Lethal and Sublethal Effects of Contact Insecticides and Horticultural Oils on the Hibiscus Bud Weevil, Anthonomus testaceosquamosus Linell (Coleoptera: Curculionidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hibiscus Plants
2.2. Hibiscus Bud Weevil Colony
2.3. Laboratory Bioassays
2.4. Contact Toxicity Experiments
2.5. Greenhouse Experiments
2.6. Statistical Analyses
2.6.1. Mortality Measurements
2.6.2. Biological Measurements
2.6.3. Contact Insecticide Toxicity Analysis
2.6.4. Software
3. Results
3.1. Laboratory Experiments
3.1.1. Fixed Experiments
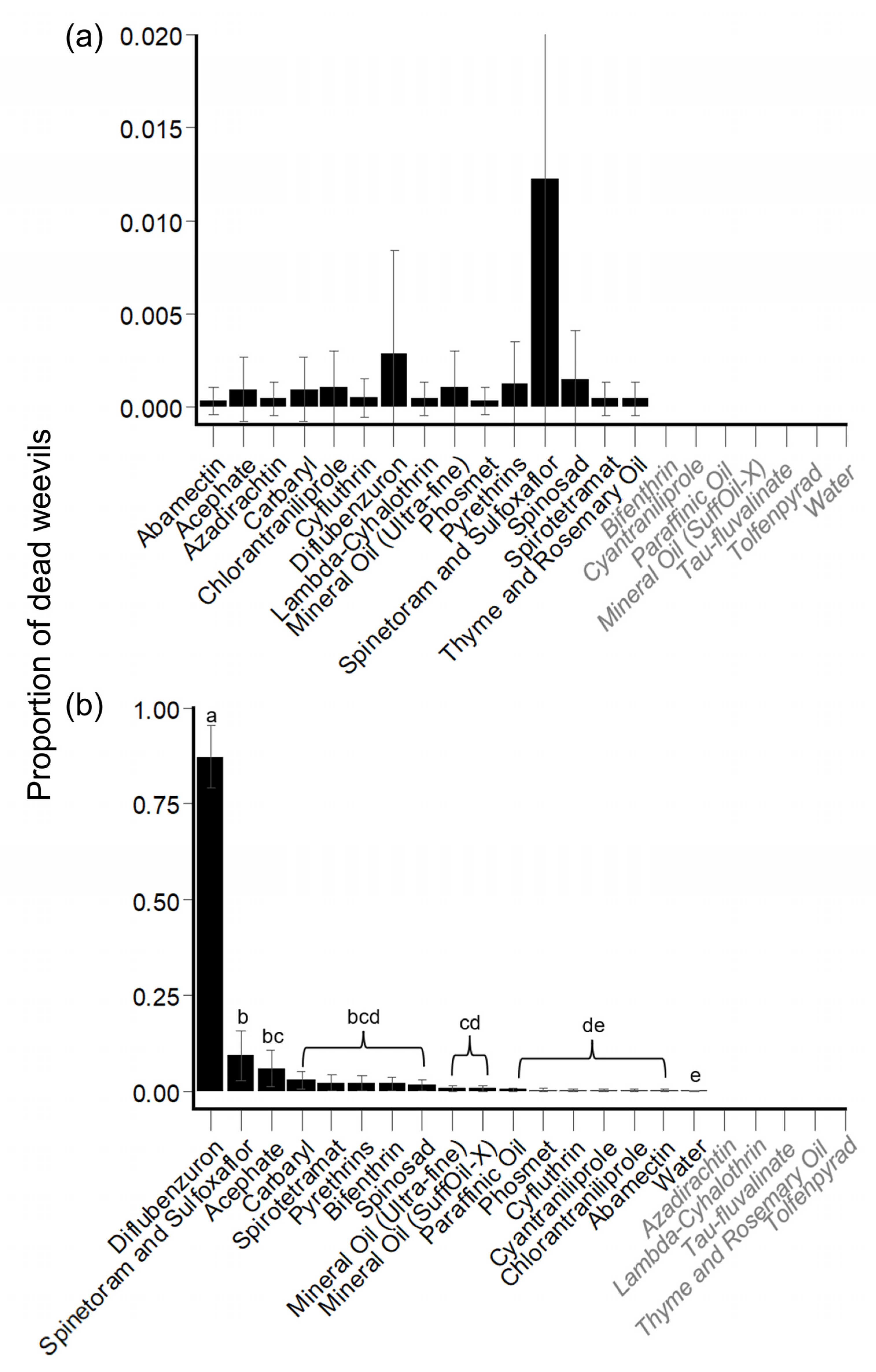
Hibiscus Bud Experiments: Mortality
Hibiscus Leaf Experiments: Mortality
3.1.2. Direct Mortality
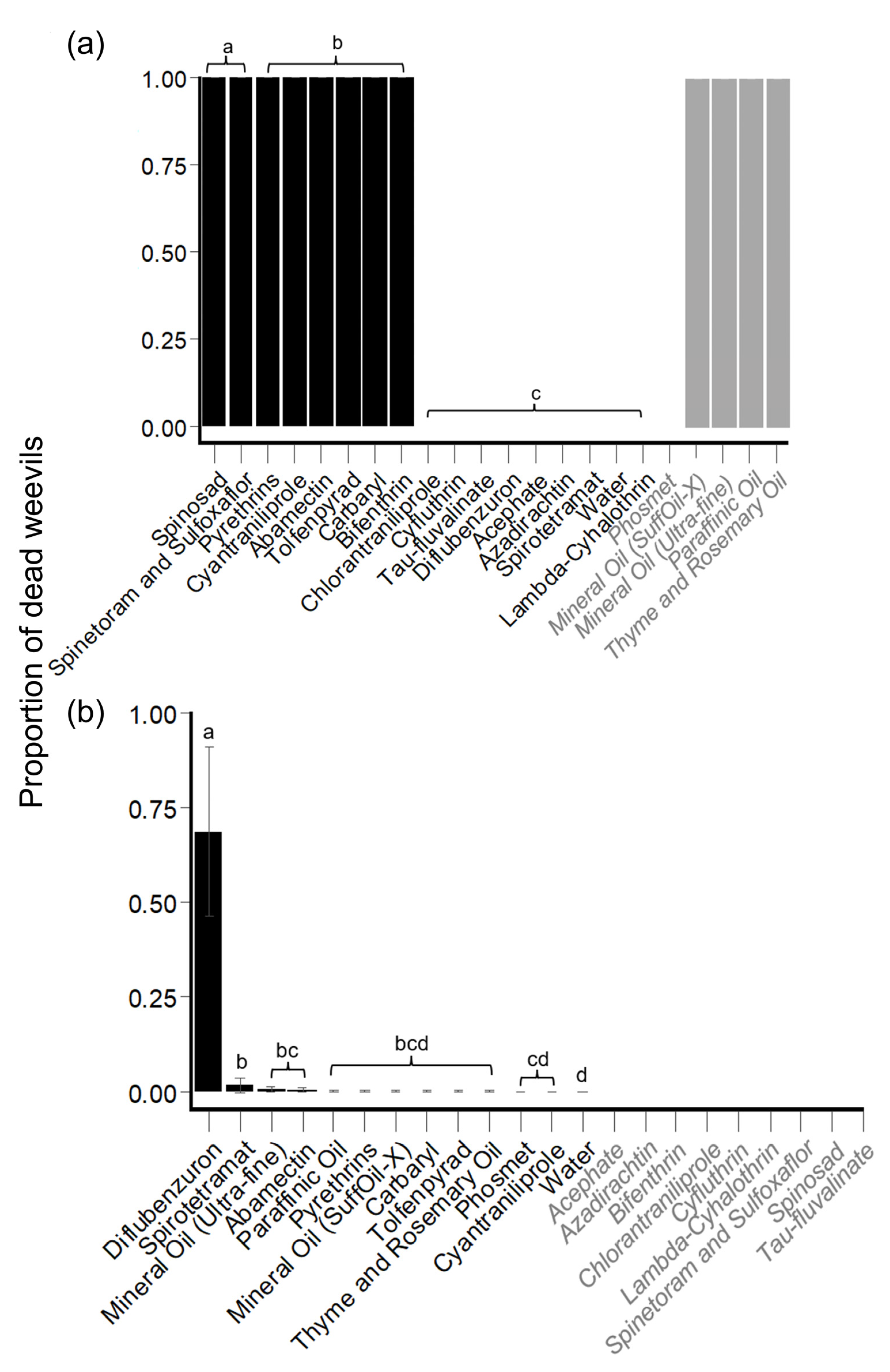
3.1.3. Replacement Experiments
Hibiscus Bud Experiments: Mortality
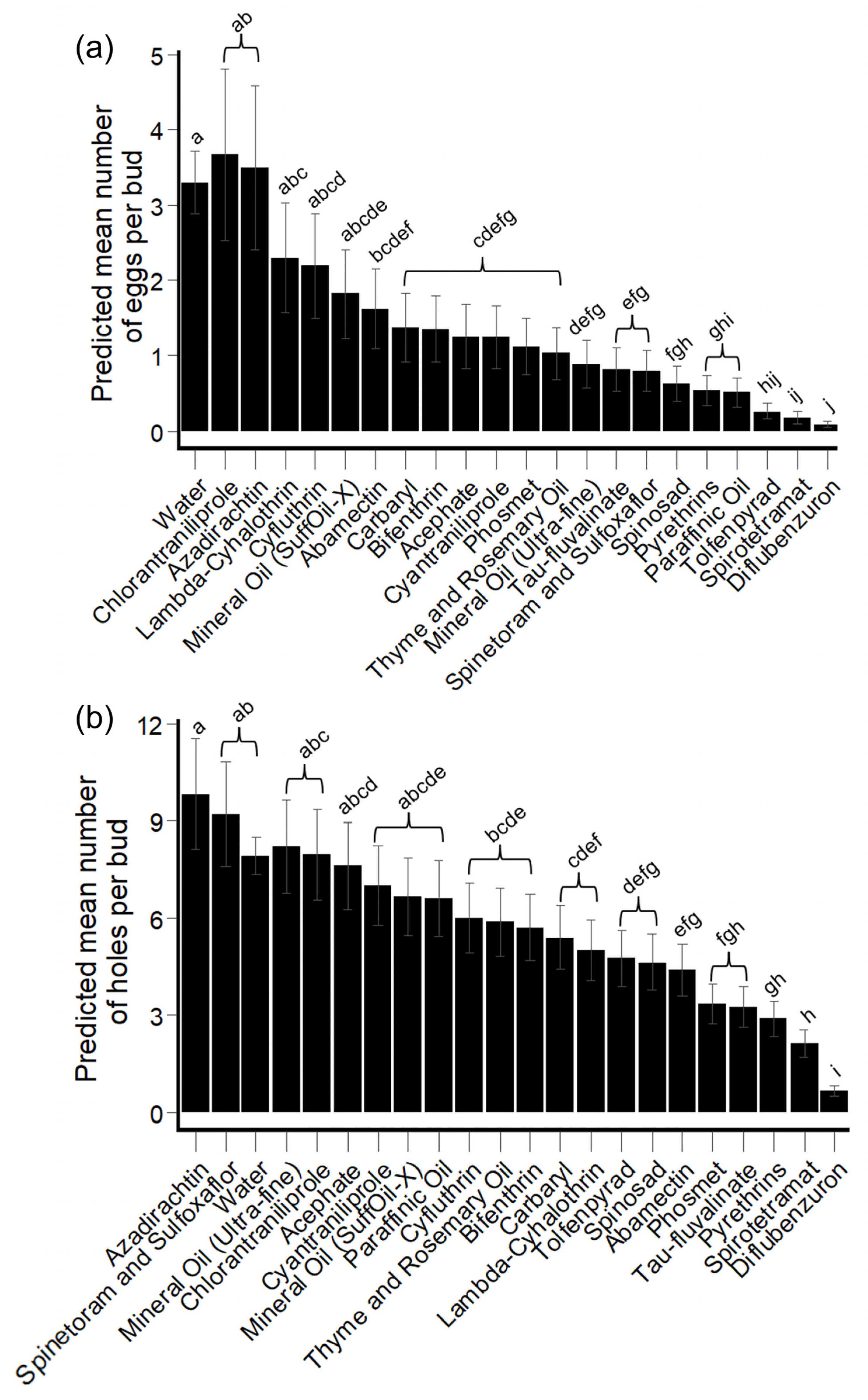
Hibiscus Bud Experiments: HBW Eggs
Hibiscus Bud Experiments: HBW Feeding/Oviposition Holes
3.1.4. Contact Insecticide Toxicity
3.2. Greenhouse Experiments
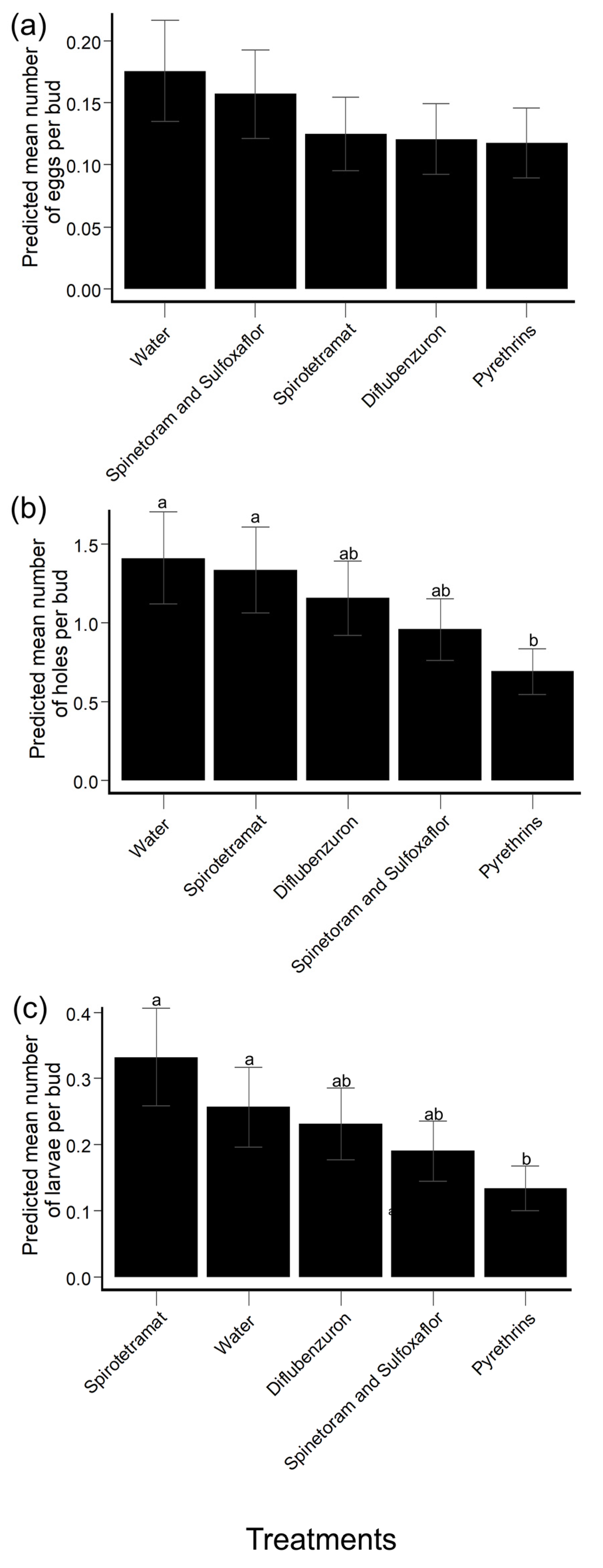
3.2.1. Collected Hibiscus Buds: HBW Eggs
3.2.2. Collected Hibiscus Buds: HBW Feeding/Oviposition Holes
3.2.3. Collected Hibiscus Buds: HBW Larvae
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.; Burke, H.R. Larvae of the Weevil Tribe Anthonomini (Coleoptera: Curculionidae). Misc. Publ. Entomol. Soc. Am. 1972, 8, 1–81. [Google Scholar]
- Bográn, C.E.; Helnz, K.M.; Ludwlg, S. The Bud Weevil Anthonomus testaceosquamosus, a Pest of Tropical Hibiscus. In Proceedings of the SNA Research Conference Entomology, Atlanta, GA, USA, December 2003; Volume 48, pp. 147–149. [Google Scholar]
- Skelly, P.E.; Osborne, L.S. Anthonomus Testaceosquamosus Linell, the Hibiscus Bud Weevil, New in Florida; Florida Department of Agriculture and Consumer Services Division of Plant Industry: Gainesville, FL, USA, 2018; pp. 1–2. [Google Scholar]
