Are Appearances Deceiving? Morpho-Genetic Complexity of the Eumerus tricolor Group (Diptera: Syrphidae) in Europe, with a Focus on the Iberian Peninsula †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
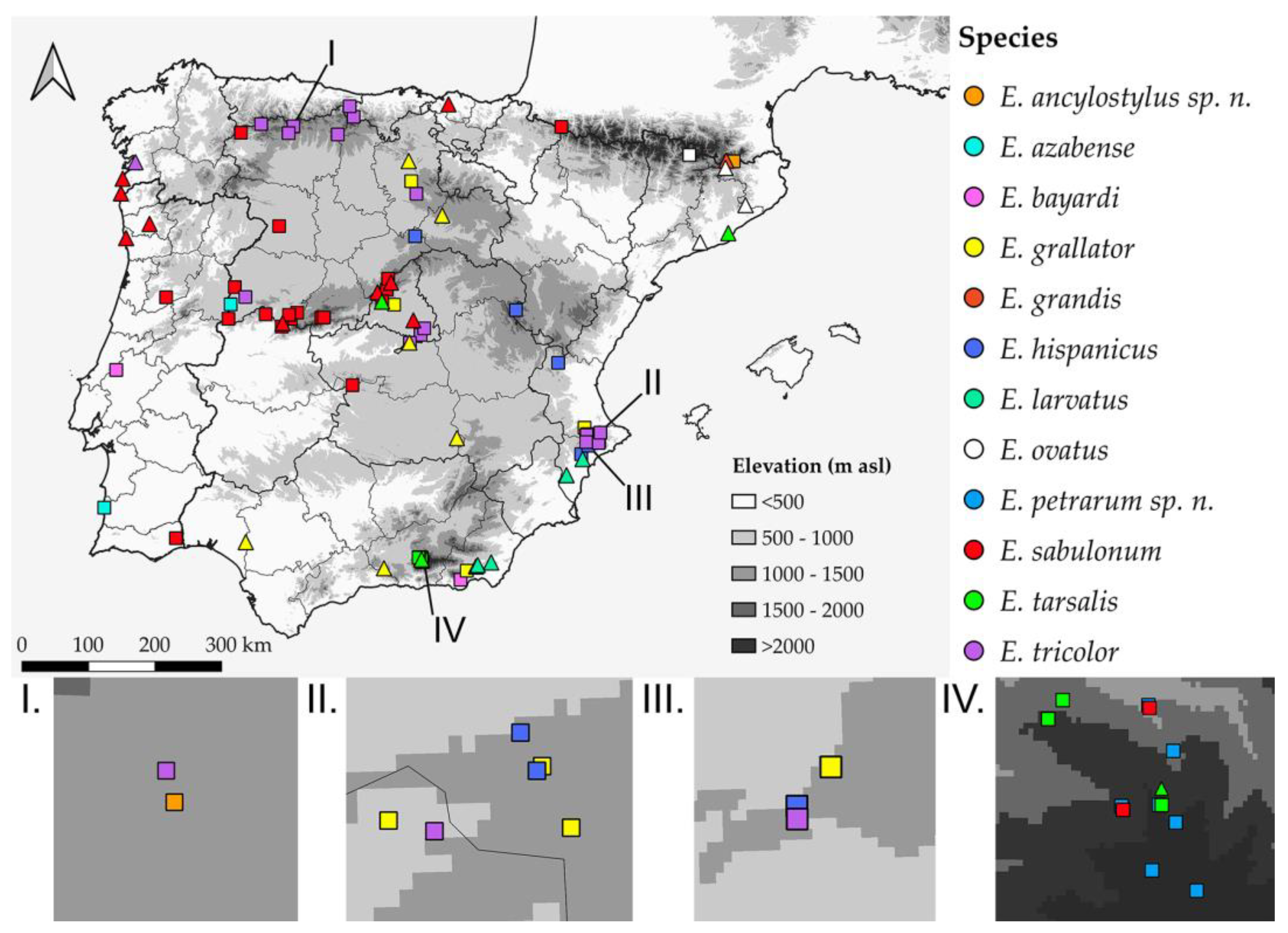
2.1. Study Area
2.2. Examined Material
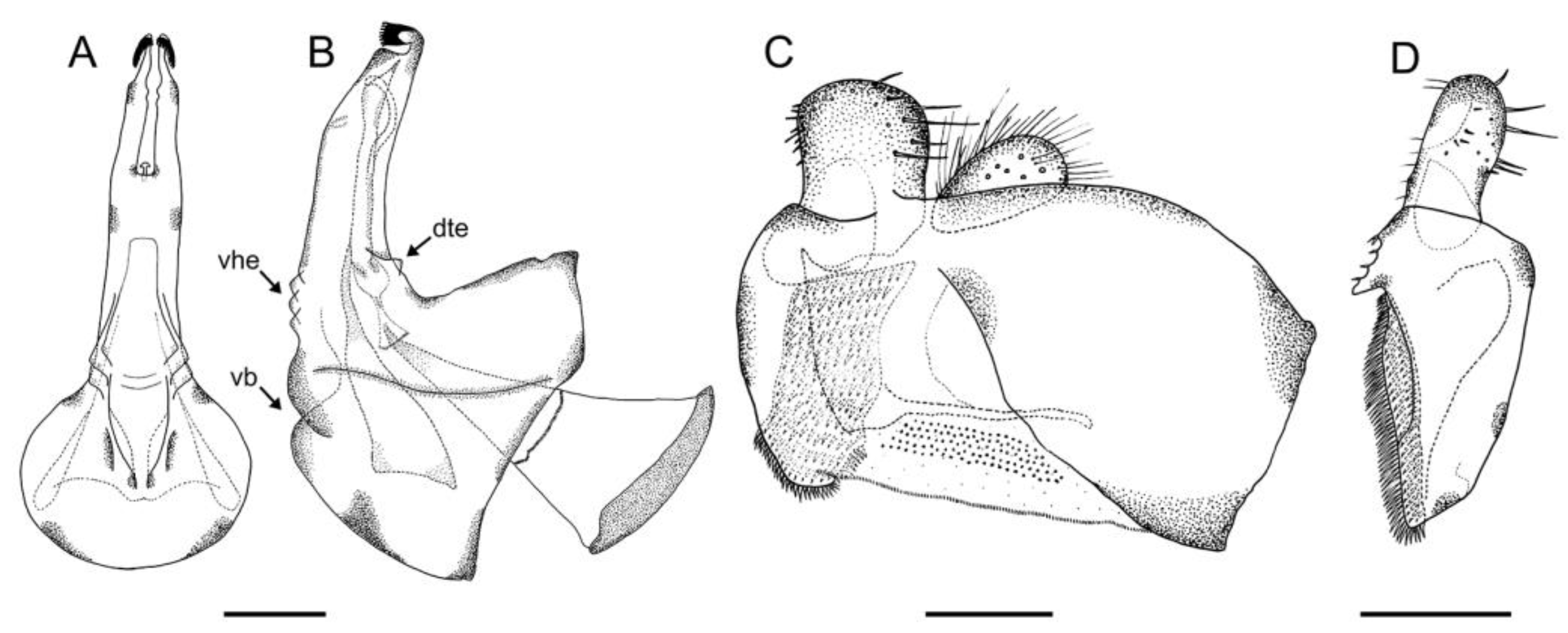
2.3. Morphological Study
2.4. Molecular Study
| Gene Region | Primer | Sequence | Reference |
|---|---|---|---|
| COI-5′ (complete) | LCO1490 | 5′-GGTCAACAAATCATAAAGATATTGG-3′ | [41] |
| HCO2198 | 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ | ||
| COI-5′ (“A”) | Heb-F | 5′-GGTCAACAAATCATAAAGATATTGG-3′ | [41] |
| 1762-R | 5′-CGDGGRAADGCYATRTCDGG-3′ | Kelso (in prep.) | |
| COI-5′ (“B”) | 342-F | 5′-TGTAAAACGACGGCCAGTGGDKCHCCNGAYATRGC-3′ | Kelso (in prep.) |
| 1976-R | 5′-GWAATRAARTTWACDGCHCC-3′ | ||
| COI-5′ (“C”) | 1957-F | 5′-GGDATWTCHTCHATYYTAGG-3′ | Kelso (in prep.) |
| 780-R | 5′-CCAAAAAATCARAATARRTGYTG-3′ | [42] | |
| COI-3′ | 780-F | 5′-CARCAYYTATTYTGATTTTTTGG-3′ | Kelso (in prep.) |
| C1-J-2183 (Jerry) | 5′-CAACATTTATTTTGATTTTTTGG-3′ | [43] | |
| TL2-N-3014 (Pat) | 5′-TCCAATGCACTAATCTGCCATATTA-3′ |
3. Results
3.1. Integrative Approach
| Species | Type Locality | IUCN Red List Category |
|---|---|---|
| E. alajensis Peck, 1966 | Kyrgyzstan | ⸺ |
| E. ancylostylus Aguado-Aranda & Ricarte, sp. n. | Spain | ⸺ |
| E. arctus van Steenis in Grković et al., 2021 | Switzerland | NE |
| E. arkadii Mutin in Mutin & Barkalov, 1999 | Russia | ⸺ |
| E. arkitensis Peck, 1969 | Kyrgyzstan | ⸺ |
| E. armatus Ricarte & Rotheray in Ricarte et al., 2012 | Greece | VU |
| E. armenorum Stackelberg, 1960 | Armenia | ⸺ |
| E. atricolorus Gilasian & van Steenis in Gilasian et al., 2020 | Iran | ⸺ |
| E. aurofinis Grković, Vujić & Radenković in Grković et al., 2016 | Greece | EN |
| E. azabense Ricarte & Marcos-García in Ricarte et al., 2018 | Spain | CR |
| E. badkhyziensis Mutin, 2019 | Turkmenistan | ⸺ |
