The Role of Ascorbate–Glutathione System and Volatiles Emitted by Insect-Damaged Lettuce Roots as Navigation Signals for Insect and Slug Parasitic Nematodes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Glasshouse Experiment
2.2. Determination of Antioxidants and Photosynthetic Pigments
2.3. Lettuce Roots Volatile Organic Compounds Analyses
2.4. Source and Maintenance of Parasitic Nematodes and Synthetic Volatile Organic Compounds
2.5. Chemotaxis Assay
2.6. Statistical Analysis
2.6.1. Biochemical Analyses of Photosynthetic Pigments and Antioxidants
2.6.2. Chemotaxis Assay
3. Results
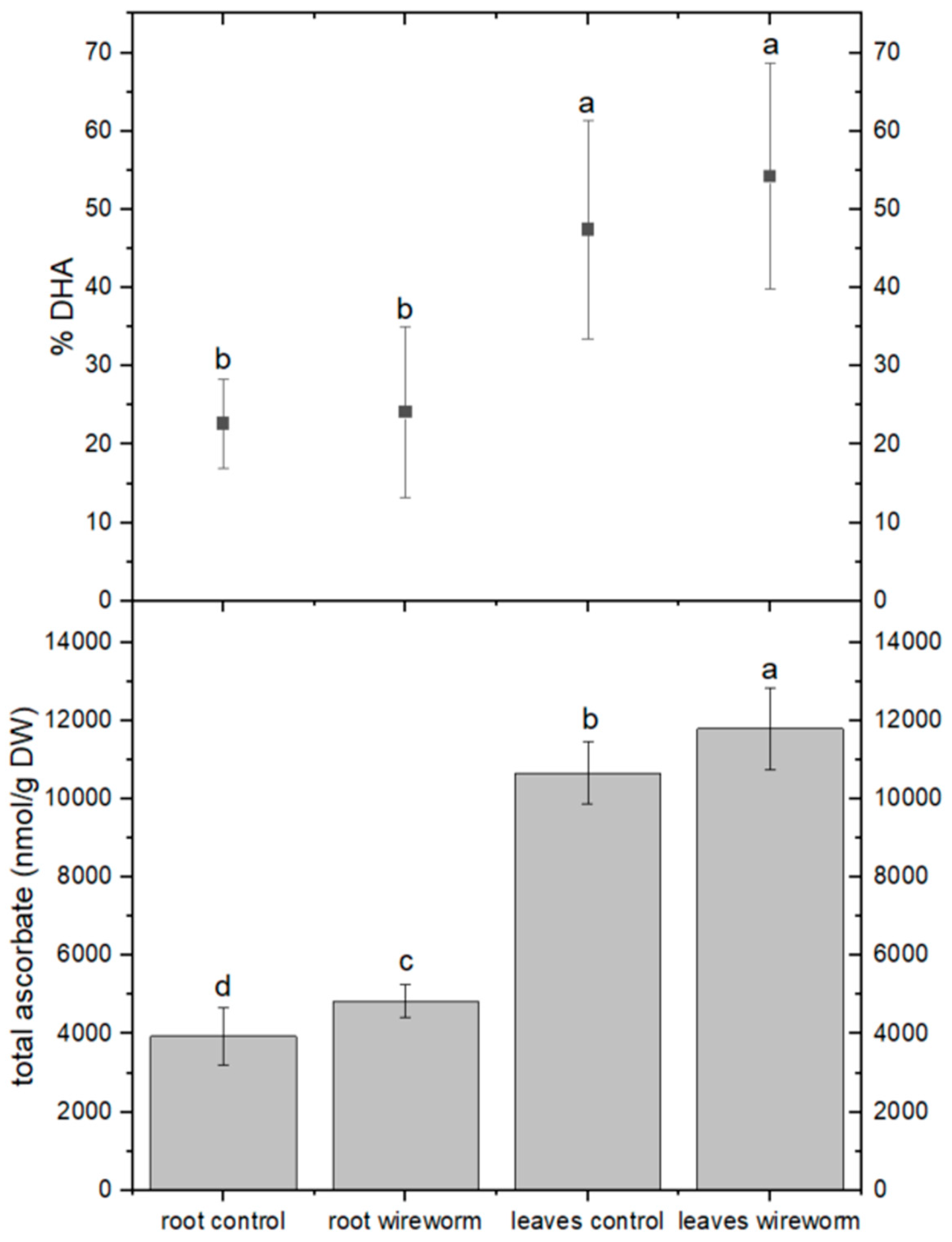
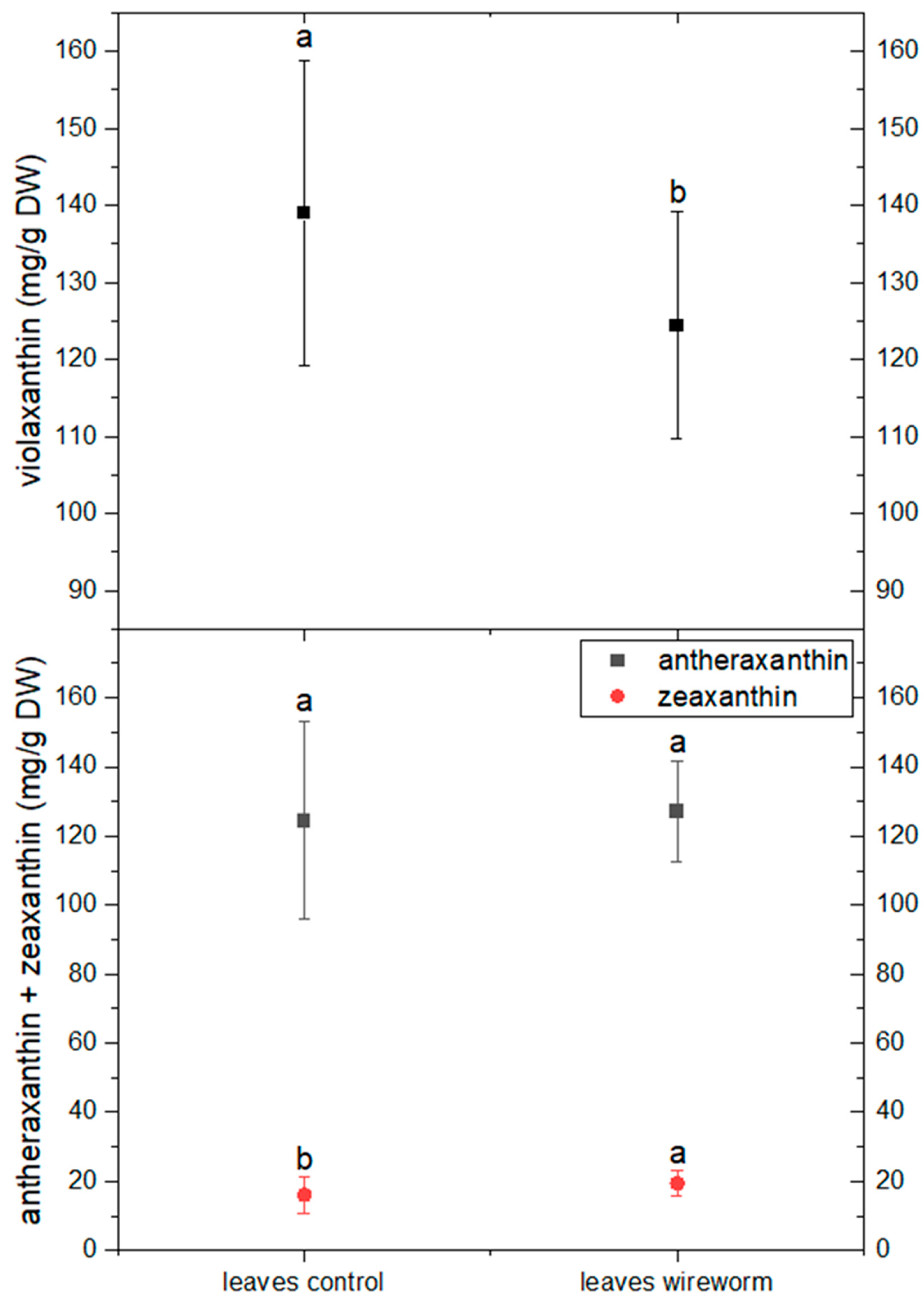
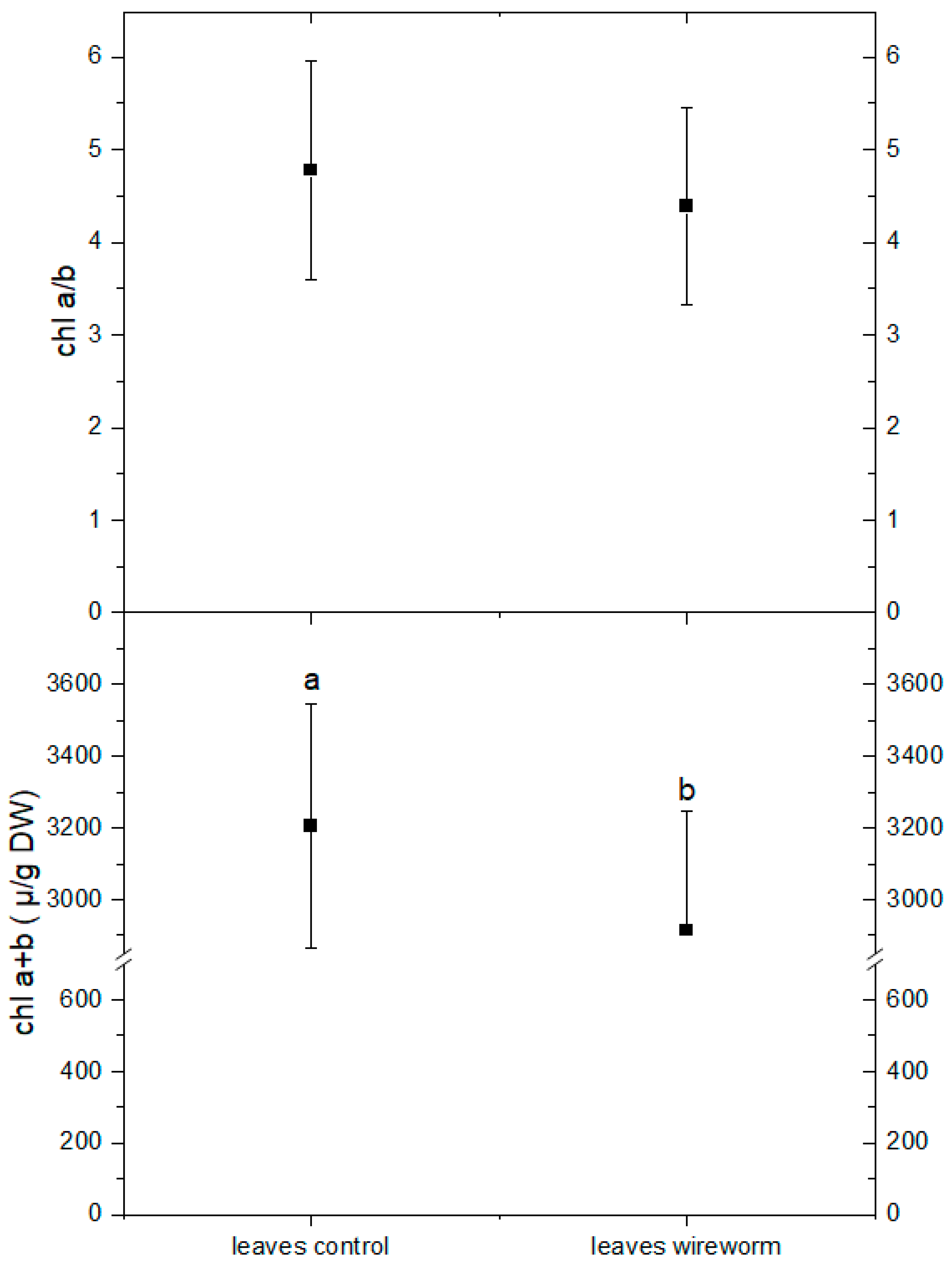
3.1. Antioxidant Concentrations and Redox State
3.2. Lettuce Root Volatile Organic Compound Analyses
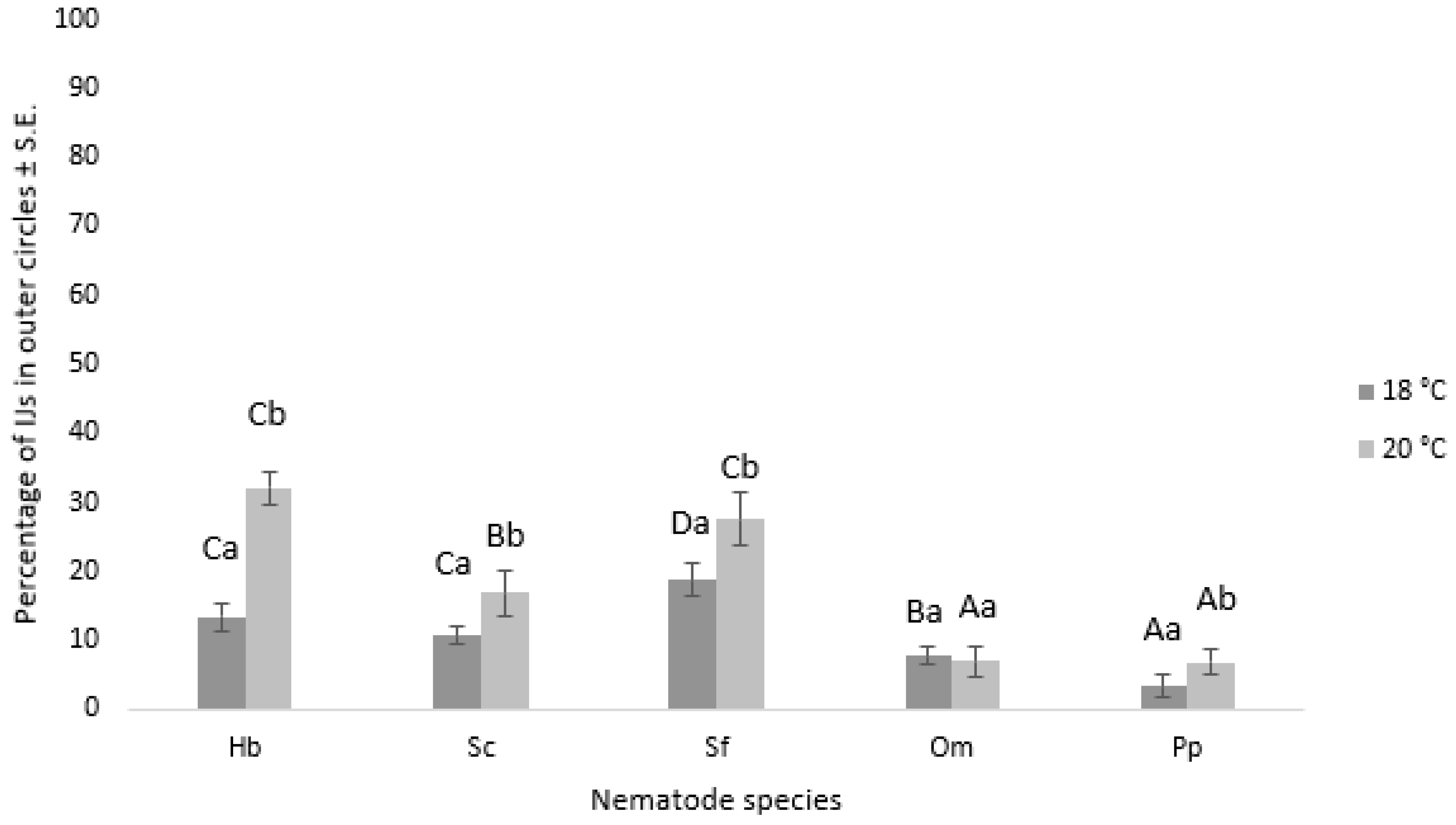
3.3. Nematode Chemoattraction towards VOCs vs. Water
3.3.1. Nematode Motility
3.3.2. Chemotaxis Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, J.G.; Alborn, H.T.; Stelinski, L.L. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J. Chem. Ecol. 2010, 36, 361–368. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; Wae, M.Y.; Ignacimuthu, S. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signal. Behav. 