- Clark, W.E.; Burke, H.R.; Jones, R.W.; Anderson, R.S. The North American Species of the Anthonomus squamosus Species-Group (Coleoptera: Curculionidae: Curculioninae: Anthonomini). Coleopt. Bull. 2019, 73, 773–827. [Google Scholar] [CrossRef]
- Revynthi, A.M.; Velazquez Hernandez, Y.; Canon, M.A.; Greene, A.D.; Vargas, G.; Kendra, P.E.; Mannion, C.M. Biology of Anthonomus testaceosquamosus Linell, 1897 (Coleoptera: Curculionidae): A New Pest of Tropical Hibiscus. Insects 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- USDA-NASS. 2019 Census of Horticultural Specialties; 2017 Census of Agriculture; United States Department of Agriculture-National Agricultural Statistics Service: Washington, DC, USA, 2020; pp. 1–604. [Google Scholar]
- USDA-NASS. County Profile-Miami-Dade County Florida; 2017 Census of Agriculture; United States Department of Agriculture-National Agricultural Statistics Service: Washington, DC, USA, 2017; pp. 1–2. [Google Scholar]
- Costello, R.A.; Gillespie, D.R. The Pepper Weevil, Anthonomus eugenii Cano as a Greenhouse Pest in Canada. Pepper Weevil 1993, 16, 31–34. [Google Scholar]
- Caballero, R.; Schuster, D.J.; Smith, H.A.; Mangandi, J.; Portillo, H.E. A Systemic Bioassay to Determine Susceptibility of the Pepper Weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae) to Cyantraniliprole and Thiamethoxam. Crop Prot. 2015, 72, 16–21. [Google Scholar] [CrossRef]
- Barros, E.M.; Rodrigues, A.R.d.S.; Batista, F.C.; Machado, A.V.d.A.; Torres, J.B. Susceptibility of Boll Weevil to Ready-to-Use Insecticide Mixtures. Arq. Inst. Biológico 2019, 86, e1232018. [Google Scholar] [CrossRef]
- Servin-Villegas, R.; García-Hernández, J.; Tejas-Romero, A.; Martínez-Carrillo, J.L.; Toapanta, M.A. Susceptibility of Pepper Weevil (Anthonomus Eugenii Cano)(Coleoptera: Curculionidae) to Seven Insecticides in Rural Areas of Baja California Sur, Mexico. Acta Zool. Mex. 2008, 24, 45–54. [Google Scholar] [CrossRef]