| E. bayardi Séguy, 1961 | France | ⸺ |
| E. binominatus Hervé-Bazin, 1923* | Kazakhstan | ⸺ |
| E. brevipilosus Gilasian & van Steenis in Gilasian et al., 2020 | Iran | ⸺ |
| E. chekabicus Gilasian & van Steenis in Gilasian et al., 2020 | Iran | ⸺ |
| E. coeruleithorax Peck, 1969 | Kazakhstan | ⸺ |
| E. coeruleus (Becker, 1913) | Iran | ⸺ |
| E. compertus Villeneuve in Villeneuve & Gauthier, 1924 | Tunisia | ⸺ |
| E. crispus Vujić & Grković in Grković et al., 2021 | Serbia | NE |
| E. falsus Becker, 1922 | Syria | ⸺ |
| E. grallator Smit in Grković et al., 2019a* | Spain | VU |
| E. grandis Meigen, 1822 | Europe | LC |
| E. grisescens Becker, 1921 | Russia | ⸺ |
| E. hispanicus van der Goot, 1966 | Spain | VU |
| E. hissaricus Stackelberg, 1949 | Tajikistan | ⸺ |
| E. jacobsoni Becker in Becker & Stein, 1913 | Iran | ⸺ |
| E. kazanovzkyae Paramonov, 1927 | Azerbaijan | ⸺ |
| E. kirgisorum Peck, 1966 | Kyrgyzstan | ⸺ |
| E. kopetdagicus Barkalov & Mutin, 2022 | Turkmenistan | ⸺ |
| E. larvatus Aracil, Grković & Pérez-Bañón in Aracil et al., 2023 | Spain | ⸺ |
| E. leleji Mutin, 2016 | Khakassia | ⸺ |
| E. longitarsis Peck, 1979* | Tajikistan | ⸺ |
| E. lunatus (Fabricius, 1794) | North Africa | ⸺ |
| E. mucidus Bezzi, 1921 | Africa | ⸺ |
| E. nigrifacies Becker, 1921 | Russia | ⸺ |
| E. nigrorufus Grković & Vujić in Grković et al., 2021 | Montenegro | NE |
| E. niveitibia Becker, 1921 | Bulgaria | VU |
| E. ovatus Loew, 1848 | Europe | EN |
| E. ovoformus Gilasian & van Steenis in Gilasian et al., 2020 | Iran | ⸺ |
| E. palaestinensis Stackelberg, 1949 | Israel | ⸺ |
| E. pamirorum Stackelberg, 1949 | Tajikistan | ⸺ |
| E. pavlovskii Stackelberg, 1964 | Armenia | ⸺ |
| E. persarum Stackelberg, 1961 | Iran | ⸺ |
| E. persicus Stackelberg, 1949 | Iran | ⸺ |
| E. petrarum Aguado-Aranda, Nedeljković & Ricarte, sp. n. | Spain | ⸺ |
| E. pilosipedes Gilasian & van Steenis in Gilasian et al., 2020 | Iran | ⸺ |