2011, 6, 1973–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo, E.; Hordijk, C.A.; de Graff, R.M.; Samudrala, D.; Cristescu, S.M.; Harren, F.J.M.; van Dam, N.M. On-line detection of root-induced volatiles in Brassica nigra plants infested with Delia radicum L. root fly larvae. Phytochemistry 2012, 84, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copolovici, L.; Kannaste, A.; Remmel, T.; Niinemets, U. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Laznik, Ž.; Trdan, S. Attraction behaviors of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) to synthetic volatiles emitted by insect-damaged carrot roots. J. Pest Sci. 2016, 89, 977–984. [Google Scholar] [CrossRef]
- Grunseich, J.M.; Thompson, M.N.; Hay, A.A.; Gorman, Z.; Kolomiets, M.V.; Eubanks, M.D.; Helms, A.M. Risky roots and careful herbivores: Sustained herbivory by a root-feeding herbivore attenuates indirect plant defences. Funct. Ecol. 2020, 34, 1779–1789. [Google Scholar] [CrossRef]
- Sendel, S.; Bocianoeski, J.; Buszewski, B.; Piesik, M.; Mayhew, C.A.; Piesik, D. Volatile organic compounds released by wheat as a result of striped shieldbug feeding an insect behaviour. J. Appl. Entomol. 2022, 146, 710–724. [Google Scholar] [CrossRef]
- Hallem, E.A.; Dillman, A.R.; Hong, A.V.; Zhang, Y.; Yano, J.M.; DeMarco, S.F.; Sternberg, P.W. A sensory code for host seeking in parasitic nematodes. Curr. Biol. 2011, 21, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquires resistance. Front. Plant Sci. 2015, 6, 228. [Google Scholar] [CrossRef] [Green Version]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-Transferase Enzymes in Plant-Pathogen Interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef] [Green Version]