- Rolim, G.G.; Coelho, R.R.; Antonino, J.D.; Arruda, L.S.; Rodrigues, A.S.; Barros, E.M.; Torres, J.B. Field-evolved Resistance to Beta-cyfluthrin in the Boll Weevil: Detection and Characterization. Pest Manag. Sci. 2021, 77, 4400–4410. [Google Scholar] [CrossRef]
- FAO. Guidelines on Prevention and Management of Pesticide Resistance; Food and Agriculture Organization of the United Nations (FAO Rome): Rome, Italy, 2012. [Google Scholar]
- Cowles, R.S.; Eitzer, B.D. Residues of Neonicotinoid Insecticides in Pollen and Nectar from Model Plants. J. Environ. Hortic. 2017, 35, 24–34. [Google Scholar] [CrossRef]
- Joseph, S.V. Transovarial Effects of Insect Growth Regulators on Stephanitis pyrioides (Hemiptera: Tingidae). Pest Manag. Sci. 2019, 75, 2182–2187. [Google Scholar] [CrossRef]
- Joseph, S.V. Insect Growth Regulators Elicit Transovarial Effects on Teleonemia scrupulosa (Hemiptera: Tingidae). Pest Manag. Sci. 2022, 78, 1800–1805. [Google Scholar] [CrossRef]
- Bogran, C.E.; Ludwig, S.; Metz, B. Using Oils as Pesticides. Tex. AM AgriLife Ext. Serv. 2006, E419. [Google Scholar]
- Robinson, J.V. Horticultural Oils and Pest Control; Texas Agricultural Extension Service, Texas A&M University System: College Station, TX, USA, 2011. [Google Scholar]
- Cranshaw, W.S.; Baxendale, B. Insect Control: Horticultural Oils; Insect Series; Home and Garden No. 5.569; Colorado State University: Fort Collins, CO, USA, 2005. [Google Scholar]
- Wright, P.J.; Walker, G.P.; MacDonald, F.H.; Gardner-Gee, R.; Hedderley, D.I. Mineral Oil Foliar Applications in Combination with Insecticides Affect Tomato Potato Psyllid (Bactericera cockerelli) and Beneficial Insects in Potato Crops. N. Z. J. Crop Hortic. Sci. 2017, 45, 263–276. [Google Scholar] [CrossRef]
- Phillips, D.A.; Liburd, O.E.; Duncan, L.W. Diaprepes Root Weevil on Southern Highbush Blueberry in Florida: ENY999/IN1241; EDIS: Gainesville, FL, USA, 2019. [Google Scholar]