| E. platycodon Choi & Hong in Choi et al., 2021 | South Korea | ⸺ |
| E. richteri Stackelberg, 1960 | Azerbaijan | ⸺ |
| E. rubrum Grković & Vujić in Grković et al., 2017 | Greece | EN |
| E. rubescens Villeneuve, 1912 | Syria | ⸺ |
| E. rufipilus Peck, 1969 | Kyrgyzstan | ⸺ |
| E. rufomaculatus Peck, 1966 | Kyrgyzstan | ⸺ |
| E. ryzhik Barkalov & Mutin, 2022 | Uzbekistan | ⸺ |
| E. sabulonum (Fallén, 1817) | Sweden | LC |
| E. selevini Stackelberg, 1949 | Kazakhstan | ⸺ |
| E. sinuatus Loew, 1855 | Austria | EN |
| E. tadzhikorum Stackelberg, 1949* | Tajikistan | ⸺ |
| E. tarsalis Loew, 1848 | Europe | EN |
| E. tauricus Stackelberg, 1952 | Ukraine | EN |
| E. tenuitarsis Grković & Vujić in Grković et al., 2019a* | Greece | CR |
| E. tricolor (Fabricius, 1798) | Switzerland | LC |
| E. turcmenorum Paramonov, 1927 | Turkmenistan | ⸺ |
| E. urartorum Stackelberg, 1960 | Armenia | ⸺ |
| E. ursiculus Stackelberg, 1949 | Tadzhikistan | ⸺ |
| E. ussuriensis Stackelberg, 1952 | Russia | ⸺ |
| E. vallicolus Gilasian & van Steenis in Gilasian et al., 2020 | Iran | ⸺ |
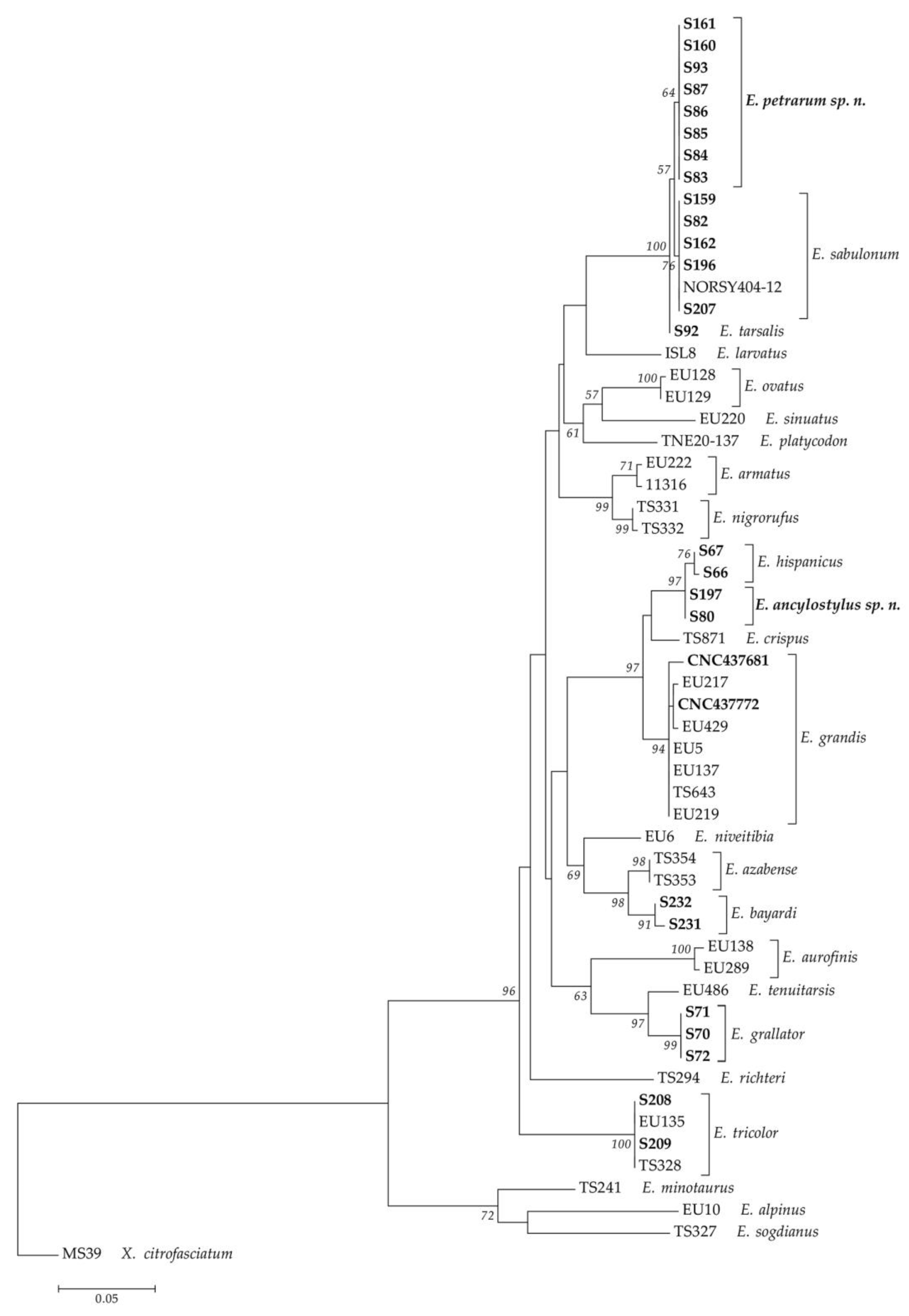
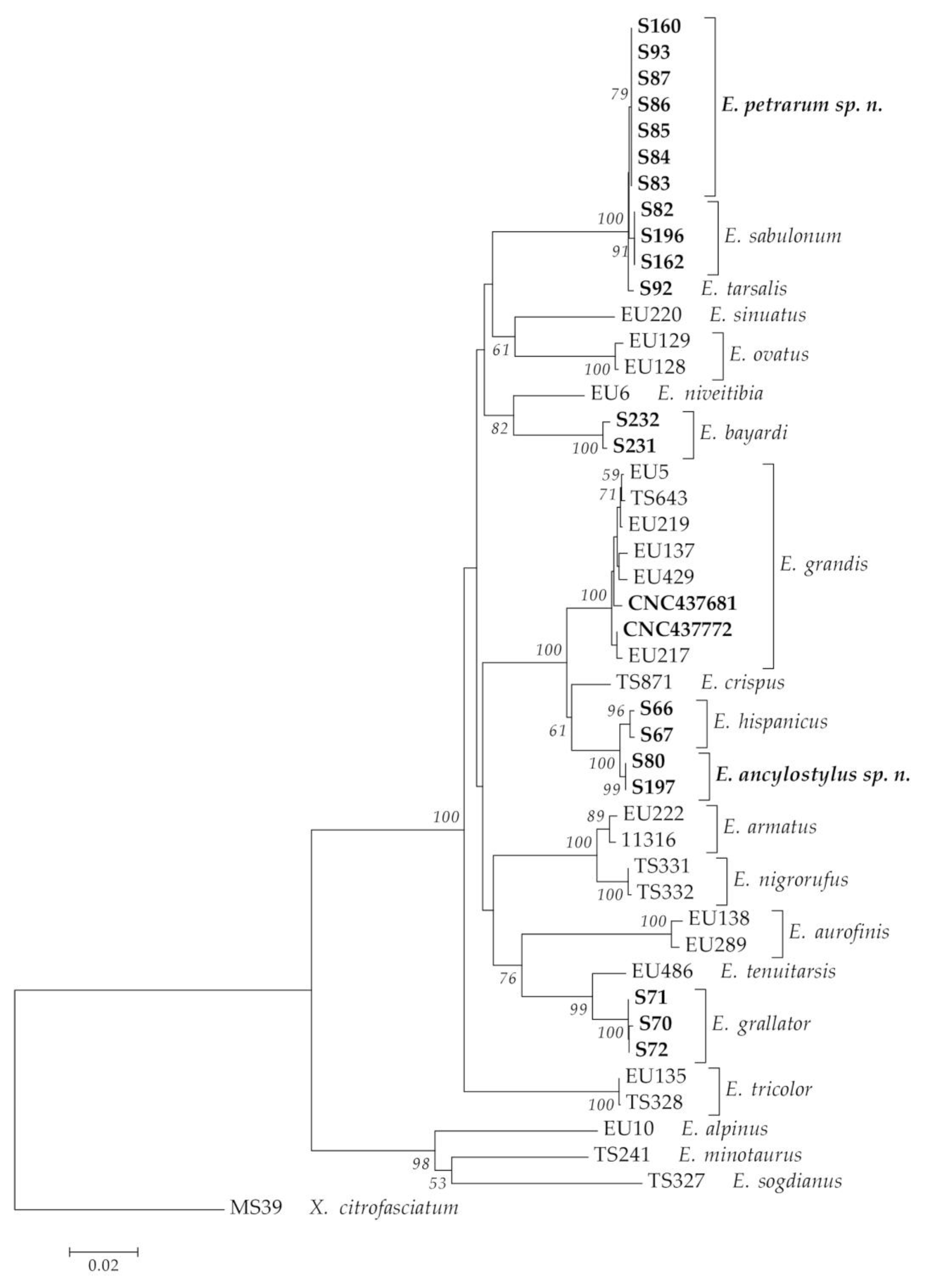
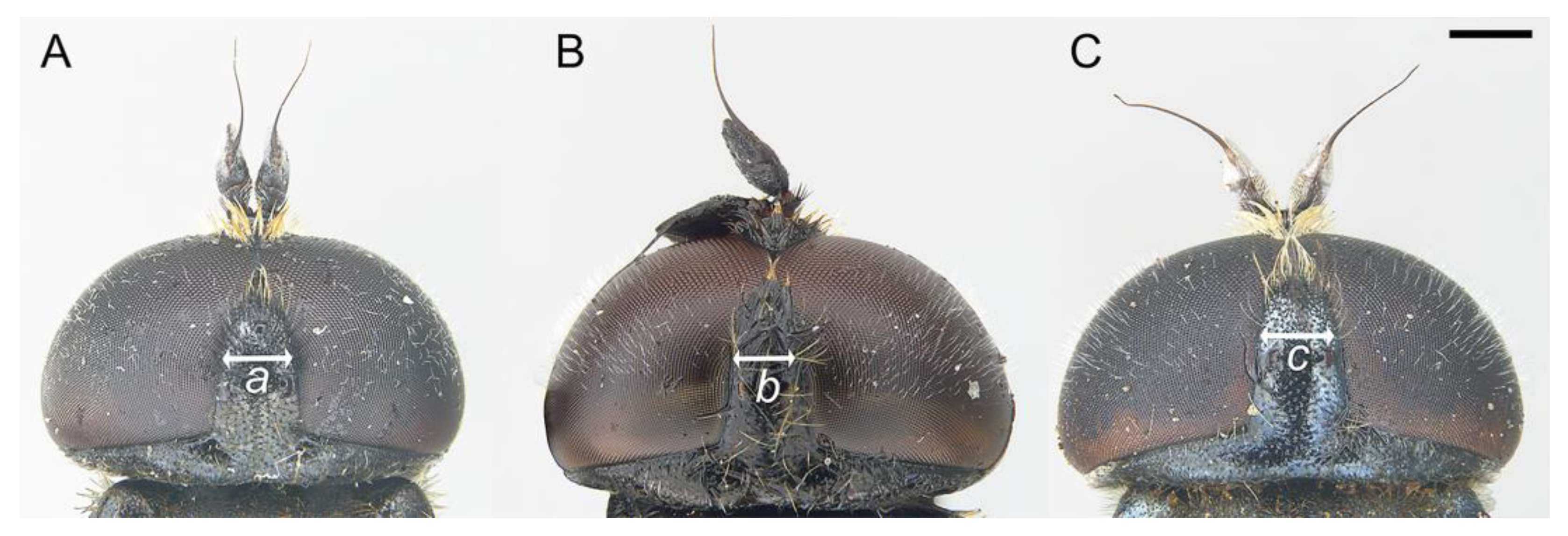
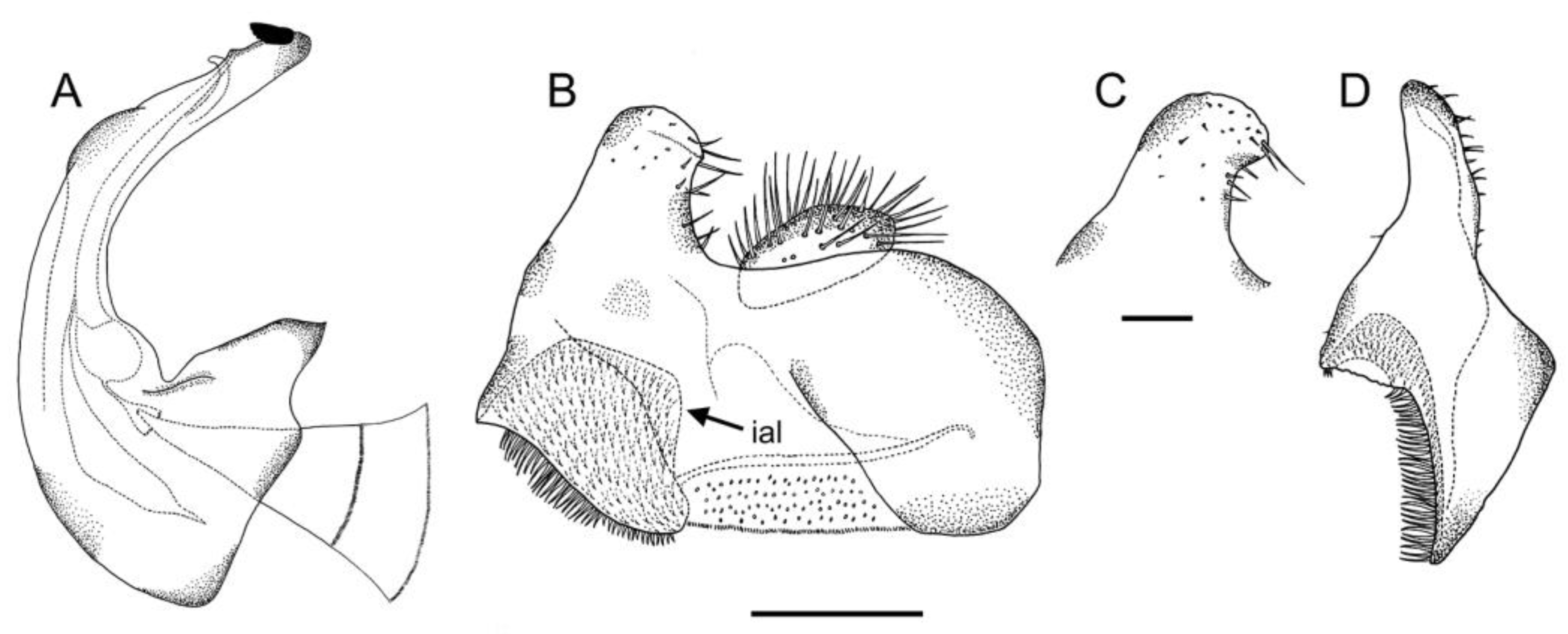
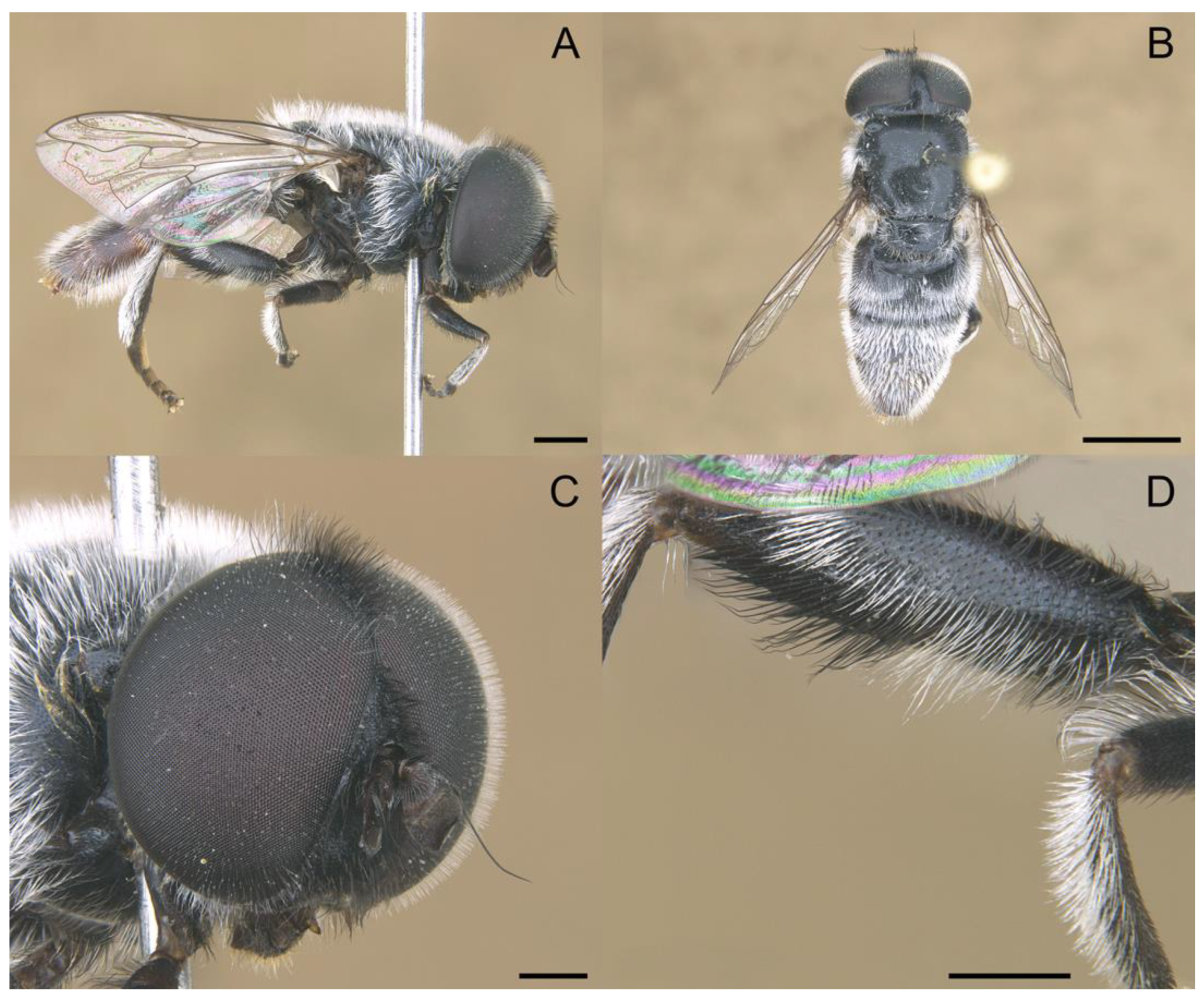
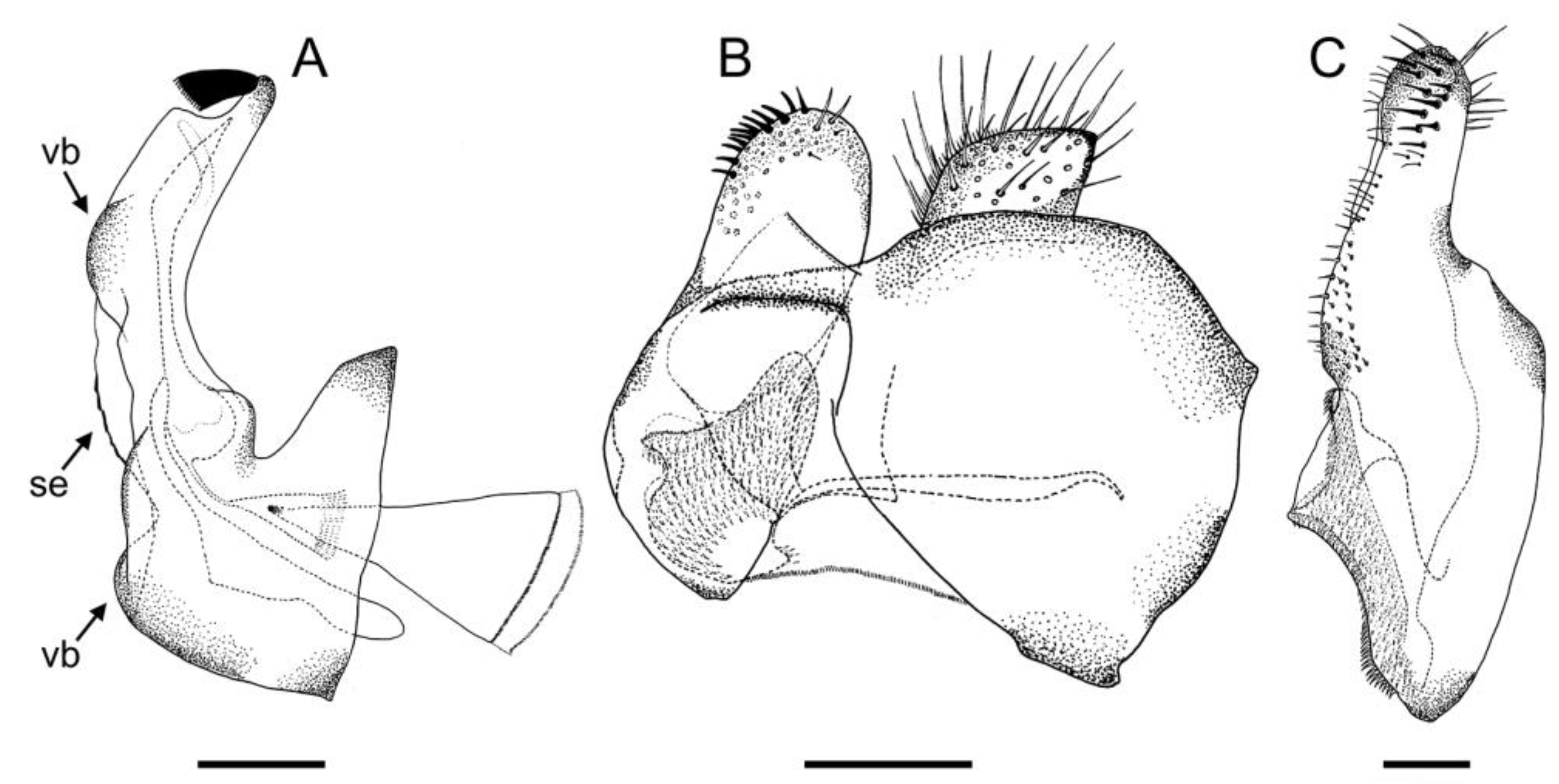
3.2. New Species Descriptions

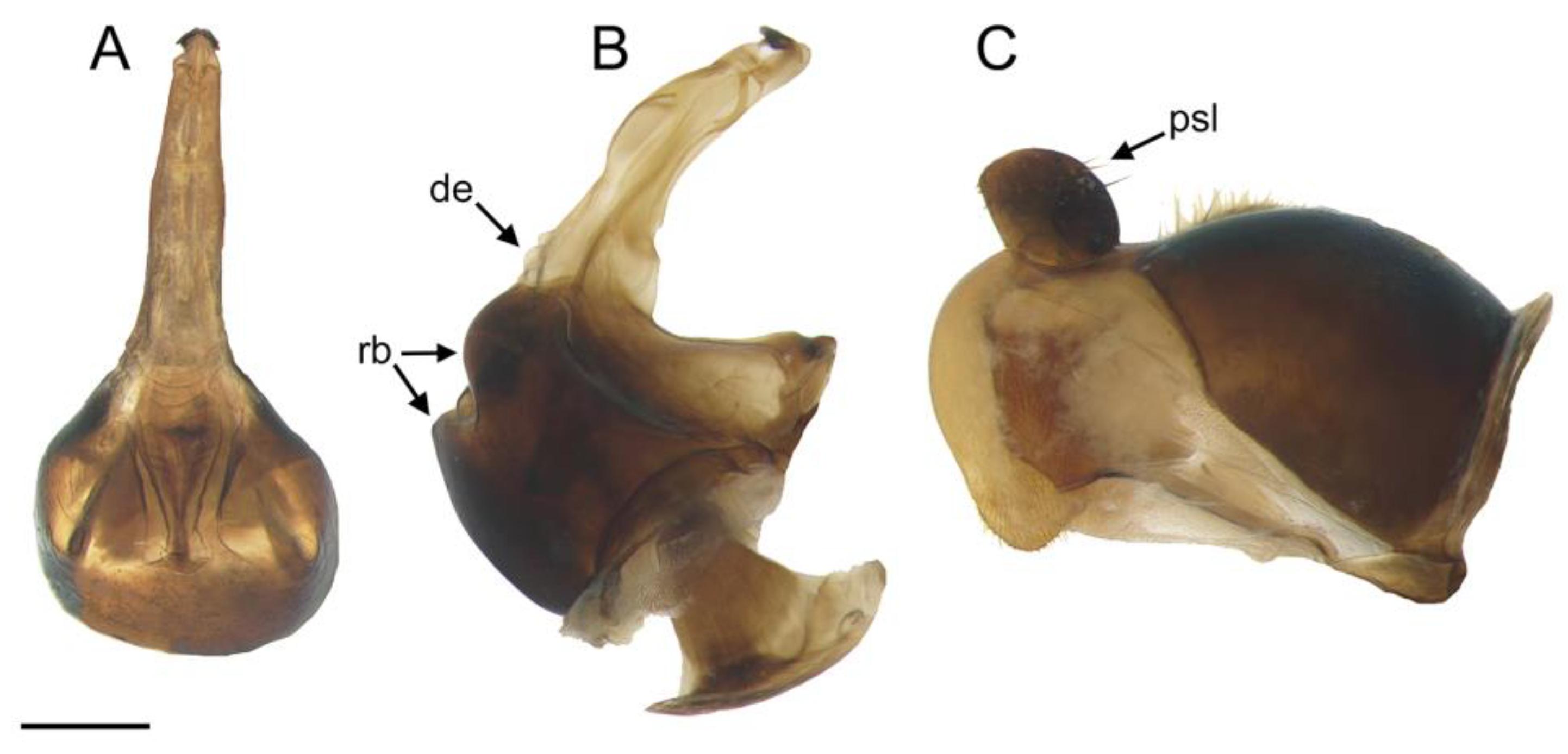

- Holotype
- Paratypes

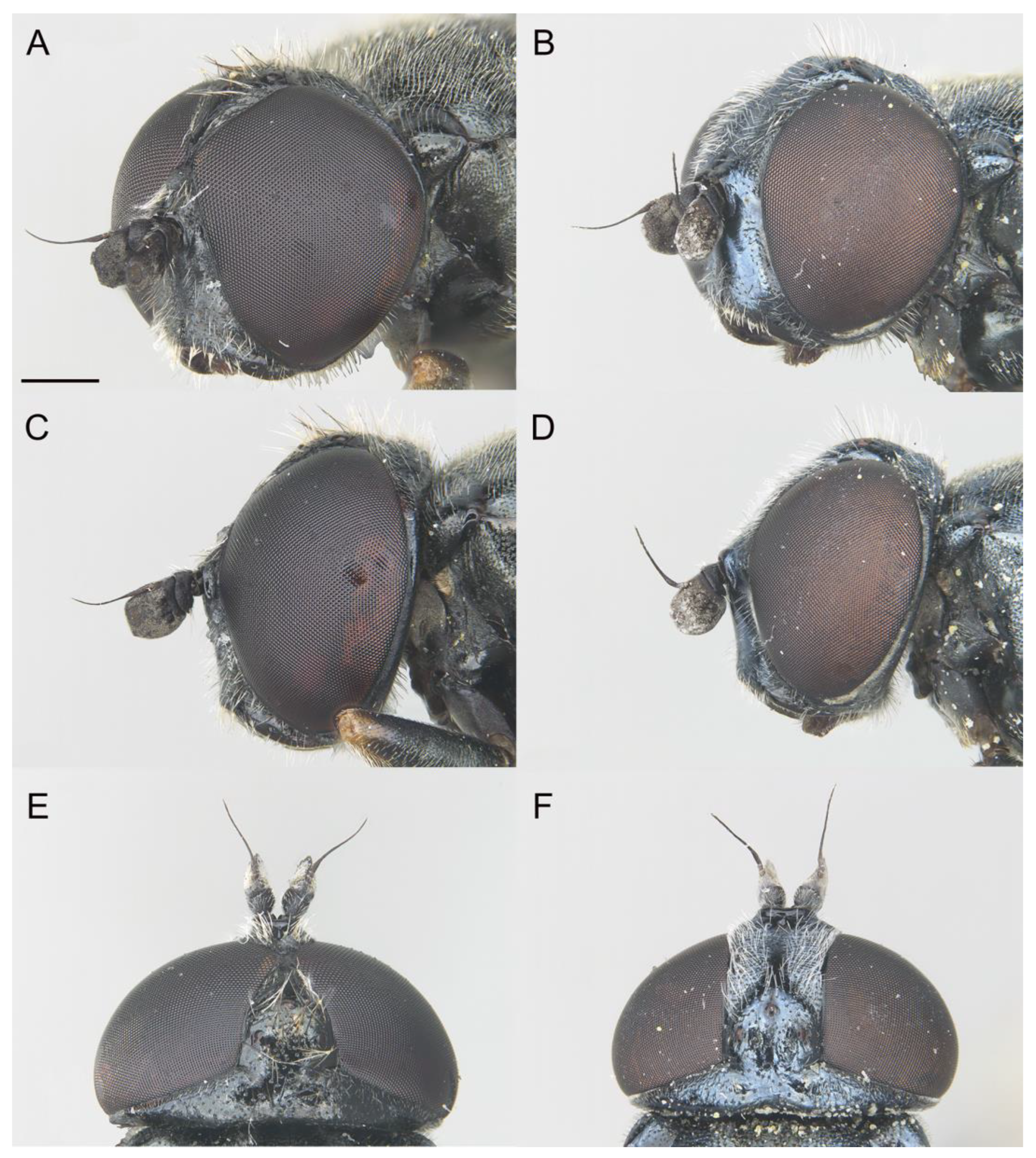
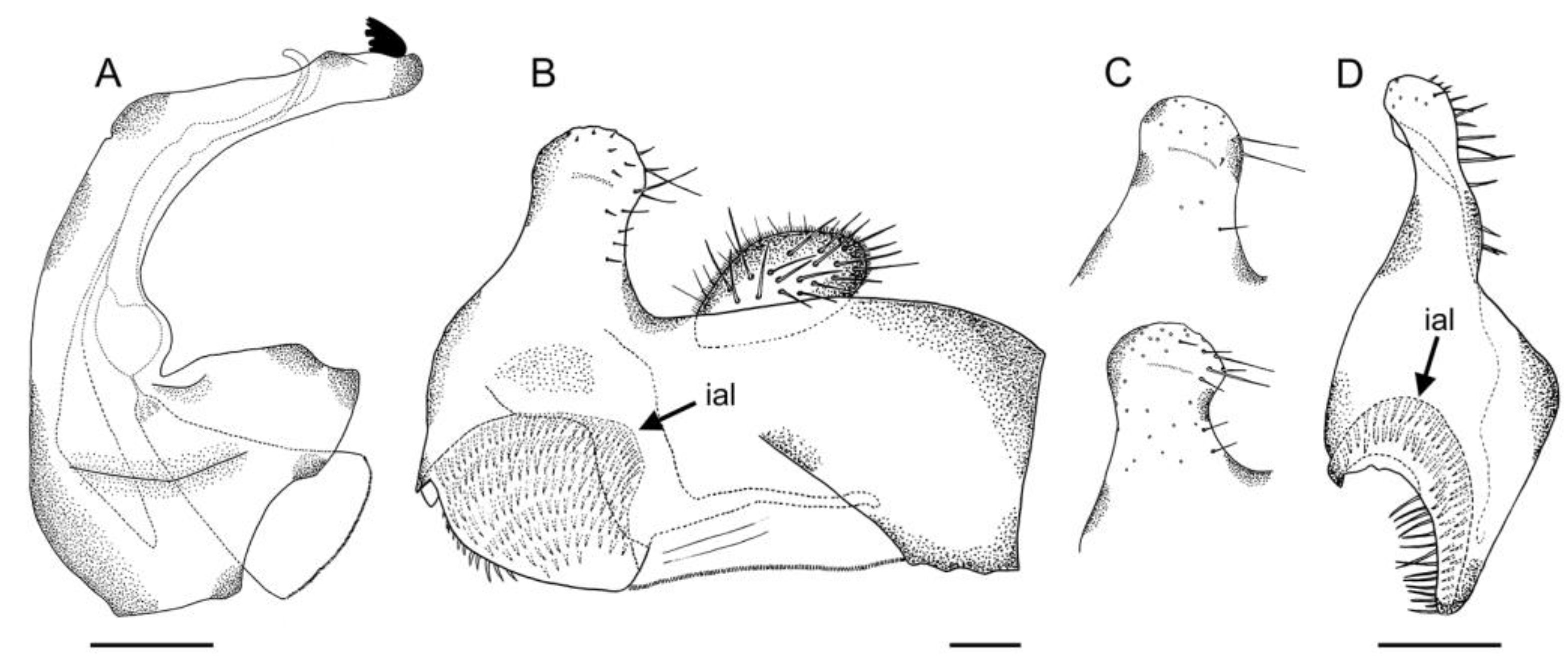
- Holotype
- Paratypes
3.3. Remarks on other Iberian Species of the E. tricolor Group
3.3.1. Eumerus azabense Ricarte & Marcos–García in Ricarte et al., 2018
3.3.2. Eumerus bayardi Séguy, 1961
3.3.3. Eumerus grallator Smit in Grković et al., 2019a
3.3.4. Eumerus grandis Meigen, 1822
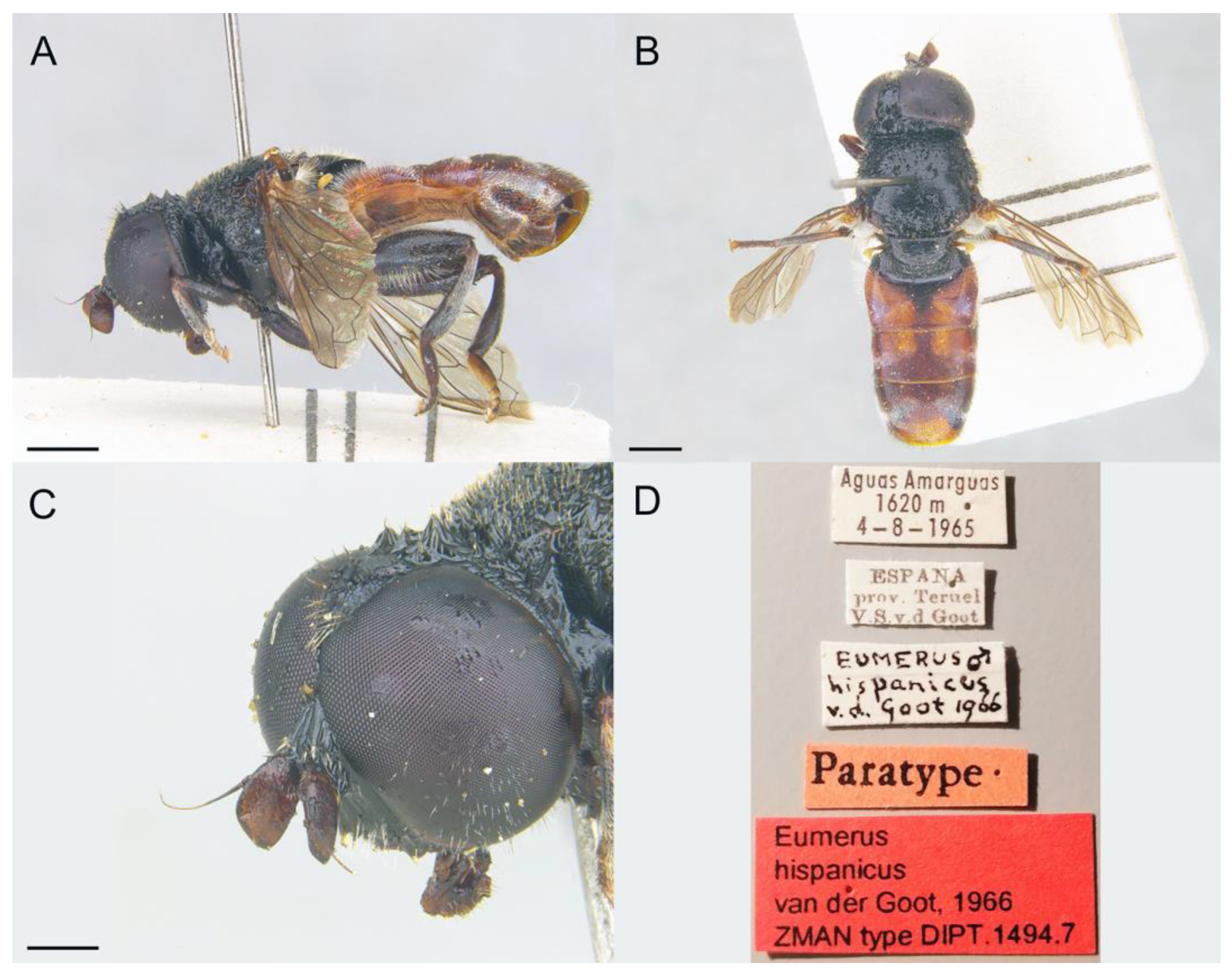
3.3.5. Eumerus hispanicus van der Goot, 1966
3.3.6. Eumerus larvatus Aracil, Grković & Pérez-Bañón in Aracil et al., 2023