- Bloem, E.; Haneklaus, S.; Schnug, E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015, 5, 779. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Whitworth, R.J.; Stuart, J.J.; Chen, M.S. Unbalanced Activation of Glutathione Metabolic Pathways Suggests Potential Involvment in Plant Defense against the Gall Midge Mayetiola destructor in Wheat. Sci. Rep. 2015, 5, 8092. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pang, Q.; Dai, S.; Wang, Y.; Chen, S.; Yan, X. Proteomic identification of differentially expressed proteins in Arabidopsis in response to methyl jasmonate. J. Plant Physiol. 2011, 168, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.M.; Cahoon, R.E.; Bonner, E.R.; Rivard, R.S.; Sheffield, J.; Jez, J.M. Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana. Plant Cell 2007, 19, 2653–2661. [Google Scholar] [CrossRef] [Green Version]
- Tu, S.; Ma, L.; Luongo, T. Root exudates and arsenic accumulation in arsenic hyperaccumulatin Pteris vittate and non-hyperaccumulating Nephrolepis exaltata. Plan Soil 2004, 285, 9–19. [Google Scholar] [CrossRef]
- Rani, R.; Juwarkar, A. Influence of insecticide phorate on chemical composition and enyzme profile of root exudates and root extracts of Brassica juncea. J. Environ. Biol. 2016, 37, 413–419. [Google Scholar]