- Sánchez-Bayo, F.; Tennekes, H.A.; Goka, K. Impact of Systemic Insecticides on Organisms and Ecosystems. Insectic.-Dev. Safer More Eff. Technol. 2013, 365–414. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; CRAN: Global, CDN, 2022. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Rup, P.J.; Chopra, P.K. Effect of Diflubenzuron on Egg Viability, Fecundity, and Adult Longevity of the Banana Fruit Fly, Zaprionus paravittiger (Diptera: Drosophilidae). J. Econ. Entomol. 1985, 78, 1118–1120. [Google Scholar] [CrossRef]
- Tail, G.; Porcheron, P.; Doumandji-Mitiche, B.; Blais, C. Diflubenzuron Effects on Reproduction and Hemolymph Ecdysteroid Levels in Female Locusts Schistocerca gregaria (Forskål, 1775)(Orthoptera, Acrididae). J. Orthoptera Res. 2008, 17, 89–95. [Google Scholar] [CrossRef]
- Ashouri, S.; Pourabad, R.F.; Ebadollahi, A. The Effect of Diflubenzuron and Hexaflumuron on the Last Larval Instars of the Mediterranean Flour Moth Anagasta kuehniella (Zeller)(Lepidoptera: Pyralidae) under Laboratory Conditions. Arch. Phytopathol. Plant Prot. 2014, 47, 75–81. [Google Scholar] [CrossRef]
- Domingues, L.N.; Mendes, J. Susceptibility of African Dung Beetle to Insect Growth Regulators. Arq. Bras. Med. Veterinária E Zootec. 2009, 61, 1077–1084. [Google Scholar] [CrossRef]
- He, F.; Sun, S.; Sun, X.; Ji, S.; Li, X.; Zhang, J.; Jiang, X. Effects of Insect Growth-Regulator Insecticides on the Immature Stages of Harmonia axyridis (Coleoptera: Coccinellidae). Ecotoxicol. Environ. Saf. 2018, 164, 665–674. [Google Scholar] [CrossRef]
- Wright, J.E.; Roberson, J.; Dawson, J.R. Boll Weevil: Effects of Difluhenzuron on Sperm Transfer, Mortality, and Sterility. J. Econ. Entomol. 1980, 73, 803–805. [Google Scholar] [CrossRef]