3.3.7. Eumerus ovatus Loew, 1848
3.3.8. Eumerus sabulonum (Fallén, 1817)
3.3.9. Eumerus tarsalis Loew, 1848
3.3.10. Eumerus tricolor (Fabricius, 1798)
3.4. Key to the European Species of the E. tricolor Group
Males
- The segments of the metaleg are remarkably slender (Grković et al. [15]: figure 4) … 2 (E. binominatus subgroup)
- −
- Segments of the metaleg of normal length … 3 (other species)
- The greatest width of the metafemur is approximately equal to one-fifth of the length of the metafemur (Grković et al. [15]: figure 4) … E. grallator
- −
- The greatest width of the metafemur is approximately equal to one-eighth of the length of the metafemur (Grković et al. [15]: figure 4) … E. tenuitarsis
- The eyes are bare or nearly bare (i.e., with very short and dispersed hairs on the eye surface) … 4
- −
- The eyes are hairy … 10
- The basoflagellomere is large, about one-third of the height of the head, and yellow (Grković et al. [11]: figure 4). Tarsomeres are short, and the metabasotarsomere is longer than the other four tarsomeres of the same leg combined (Grković et al. [11]: figure 4). The ocellar triangle is isosceles in nature and closer to eye contiguity than to the upper eye margin … 5
- −
- The basoflagellomere is small, about one-quarter of the height of the head, and yellow or brown (Grković et al. [11]: figure 6). Tarsi of normal length. The ocellar triangle is wider than it is long or equilateral and closer to the upper corner of the eye than to eye contiguity … 6
- Basoflagellomere yellow. Tergum I yellow posteriorly (Grković et al. [13]: figure 10) … E. rubrum
- −
- Basoflagellomere black or dark brown. Tergum I is entirely black … 11
- Hairs on the ventral side of the metafemur are very long and dense and clearly longer than the spinae … E. tauricus
- −
- Hairs on the ventral side of the metafemur are shorter, or only slightly longer, than the spinae … 8