- Contreras, F.; Diaz, J.; Rombola, A.D.; de la Luz Mora, M. Prospecting intercropping between subterranean clover and grapevine as potential strategy for improving grapevine preformance. Curr. Plant Biol. 2019, 19, 100–110. [Google Scholar] [CrossRef]
- Zechmann, B. Diurnal changes of subcellular glutathione contents in Arabidopsis thaliana. Biol. Plant. 2017, 61, 791–796. [Google Scholar] [CrossRef]
- Massacci, A.; Nabiev, S.M.; Pietrosanti, L.; Nematov, S.K.; Chernikova, T.N.; Thor, K.; Leipner, J. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll flourescence imaging. Plant Physiol. Biochem. 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Caferri, R.; Guardini, Z.; Bassi, R.; Dall’Osto, L. Chapter Two–Assessing photoprotective functions of carotenoids in photosynthetic systems of plants and green algae. Methods Enzymol. 2022, 674, 53–84. [Google Scholar]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Dillman, A.R.; Sternberg, P.W. Entomopathogenic nematode. Curr. Biol. 2012, 22, R430. [Google Scholar] [CrossRef] [Green Version]
- Dillman, A.R.; Guillermin, M.L.; Lee, J.H.; Kim, B.; Sternberg, P.W.; Hallem, E.A. Olfaction shapes host-parasite interactions in parasitic nematodes. Proc. Natl. Acad. Sci. USA 2012, 109, E2324–E2333. [Google Scholar] [CrossRef] [Green Version]
- Shapiro-Ilan, D.; Han, R.; Qiu, Q. Chapter 10–Production of Entomopathogenic Nematodes. In Mass Production of Beneficial Organisms: Invertabrates and Entomopathogens; Elsevier: Amsterdam, The Netherlands, 2014; pp. 321–355. [Google Scholar]