- Villavaso, E.J.; Haynes, J.W.; McGovern, W.L.; Jones, R.G.; Smith, J.W. Diflubenzuron Effects on Boll Weevils (Coleoptera: Curculionidae) in Small Field Cages. J. Econ. Entomol. 1995, 88, 1631–1633. [Google Scholar] [CrossRef]
- De Clercq, P.; De Cock, A.; Tirry, L.; Vinuela, E.; Degheele, D. Toxicity of Diflubenzuron and Pyriproxyfen to the Predatory Bug Podisus maculiventris. Entomol. Exp. Appl. 1995, 74, 17–22. [Google Scholar] [CrossRef]
- Dively, G.P.; Patton, T.; Barranco, L.; Kulhanek, K. Comparative Efficacy of Common Active Ingredients in Organic Insecticides against Difficult to Control Insect Pests. Insects 2020, 11, 614. [Google Scholar] [CrossRef]
- Vafaie, E.K. Insecticide Efficacy Against Crapemyrtle Bark Scale (Acanthococcus lagerstroemiae) in Containerized Production, 2020. Arthropod Manag. Tests 2021, 46, tsab116. [Google Scholar] [CrossRef]
- Gill, G.S.; Chong, J.H. Efficacy of Selected Insecticides as Replacement for Neonicotinoids in Managing Sweetpotato Whitefly on Poinsettia. HortTechnology 2021, 31, 745–752. [Google Scholar] [CrossRef]
- Nauen, R.; Reckmann, U.; Thomzik, J.; Thielert, W. Biological Profile of Spirotetramat (Movento®)–a New Two-Way Systemic (Ambimobile) Insecticide against Sucking Pest Species. Bayer Crop. J. 2008, 61, 245–278. [Google Scholar]
- Nauen, R.; Bretschneider, T.; Elbert, A.; Fischer, R.; Reckmann, U.; Van Waetermeulen, X. Biological and Mechanistic Considerations on the Mode of Action of Spirotetramat. In Proceedings of the 11th IUPAC Int. Congress of Pesticide Chemistry, 6–11 August 2006; pp. 6–10. [Google Scholar]
- Wang, Z.-H.; Gong, Y.-J.; Jin, G.-H.; Zhu, L.; Wei, S.-J. Effects of Spirotetramat on Development and Reproduction of Myzus persicae (Hemiptera: Aphididae). Austral Entomol. 2016, 55, 235–241. [Google Scholar] [CrossRef]