- The basotarsomere and tarsomeres II–IV of the proleg each bear a long black seta posterolaterally. The male genitalia, as in figure 5d [11] … E. tarsalis
- −
- Protarsus without a long black seta … 9
- The eyes are separated, at least, by the diameter of an ocellus (Grković et al. [11]: figures 4, 6) … 11
- −
- The eyes are holoptic or, at most, separated by less than the diameter of an ocellus (Grković et al. [11]: figures 9, 11) … 14
- The basoflagellomere is yellow or red. The terga are black … 12
- −
- The basoflagellomere is black or dark brown. The terga have red maculae … 13
- Tergum IV is covered with short, golden yellow hairs (Grković et al. [11]: figure 6). Posterior surstylar lobe of male genitalia erect (Grković et al. [11]: figure 3) … E. aurofinis
- −
- Tergum IV is not covered with golden yellow hairs. The posterior surstylar lobe of the male genitalia is bent posteriorly (Grković et al. [11]: figure 3) … E. richteri
- The hairs on the mesonotum are very long. Terga II-III have large, black maculae centrally (Grković et al. [11]: figure 11) … E. sinuatus
- The ventral side of the metafemur is covered with white hairs basally and black hairs distally (Figure 17D) … E. bayardi
- −
- The ventral side of the metafemur is entirely covered with unicolorous hairs… 15
- The basoflagellomere is square-shaped. Metafemur ventrally covered with long black hairs … E. larvatus
- −
- The basoflagellomere is not square-shaped. Metafemur covered with hairs of different dispositions and/or colours … 16
- The mesonotum is covered with moderately long hairs (e.g., Figure 3A) … 17
- −
- The mesonotum is covered with very long hairs (Figure 15A) … 21
- The terga are entirely black. Posterior surstylar lobe of male genitalia with a distinct lateral wing-like expansion (Grković et al. [11]: figure 3) … 18
- −
- The terga usually have red maculae laterally. The posterior surstylar lobe of the male genitalia does not have a lateral wing-like protrusion … 19