- Kumar Singh, A.; Kumar, M.; Ahuja, A.; Vinay, B.K.; Kumar Kommu, K.; Thakur, S.; Paschapur, A.U.; Jeevan, B.; Mishra, K.K.; Prasad Meena, R.; et al. Chapter 6–Entomopathogenic Nematodes: A Sustainable Option for Insect Pest Management. In Biopesticides; Woodhead Publishing: Sawston, UK, 2022; pp. 73–92. [Google Scholar]
- Wilson, M.J.; Rae, R. Phasmarhabditis hermaphrodita as a control agent for Slugs. In Nematode Pathogenesis of Insects and Other Pests; Campos-Herrera, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 509–521. [Google Scholar]
- Ivanova, E.S.; Spiridonov, S.E. Phasmarhabditis quinamensis sp. n. (Nematoda: Rhabditidae) from tropical terrestrial gastropods in southern Vietnam. Nematology 2021, 24, 225–239. [Google Scholar] [CrossRef]
- Tandingan De Ley, I.; Schurkman, J.; Wilen, C.; Dillman, A.R. Mortality of the invasive white garden snail Theba pisana exposed to three US isolates of Phasmarhabditis spp. (P. hermaphrodita, P. californica, and P. papillosa). PLoS ONE 2020, 15, e0225244. [Google Scholar] [CrossRef] [Green Version]
- Andrássy, I. A Taxonomic Review of the Suborder Rhabditina (Nematoda: Secernentia); ORSTOM: Paris, France, 1983; 241p. [Google Scholar]
- Campos-Herrera, R.; Půža, V.; Jaffuel, G.; Blanco-Pérez, R.; Čepulyte Rakauskiene, R.; Turlings, T. Unraveling the intraguild competition between Oscheius spp. nematodes and entomopathogenic nematodes: Implications for their natural distribution in Swiss agricultural soils. J. Invertebr. Pathol. 2015, 132, 216–227. [Google Scholar] [CrossRef]