- Abbas, A.; Iqbal, J.; Zeshan, A.; Ali, Q.; Nadeem, I.; Malik, H.; Nazir, T.; Akhter, M.F.; Iqbal, B.B. Lethal and Sublethal Effects of Flonicamid (50 WG) and Spirotetramat (240 SC) on Bemisia tabaci (Homoptera: Aleyrodidae): An Age-Stage Two Sex Life Table Study. Phytoparasitica 2022, 50, 727–742. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Yang, X.-M.; Sun, L.-J.; Zhao, C.-D.; Chi, H.; Zheng, C.-Y. Sublethal Effect of Spirotetramat on the Life Table and Population Growth of Frankliniella occidentalis (Thysanoptera: Thripidae). Entomol. Gen. 2021, 41, 219–231. [Google Scholar] [CrossRef]
- Showler, A.T. The Conundrum of Chemical Boll Weevil Control in Subtropical Regions. In Insectic. Pest Eng. Croa; Perveen, F., Ed.; InTecha: Rijeka, Croatia, 2012; pp. 437–448. [Google Scholar]
- Arruda, L.S.; Torres, J.B.; Rolim, G.G.; Silva-Torres, C.S. Dispersal of Boll Weevil toward and within the Cotton Plant and Implications for Insecticide Exposure. Pest Manag. Sci. 2021, 77, 1339–1347. [Google Scholar] [CrossRef]
- NOAA-NESDIS. Global Summary of the Month-Average Temperature for the PERRINE 4 W, FL US USC00087020 Station in 2021; National Environmental Satellite, Data, and Information Service, National Oceanic & Atmospheric Administration, U.S.; Department of Commerce, National Centers for Environmental Information: Asheville, NC, USA, 2022. [Google Scholar]
- dos Santos, R.L.; dos Santos Neves, R.C.; Colares, F.; Torres, J.B. Parasitoides Do Bicudo Anthonomus grandis e Predadores Residentes Em Algodoeiro Pulverizado Com Caulim. Semina Ciênc. Agrár. 2013, 1, 3463–3474. [Google Scholar] [CrossRef]
- Neves, R.C.; Showler, A.T.; Pinto, É.S.; Bastos, C.S.; Torres, J.B. Reducing Boll Weevil Populations by Clipping Terminal Buds and Removing Abscised Fruiting Bodies. Entomol. Exp. Appl. 2013, 146, 276–285. [Google Scholar] [CrossRef]
- Labbé, R.M.; Gagnier, D.; Rizzato, R.; Tracey, A.; McCreary, C. Assessing New Tools for Management of the Pepper Weevil (Coleoptera: Curculionidae) in Greenhouse and Field Pepper Crops. J. Econ. Entomol. 2020, 113, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Benda, N.; Dale, A.G. Managing Insecticide and Miticide Resistance in Florida Landscapes: ENY-842/IN714, Rev. 7/2018; EDIS: Gainesville, FL, USA, 2018. [Google Scholar]