- The ocellar triangle has black and white hairs intermixed. The terga are mainly red … E. hispanicus
- −
- The ocellar triangle has black hairs. The terga are black with red maculae laterally, at least, on tergum II … 20
- Terga III-IV are covered with characteristic silver-white hairs … E. ovatus
- −
- The hairs on terga II–IV are in a different arrangement … 22
- The metatibia is covered with characteristic snow-white hairs dorsally (Grković et al. [11]: figure 7). Terga II–IV entirely black with metallic blue shine (Grković et al. [11]: figure 11) … E. niveitibia
- −
- The metatibia is not covered with snow-white hairs. Terga II–IV do not have a metallic blue shine … E. azabense
Females
- The metafemur and metatibia are remarkably slender. The spinae on the ventral side of the metafemur are tiny, short and sparse (Grković et al. [11]: figure 8) … 2 (E. binominatus subgroup)
- −
- The metafemur and metatibia are more or less thickened. The spinae on the ventral side of the metafemur are arranged in rows … 3 (other species)
- The ocellar triangle has white hairs. The sterna have white hairs … E. grallator
- −
- The ocellar triangle has black hairs. The sterna have black hairs except sternum IV, covered with white hairs … E. tenuitarsis
- The eyes are bare or nearly bare … 4
- −
- The eyes are covered with conspicuous hairs … 9
- The basoflagellomere is dark brown (Grković et al. [11]: figure 6). Pro- and mesotarsomeres II-III yellow, with a black spot basally (Grković et al. [11]: figure 6) … 5
- −
- The basoflagellomere is yellow (Grković et al. [11]: figure 4). The pro- and mesotarsus are black … 7
- Protarsomeres II–IV each have a long black seta posterolaterally … E. tarsalis
- −
- The protarsus does not bear a long black seta … 6
- The basoflagellomere usually tapers toward the apex. Vertical triangle slightly elevated dorsally (Figure 10D) … E. petrarum sp. n.
