae) at constant laboratory temperatures. J. Med. Entomol. 1994, 31, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Nassu, M.P.; Thyssen, P.J.; Linhares, A.X. Developmental rate of immatures of two fly species of forensic importance: Sarcophaga (Liopygia) ruficornis and Microcerella halli (Diptera: Sarcophagidae). Parasitol. Res. 2014, 113, 217–222. [Google Scholar] [CrossRef]
- Adhikari, K.; Khanikor, B.; Sarma, R.; Mahanta, S.; Kalita, J. Study on the life history and protein content of Sarcophaga ruficornis (Diptera: Sarcophagidiae) a forensically important insect. J. Zool. Stud. 2016, 3, 1–8. [Google Scholar]
- Bansode, S.A.; More, V.R.; Zambare, S.P. Effect of Constant Temperature (20 °C, 25 °C, 30 °C, 35 °C, 40 °C) on the Development of Sarcophagidae: Diptera (FAB) (Sarcophagidae: Diptera). J. Pet. Environ. Biotechnol. 2016, 7, 2. [Google Scholar]
- Barbosa, T.M.; Cruz, M.R.P.; Pontes, W.J.T.; Vasconcelos, S.D. Aspects of the reproductive behaviour and development of two forensically relevant species, Blaesoxipha (Gigantotheca) stallengi (Lahille, 1907) and Sarcophaga (Liopygia) ruficornis (Fabricius, 1794) (Diptera: Sarcophagidae). Rev. Bras. Entomol. 2019, 63, 124–129. [Google Scholar] [CrossRef]
- Prawirodisastro, M.; Benjamin, D.M. Laboratory study on the biology and ecology of Megaselia scalaris (Diptera: Phoridae). J. Med. Entomol. 1979, 16, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Trumble, J.T.; Pienkowski, R.L. Development and survival of Megaselia scalaris (Diptera: Phoridae) at selected temperatures and photoperiods. Proc. Entomol. Soc. Wash. 1979, 81, 207–210. [Google Scholar]
- Greenberg, B.; Wells, J.D. Forensic use of Megaselia abdita and M. scalaris (Phoridae: Diptera): Case studies, development rates, and egg structure. J. Med. Entomol. 1998, 35, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.A.; Cooper, R.L. Characterization of development, behavior and neuromuscular physiology in the phorid fly, Megaselia scalaris. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Zuha, R.M.; Razak, T.A.; Ahmad, N.W.; Omar, B. Interaction effects of temperature and food on the development of forensically important fly, Megaselia scalaris (Loew) (Diptera: Phoridae). Parasitol. Res. 2012, 111, 2179–2187. [Google Scholar] [CrossRef]
- Zuha, R.M.; Omar, B. Developmental rate, size, and sexual dimorphism of Megaselia scalaris (Loew) (Diptera: Phoridae): Its possible implications in forensic entomology. Parasitol. Res. 2014, 113, 2285–2294. [Google Scholar] [CrossRef]
- Chakraborty, A.; Naskar, A.; Parui, P.; Banerjee, D. Developmental variation of Indian thermophilic variety of scuttle fly Megaselia (Megaselia) scalaris (Loew, 1866) (Diptera: Phoridae) on different substrates. Scientifica 2016, 2016, 4257081. [Google Scholar] [CrossRef]
- Thomas, J.K.; Sanford, M.R.; Longnecker, M.; Tomberlin, J.K. Effects of temperature and tissue type on the development of Megaselia scalaris (Diptera: Phoridae). J. Med. Entomol. 2016, 53, 519–525. [Google Scholar] [CrossRef]
- Ong, S.Q.; Ahmad, H.; Tan, E.H. Substrate moisture affects the development of Megaselia scalaris (Diptera: Phoridae): An implication of the growth circumstances of the fly in forensic entomology. Environ. Entomol. 2018, 47, 1582–1585. [Google Scholar] [CrossRef]
- Castillo-Alanis, L.A.; González-Hernández, A.E.; Quijano-Mateos, A.; Pedraza-Lara, C.S.; Villavicencio-Queijeiro, A.; Bravo-Gómez, M.E. Standardization of a Culture Medium for Megaselia scalaris (Diptera: Phoridae) for Entomotoxicological Studies. J. Med. Entomol. 2020, 57, 1421–1431. [Google Scholar] [CrossRef]
- Matuszewski, S.; Hall, M.J.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int. J. Legal Med. 2020, 134, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.C.; Cook, D.F.; Dadour, I.R. Decomposition and insect succession of clothed and unclothed carcasses in Western Australia. Forensic Sci. Int. 2011, 211, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kotzé, Z.; Aimar, S.; Amendt, J.; Anderson, G.S.; Bourguignon, L.; Hall, M.J.; Tomberlin, J.K. The forensic entomology case report—A global perspective. Insects 2021, 12, 283. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).