- Fishel, F.M. The EPA Conventional Reduced Risk Pesticide Program; EDIS: Gainesville, FL, USA, 2013. [Google Scholar]
- Kraiss, H.; Cullen, E.M. Efficacy and Nontarget Effects of Reduced-Risk Insecticides on Aphis glycines (Hemiptera: Aphididae) and Its Biological Control Agent Harmonia axyridis (Coleoptera: Coccinellidae). J. Econ. Entomol. 2014, 101, 391–398. [Google Scholar] [CrossRef]
- Mead, F.W.; Fasulo, T.R. Cotton Stainer, Dysdercus suturellus (Herrich Schaeffer)(Insecta: Hemiptera: Pyrrhocoridae): EENY-330/IN606, Rev. 3/2005; EDIS: Gainesville, FL, USA, 2005. [Google Scholar]
- Howard, F.W.; Pemberton, R.; Hamon, A.; Hodges, G.S.; Steinberg, B.; Mannion, C.M.; McLean, D.; Wofford, J. Lobate Lac Scale, Paratachardina lobata lobata (Chamberlin)(Hemiptera: Sternorrhyncha: Coccoidea: Kerriidae): EENY-276/IN471, Rev. 5/2004; EDIS: Gainesville, FL, USA, 2005. [Google Scholar]
- Capinera, J.L. Melon Aphid or Cotton Aphid, Aphis gossypii Glover (Insecta: Hemiptera: Aphididae); University of Florida IFAS Extension: Gainesville, FL, USA, 2000. [Google Scholar]
- Walker, A.; Hoy, M.; Meyerdirk, D. Papaya Mealybug (Paracoccus marginatus Williams and Granara de Willink (Insecta: Hemiptera: Pseudococcidae)). Papaya Mealybug (Paracoccus marginatus Williams and Granara de Willink (Insecta: Hemiptera: Pseudococcidae)) 2006. [CrossRef]
- Hoy, M.A.; Hamon, A.; Nguyen, R. Pink Hibiscus Mealybug, Maconellicoccus hirsutus (Green); University of Florida IFAS Extension: Gainesville, FL, USA, 2002. [Google Scholar]
- Mannion, C.; Hunsberger, A.; Gabel, K.; Buss, E.; Buss, L. Hibiscus Bud Midge (Contarinia maculipennis); University of Florida IFAS Extension: Gainesville, FL, USA.