- −
- The basoflagellomere is usually axe-shaped. Vertical triangle not elevated dorsally (Figure 21B) … E. sabulonum
- The ocellar triangle is wider than it is long (Grković et al. [13]: figure 12). The metafemur has a transverse suture anteriorly (Grković et al. [13]: figure 12) … E. rubrum
- −
- The ocellar triangle is equilateral or almost so (Grković et al. [13]: figure 12). The metafemur is without a transverse suture anteriorly … E. tauricus
- −
- Hairs on the mesonotum are very long (Figure 15A) … 16
- The basoflagellomere is yellow or red (Grković et al. [11]: figure 6) … 17
- −
- The basoflagellomere is black or dark brown … 12
- The basoventral hairs of the metafemur are longer than the spinae … 13
- −
- Basoventral hairs of the metafemur are clearly shorter than the spinae … 15
- The basoflagellomere is square-shaped … E. larvatus
- −
- The basoflagellomere is rounded … 14
- The basoflagellomere has a yellow macula basally … E. grandis
- −
- The basoflagellomere is unicolorous … E. hispanicus
- The metatibia is dorsally covered with conspicuous snow-white hairs (Grković et al. [11]: figure 8d). Terga II–III are mainly black, sometimes with reduced red maculae … E. niveitibia
- −
- The metatibia is without conspicuous snow-white hairs. Terga II–III are mainly red … 17
- The basoflagellomere is oval (Figure 16B) … E. azabense
- −
- The basoflagellomere is rounded (Figure 16A) … 18
- The basoflagellomere is moderately striated (Grković et al. [11]: figure 11) … E. ovatus
- −
- The basoflagellomere is densely striated (Grković et al. [11]: figure 11) … E. sinuatus
4. Discussion
5. Conclusions
- (1)
- A total of 12 species of the E. tricolor group are present in the Ibero-Balearic region. A lectotype is designated for E. lateralis;
- (2)
- Two new species, E. ancylostylus sp. n. and E. petrarum sp. n., are described, illustrated and discussed;
- (3)
- From a morphological point of view, levels of intraspecific variability were high for both E. sabulonum and E. petrarum sp. n., while the interspecific variability was low between E. grandis and E. ancylostylus sp. n.;
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, S.A. Flies: The Natural History and Diversity of Diptera; Firefly Books Ltd.: Richmond Hill, ON, Canada, 2012; pp. 1–616. [Google Scholar]
- Ballester-Torres, I.; Ricarte, A.; Nedeljković, Z.; Marcos-García, M.Á. High phenotypic diversity does not always hide taxonomic diversity: A study case with Cheilosia soror (Zetterstedt, 1843) (Diptera: Syrphidae) in the Iberian Peninsula. J. Zool. Syst. Evol. Res. 2022, 2022, 8378483. [Google Scholar] [CrossRef]
- Vujić, A.; Radenković, S.; Likov, L.; Veselić, S. Taxonomic complexity in the genus Merodon Meigen, 1803 (Diptera, Syrphidae). ZooKeys 2021, 1031, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Ennos, R.A.; French, G.C.; Hollingsworth, P.M. Conserving taxonomic complexity. TRENDS Ecol. Evol. 2005, 20, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Struck, T.H.; Cerca, J. Cryptic species and their evolutionary significance. Encycl. Life Sci. 2019, 2019, 1–9. [Google Scholar]
- Nedeljković, Z.; Ricarte, A.; Šašić Zorić, L.; Djan, M.; Hayat, R.; Vujić, A.; Marcos-García, M.Á. Integrative taxonomy confirms two new West-Palaearctic species allied with Chrysotoxum vernale Loew, 1841 (Diptera: Syrphidae). Org. Divers. Evol. 2020, 20, 821–833. [Google Scholar] [CrossRef]
- Ricarte, A.; Nedeljković, Z.; Aguado-Aranda, P.; Marcos-García, M.Á. Assessing the Diversity and Systematics of Brachyopini Hoverflies (Diptera: Syrphidae) in the Iberian Peninsula, Including the Descriptions of Two New Species. Insects 2022, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, N. Cryptic and Sibling Species among the Decapod Crustacea. J. Crustacean Biol. 1986, 6, 356–363. [Google Scholar] [CrossRef]
- Vujić, A.; Radenković, S.; Likov, L.; Andrić, A.; Janković, M.; Ačanski, J.; Popov, G.; de Courcy Williams, M.; Šašić Zorić, L.; Djan, M. Conflict and congruence between morphological and molecular data: Revision of the Merodon constans group (Diptera: Syrphidae). Invertebr. Syst. 2020, 34, 406–448. [Google Scholar] [CrossRef]
- Ricarte, A.; Souba-Dols, G.J.; Hauser, M.; Marcos-García, M.Á. A review of the early stages and host plants of the genera Eumerus and Merodon (Diptera: Syrphidae), with new data on four species. PLoS ONE 2017, 12, e0189852. [Google Scholar] [CrossRef]
- Grković, A.; van Steenis, J.; Miličić, M.; Kočiš Tubić, N.; Djan, M.; Radenković, S.; Vujić, A. Taxonomic revision of the highly threatened Eumerus tricolor species group (Diptera: Syrphidae) in Southeast Europe, with insights into the conservation of the genus Eumerus. Eur. J. Entomol. 2021, 118, 368–393. [Google Scholar] [CrossRef]
- Chroni, A.; Djan, M.; Vidaković, D.O.; Petanidou, T.; Vujić, A. Molecular species delimitation in the genus Eumerus (Diptera: Syrphidae). Bull. Entomol. Res. 2017, 107, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Grković, A.; Vujić, A.; Chroni, A.; van Steenis, J.; Djan, M.; Radenković, S. Taxonomy and systematics of three species of the genus Eumerus Meigen, 1822 (Diptera: Syrphidae) new to southeastern Europe. Zool. Anz. 2017, 270, 176–192. [Google Scholar] [CrossRef]
- Ricarte, A.; Nencioni, A.; Kočiš Tubić, N.; Grković, A.; Vujić, A.; Marcos-García, M.Á. The hoverflies of an oak dehesa from Spain, with a new species and other insights into the taxonomy of the Eumerus tricolor group (Diptera: Syrphidae). Ann. Zool. 2018, 68, 259–280. [Google Scholar] [CrossRef]
- Grković, A.; Smit, J.; Radenković, S.; Vujić, A.; van Steenis, J. Two new European long-legged hoverfly species of the Eumerus binominatus species subgroup (Diptera, Syrphidae). ZooKeys 2019, 858, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Gilasian, E.; van Steenis, J.; Parchami-Araghi, M. Review of the Eumerus tricolor species group (Diptera: Syrphidae) in Iran, with description of six new species. Eur. J. Taxon. 2020, 722, 106–152. [Google Scholar] [CrossRef]
- Vujić, A.; Gilbert, F.; Flinn, G.; Englefield, E.; Ferreira, C.C.; Varga, Z.; Eggert, F.; Woolcock, S.; Böhm, M.; Mergy, R.; et al. Pollinators on the Edge: Our European Hoverflies; The European Red List of Hoverflies; European Commission: Brussels, Belgium, 2022; pp. 1–96. [Google Scholar]
- Ricarte, A.; Marcos-García, M.Á. A checklist of the Syrphidae (Diptera) of Spain, Andorra and Gibraltar. Zootaxa 2017, 4216, 401–440. [Google Scholar] [CrossRef] [PubMed]
- Ricarte, A.; Aguado-Aranda, P.; Nedeljković, Z. Some remarkable findings within the subtribe Helophilina (Diptera, Syrphidae) from the island of Menorca, Spain. Bol. Asoc. Esp. Entomol. 2022, 46, 327–329. [Google Scholar]
- van Eck, A. A checklist of the hoverflies of Portugal (Diptera, Syrphidae). Bol. Soc. Entomol. Aragonesa (S.E.A.) 2011, 49, 127–144. [Google Scholar]
- van Eck, A. Hoverflies (Diptera, Syrphidae) new to the fauna of Mainland Portugal, with and updated hoverfly checklist. Bol. Soc. Entomol. Aragonesa (S.E.A.) 2016, 59, 187–203. [Google Scholar]
- Aguado-Aranda, P.; Ricarte, A.; Marcos-García, M.Á. Present and future of the knowledge about the genus Eumerus Meigen, 1822 (Diptera: Syrphidae) in the Iberian Peninsula. Cuad. Biodivers. 2022, 63, 40–48. [Google Scholar] [CrossRef]
- Aguado-Aranda, P.; Ricarte, A.; Nedeljković, Z.; Marcos-García, M.Á. An overlooked case for a century: Taxonomy and systematics of a new Iberian species of Eumerus Meigen, 1822 (Diptera, Syrphidae). Eur. J. Taxon. 2022, 817, 35–57. [Google Scholar] [CrossRef]
- Aracil, A.; Grković, A.; Pérez-Bañón, C.; Kočiš Tubić, N.; Juan, A.; Radenković, S.; Vujić, A.; Rojo, S. A new species of phytophagous flower fly (Diptera, Syrphidae), feeding on holoparasitic broomrape plants (Orobanchaceae) for the first time in Europe. Arthropod-Plant Interact. 2023, 2023, 18. [Google Scholar] [CrossRef]
- Grković, A.; van Steenis, J.; Kočiš Tubić, N.; Nedeljković, Z.; Hauser, M.; Hayat, R.; Demirözer, O.; Djan, M.; Vujić, A.; Radenković, S. Revision of the bactrianus subgroup of the genus Eumerus Meigen (Diptera: Syrphidae) in Europe, inferred from morphological and molecular data with descriptions of three new species. Arthropod Syst. Phylogeny 2019, 77, 21–37. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; Díaz-González, T.E.; del Río, S.; Cantó, P.; Herrero, L.; Pinto-Gomes, C.; Costa, J.C. Biogeography of Spain and Portugal. Preliminary typological synopsis. Int. J. Geobot. Res. 2014, 4, 1–64. [Google Scholar]
- García-Codron, J.C. El medio físico. In La Naturaleza de España; Reyero, J.M., Ed.; Ministerio de Medio Ambiente: Madrid, Spain, 2002; pp. 18–33. [Google Scholar]
- QGIS Geographic Information System. Ver. 3.22. Open Source Geospatial Foundation. Available online: https://qgis.org (accessed on 8 March 2023).
- Séguy, E. Diptères syrphides d’Europe Occidentale; Muséum National d’Historie Naturelle: Paris, France, 1961; pp. 1–248. [Google Scholar]
- Speight, M.C.D.; Fisler, L.; Pétremand, G.; Hauser, M. A key to the males of the Eumerus species known from Switzerland & surrounding parts of central Europe (Diptera: Syrphidae). Syrph Net Database Eur. Syrphidae 2021, 112, 1–36. [Google Scholar]
- Stackelberg, A.A. Palaearctic species of the genus Eumerus Mg. (Diptera, Syrphidae). Tr. Vsesojuoznogo Entomol. Obs. 1961, 48, 181–229. [Google Scholar]
- Ricarte, A.; Nedeljković, Z.; Rotheray, G.E.; Lyszkowski, R.; Hancock, E.; Watt, K.; Hewitt, S.; Horsfield, D.; Wilkinson, G. Syrphidae (Diptera) from the Greek island of Lesvos, with description of two new species. Zootaxa 2012, 3175, 1–23. [Google Scholar] [CrossRef]
- Thompson, F.C. A key to the genera of the flower flies (Diptera: Syrphidae) of the Neotropical Region including descriptions of new genera and species and a glossary of taxonomic terms. Contrib. Entomol. Int. 1999, 3, 321–378. [Google Scholar]
- Doczkal, D.; Pape, T. Lyneborgimyia magnifica gen. et sp.n. (Diptera: Syrphidae) from Tanzania, with a phylogenetic analysis of the Eumerini using new morphological characters. Syst. Entomol. 2009, 34, 559–573. [Google Scholar] [CrossRef]
- Doczkal, D. Description of two new species of the genus Eumerus Meigen (Diptera, Syrphidae) from Corsica. Volucella 1996, 2, 3–19. [Google Scholar]
- Chandler, A.E.F. A preliminary key to the eggs of some of the commoner aphidophagous Syrphidae (Diptera) occurring in Britain. Trans. R. Entompl. Soc. Lond. 1968, 120, 199–217. [Google Scholar] [CrossRef]
- Grković, A.; Vujić, A.; Radenković, S.; Chroni, A.; Petanidou, T. Diversity of the genus Eumerus Meigen (Diptera, Syrphidae) on the eastern Mediterranean islands with description of three new species. Ann. Soc. Entomol. Fr. (N.S.) 2015, 51, 361–373. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data system (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Folmer, D.; Black, M.B.; Hoch, W.; Lutz, R.A.; Vrijehock, R.C. DNA primers for amplification of mitochondrial cytochrome oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Gibson, J.F.; Kelso, S.; Jackson, M.D.; Kits, J.H.; Miranda, G.F.G.; Skevington, J.H. Diptera-Specific Polymerase Chain Reaction Amplification Primers of Use in Molecular Phylogenetic Research. Ann. Entomol. Soc. Am. 2011, 104, 976–997. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Ricarte, A.; Nedeljković, Z.; Marcos-García, M.Á. An exploratory survey and assessment of the hoverfly diversity (Diptera: Syrphidae) from the Pyrenees of Girona, Spain. Rev. Suisse Zool. 2021, 128, 381–398. [Google Scholar] [CrossRef]
- Marcos-García, M.A. Nuevas especies de Eristalinae para la fauna ibérica (Diptera, Syrphidae). Nouv. Rev. D’entomol. 1990, 7, 317–323. [Google Scholar]
- Marcos-García, M.A. Catálogo preliminar de los Syrphidae (Diptera) de la Cordillera Cantábrica (España). Eos 1990, 66, 83–100. [Google Scholar]
- Peck, L.V. Syrphidae. In Catalogue of Palaearctic Diptera; Soós, A., Papp, L., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1988; Volume 8, pp. 11–230. [Google Scholar]
- Vujić, A.; Tot, T.; Andrić, A.; Ačanski, J.; Šašić Zorić, L.; Pérez-Bañón, C.; Aracil, A.; Veselić, S.; Arok, M.; Mengual, X.; et al. Review of the Merodon natans group with description of a new species, a key to the adults of known species of the natans lineage and first descriptions of some preimaginal stages. Arthropod Syst. Phylogeny 2021, 79, 343–378. [Google Scholar] [CrossRef]
- Meigen, J.W. Systematische Beschreibung der Bekannten Europäischen Zweiflugeligen Insekten. Dritter Theil; Beaufort Sohn: Aachen, Germany, 1822; pp. 1–416. [Google Scholar]
- Pedersen, T.E. Some Syrphidae from Spain, with descriptions of two new species (Insecta, Diptera). Steenstrupia 1971, 1, 229–245. [Google Scholar]
- Munk, T. The hoverfly Eumerus sabulonum (Fallén, 1817) (Syrphidae, Diptera) breeds in Sheep’s Bit (Jasione montana L.). Flora Fauna 2000, 106, 19–22. [Google Scholar]
- Pérez-Bañón, C.; Marcos-García, Á. Life history and description of the immature stages of Eumerus purpurariae (Diptera: Syrphidae) developing in Opuntia maxima. Eur. J. Entomol. 1998, 95, 373–382. [Google Scholar]
- Horn, W.; Kahle, I. Über entomologische Sammlungen (Ein Beitrag zur Geschichte der Entomo-Museologie). Teil II. Entomol. Beih. Berl.-Dahl. 1936, 3, 161–306. [Google Scholar]
- van Eck, A.P.W.; Andrade, R.A.M.; van Steenis, W.; van der Ent, L.-J. New additions to the hoverflies of mainland Portugal (Diptera, Syrphidae) with some observations on flower visits. Bol. Soc. Entomol. Aragonesa (S.E.A.) 2020, 66, 193–198. [Google Scholar]
- Speight, M.C.D.; Claussen, C.; Hurkmans, W. Révision des syrphes de la faune de France: III—Liste alphabétique des espèces des genres Cheilosia, Eumerus et Merodon et Supplemént (Diptera, Syrphidae). Bull. Soc. Entomol. Fr. 1998, 103, 401–414. [Google Scholar] [CrossRef]
- International Commission on Zoological Nomenclature. The International Code of Zoological Nomenclature, 4th ed.; The International Trust for Zoological Nomenclature: London, UK, 1999; pp. 1–156. [Google Scholar]
- Mengual, X. Diversidad Biológica de los Sírfidos (Diptera: Syrphidae) Asociada a la Actividad Humana en áreas Protegidas de la Provincia de Alicante. Graduate Thesis, University of Alicante, Alicante, Spain, 2005. [Google Scholar]
- van der Goot, V.S. Two new species of Syrphidae (Dipt.) from Spain. Entomol. Ber. 1966, 26, 179–183. [Google Scholar]
- Pérez-Bañón, C. Estudio de la Comunidad de Sírfidos de las Sierras Valencianas del Negrete y Utiel (Diptera, Syrphidae). Graduate Thesis, University of Alicante, Alicante, Spain, 1995. [Google Scholar]
- Mutin, V.A.; Barkalov, A.V. New data on the hover-flies of the genus Eumerus (Diptera: Syrphidae) from Russia. Far East. Entomol. 2018, 363, 11–20. [Google Scholar] [CrossRef]
- Piwowarczyk, R.; Mielczarek, L. First report of Eumerus mucidus (Diptera: Syrphidae) on Cistanche armena (Orobanchaceae) and from Armenia. Fla. Entomol. 2018, 101, 519–521. [Google Scholar] [CrossRef]
- Andréu, J. Notas dipterológicas. Una lista de sírfidos para contribuir al conocimiento de los dípteros de España. Bol. Soc. Entomol. Esp. 1926, 9, 98–126. [Google Scholar]
- Gil-Collado, J. Monografía de los Sírfidos de España. Trab. Mus. Nac. Cienc. Nat. 1930, 54, 1–376. [Google Scholar]
- Ricarte, A. Biodiversidad de Sírfidos (Diptera: Syrphidae) y Conservación de los Hábitats en el Parque Nacional de Cabañeros, España. Ph.D. Thesis, University of Alicante, Alicante, Spain, 2008. [Google Scholar]
- Lorenzo, D.; Ricarte, A.; Nedeljković, Z.; Nieves-Aldrey, J.L.; Marcos-García, M.A. Hoverflies (Diptera: Syprhidae) of El Ventorrillo Biological Station, Madrid province, Spain: A perspective from a late twentieth century inventory. Rev. Suisse Zool. 2020, 127, 393–412. [Google Scholar] [CrossRef]
- van Eck, A. Poorly recorded Syrphidae (Diptera) from Spain including species new to its fauna. Bol. Soc. Entomol. Aragonesa (S.E.A.) 2010, 46, 299–300. [Google Scholar]
- Marcos-García, M.Á. Los Syrphidae (Dip.) de las sierras occidentales del Sistema Central español. Subfamilias: Eristalinae, Lampettiinae, Microdontinae, Milesiinae y Cerianinae. Bol. Asoc. Esp. Entomol. 1985, 9, 187–210. [Google Scholar]
- van der Goot, V.S. Quelques Syrphides (Dipt.) des Pyrénées et de la Sierra Nevada. Entomol. Ber. 1958, 18, 93–96. [Google Scholar]
- Sonet, L. Taxócenose des syrphes (Diptera: Syrphidae) associée à un champ d’amandiers dans le Parc Naturel du Carrascal de la Font Roja. Biology. Degree Thesis, University of Alicante, Alicante, Spain, 1994. [Google Scholar]
- Struck, T.H.; Feder, J.L.; Bendiksby, M.; Birkeland, S.; Cerca, J.; Gusarov, V.I.; Kistenich, S.; Larsson, K.-H.; Liow, L.H.; Nowak, M.D.; et al. Finding Evolutionary Processes Hidden in Cryptic Species. Trends Ecol. Evol. 2018, 33, 153–163. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Wallis, G.P.; Waters, J.M.; Upton, P.; Craw, C. Transverse Alpine Speciation Driven by Glaciation. TRENDS Ecol. Evol. 2016, 31, 916–926. [Google Scholar] [CrossRef]
- Speight, M.C.D. Species accounts of European Syrphidae, 2020. In Syrph the Net, the Database of European Syrphidae (Diptera); Syrph the Net publications: Dublin, Ireland, 2020; Volume 104, pp. 1–314. [Google Scholar]
- Grković, A. Eumerus ovatus. The IUCN Red List of Threatened Species. 2021, e.T149169669A149169679. Available online: https://doi.org/10.2305/IUCN.UK.2021-3.RLTS.T149169669A149169679.en (accessed on 28 March 2023).
- Grković, A. Eumerus azabense. The IUCN Red List of Threatened Species. 2021, e.T149169268A149169278. Available online: https://doi.org/10.2305/IUCN.UK.2021-3.RLTS.T149169268A149169278.en (accessed on 28 March 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado-Aranda, P.; Ricarte, A.; Nedeljković, Z.; Kelso, S.; van Eck, A.P.W.; Skevington, J.H.; Marcos-García, M.Á. Are Appearances Deceiving? Morpho-Genetic Complexity of the Eumerus tricolor Group (Diptera: Syrphidae) in Europe, with a Focus on the Iberian Peninsula. Insects 2023, 14, 541. https://doi.org/10.3390/insects14060541
Aguado-Aranda P, Ricarte A, Nedeljković Z, Kelso S, van Eck APW, Skevington JH, Marcos-García MÁ. Are Appearances Deceiving? Morpho-Genetic Complexity of the Eumerus tricolor Group (Diptera: Syrphidae) in Europe, with a Focus on the Iberian Peninsula. Insects. 2023; 14(6):541. https://doi.org/10.3390/insects14060541
Chicago/Turabian StyleAguado-Aranda, Pablo, Antonio Ricarte, Zorica Nedeljković, Scott Kelso, André P. W. van Eck, Jeffrey H. Skevington, and María Ángeles Marcos-García. 2023. "Are Appearances Deceiving? Morpho-Genetic Complexity of the Eumerus tricolor Group (Diptera: Syrphidae) in Europe, with a Focus on the Iberian Peninsula" Insects 14, no. 6: 541. https://doi.org/10.3390/insects14060541
APA StyleAguado-Aranda, P., Ricarte, A., Nedeljković, Z., Kelso, S., van Eck, A. P. W., Skevington, J. H., & Marcos-García, M. Á. (2023). Are Appearances Deceiving? Morpho-Genetic Complexity of the Eumerus tricolor Group (Diptera: Syrphidae) in Europe, with a Focus on the Iberian Peninsula. Insects, 14(6), 541. https://doi.org/10.3390/insects14060541