- Döker, İ.; Revynthi, A.M.; Mannion, C.; Carrillo, D. First Report of Acaricide Resistance in Tetranychus urticae (Acari: Tetranychidae) from South Florida. Syst. Appl. Acarol. 2020, 25, 1209–1214. [Google Scholar] [CrossRef]

| Trade Name | Active Ingredient(s) | Insecticide Group | Rate a | Solution Prepared | |
|---|---|---|---|---|---|
| Insecticides | Acelepryn | Chlorantraniliprole | 28 | 1.2 L/ha | 1.25 mL/L |
| Acephate 97UP WDG | Acephate | 1B | 840 g/ha | 898 mg/L | |
| AzaSol | Azadirachtin | Unknown | 420 g/ha | 899 mg/L | |
| Conserve SC | Spinosad | 5 | 1.8 L/ha | 0.468 mL/L | |
| Decathlon 20 WP | Cyfluthrin | 3A | 133 g/ha b | 143 mg/L | |
| Dimilin 25 W | Diflubenzuron | 15 | 1121 g/ha | 1.2 g/L | |
| Hachi-Hachi SC | Tolfenpyrad | 21A | 2.11 mL/L | ||
| Imidan 70-W | Phosmet | 1B | 1121 g/ha | 1.2 g/L | |
| Kontos | Spirotetramat | 23 | 0.25 L/ha | 0.264 mL/L | |
| Mainspring GNL | Cyantraniliprole | 28 | 0.59 L/ha b | 0.62 mL/L | |
| Mavrik Aquaflow | Tau-fluvalinate | 3A | 1.6 L/ha | 0.78 mL/L | |
| PyGanic Crop Protection EC 5.0 II | Pyrethrins | 3A | 1.1 L/ha | 1.22 mL/L | |
| Scimitar GC | Lambda-Cyhalothrin | 3A | 0.37 L/ha b | 0.39 mL/L | |
| Sevin SL | Carbaryl | 1A | 2.3 L/ha | 2.5 mL/L | |
| Talstar P | Bifenthrin | 3A | 1.6 L/ha | 1.7 mL/L | |
| Timectin 0.15 EC T&O | Abamectin | 6 | 1.2 L/ha | 0.2 mL/L | |
| Xxpire | Spinetoram plus Sulfoxaflor | 4C and 5 | 385 g/ha | 203 mg/L | |
| Horticultural oils | Agropest | Thyme and Rosemary Oil | Unclassified | 4.7 L/ha c | 5 mL/L |
| JMS Stylet-Oil | Paraffinic Oil | Unclassified | 7.3 L/ha b | 7.8 mL/L | |
| SuffOil-X | Mineral Oil | Unclassified | 18.7 L/ha b | 20 mL/L | |
| Ultra-fine | Mineral Oil | Unclassified | 47 L/ha | 20 mL/L |
| Insecticide | Sex | n a | Slope b ± SE | LD50, µg/Weevil (95% CI) | χ2 | df | p-Value |
|---|---|---|---|---|---|---|---|
| Diflubenzuron c | ♂ | 50 | - | - | - | - | - |
| ♀ | 50 | - | - | - | - | - | |
| Spinetoram plus Sulfoxaflor | ♂ | 145 | 3.89 ± 0.34 | 0.06 (0.05–0.07) | 1.55 | 2 | 0.46 |
| ♀ | 147 | 3.74 ± 0.33 | 0.06 (0.06–0.07) | 2.26 | 2 | 0.32 | |
| Pyrethrins | ♂ | 180 | 1.00 ± 0.11 | 0.07 (0.03–0.16) | 6.30 | 3 | 0.10 |
| ♀ | 180 | 1.01 ± 0.11 | 0.10 (0.04–0.23) | 6.88 | 3 | 0.08 | |
| Spirotetramat | ♂ | 175 | 3.58 ± 0.29 | 41.72 (37.63–45.98) | 3.58 | 2 | 0.17 |
| ♀ | 152 | 3.26 ± 0.27 | 44.36 (39.81–49.16) | 3.92 | 2 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greene, A.D.; Yang, X.; Velazquez-Hernandez, Y.; Vargas, G.; Kendra, P.E.; Mannion, C.; Revynthi, A.M. Lethal and Sublethal Effects of Contact Insecticides and Horticultural Oils on the Hibiscus Bud Weevil, Anthonomus testaceosquamosus Linell (Coleoptera: Curculionidae). Insects 2023, 14, 544. https://doi.org/10.3390/insects14060544
Greene AD, Yang X, Velazquez-Hernandez Y, Vargas G, Kendra PE, Mannion C, Revynthi AM. Lethal and Sublethal Effects of Contact Insecticides and Horticultural Oils on the Hibiscus Bud Weevil, Anthonomus testaceosquamosus Linell (Coleoptera: Curculionidae). Insects. 2023; 14(6):544. https://doi.org/10.3390/insects14060544
Chicago/Turabian StyleGreene, A. Daniel, Xiangbing Yang, Yisell Velazquez-Hernandez, German Vargas, Paul E. Kendra, Catharine Mannion, and Alexandra M. Revynthi. 2023. "Lethal and Sublethal Effects of Contact Insecticides and Horticultural Oils on the Hibiscus Bud Weevil, Anthonomus testaceosquamosus Linell (Coleoptera: Curculionidae)" Insects 14, no. 6: 544. https://doi.org/10.3390/insects14060544
APA StyleGreene, A. D., Yang, X., Velazquez-Hernandez, Y., Vargas, G., Kendra, P. E., Mannion, C., & Revynthi, A. M. (2023). Lethal and Sublethal Effects of Contact Insecticides and Horticultural Oils on the Hibiscus Bud Weevil, Anthonomus testaceosquamosus Linell (Coleoptera: Curculionidae). Insects, 14(6), 544. https://doi.org/10.3390/insects14060544