- Takabayashi, J.; Dicke, M. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1996, 1, 109–113. [Google Scholar] [CrossRef]
- Furlan, L.; Benvegnù, I.; Bilò, M.F.; Lehmhus, J.; Ruzzier, E. Species identification of wireworms (Agriotes spp.; Coleoptera: Elateridae) of agricultural importance in Europe: A new »horizontal indentification table«. Insects 2021, 12, 534. [Google Scholar] [CrossRef]
- Tausz, M.; Wonisch, A.; Grill, D.; Morales, D.; Jimenez, M.S. Measuring antioxidants in tree species in the natural environment: From sampling to data evaluation. J. Exp. Bot. 2003, 54, 1505–1510. [Google Scholar] [CrossRef]
- Rowson, B.; Turner, J.; Anderson, R.; Symondson, B. Slugs of Britain and Ireland, 1st ed.; FSC Publications: Telford, UK, 2014; pp. 1–136. [Google Scholar]
- Pieterse, A.; Tiedt, L.R.; Malan, A.P.; Ross, J.L. First record of Phasmarhabditis papillosa (Nematoda: Rhabditidae) in South Africa, and its virulence against the invasive slug, Deroceras panormitanum. Nematology 2017, 19, 1035–1050. [Google Scholar] [CrossRef]
- Laznik, Ž.; Majić, I.; Trdan, S.; Malan, A.; Pieterse, A.; Ross, J.L. Is Phasmarhabditis papillosa (Nematoda: Rhabditidae) a possible biological control agent against the Spanish slug, Arion vulgaris (Gastropoda: Arionidae)? Nematology 2020, 23, 577–585. [Google Scholar] [CrossRef]
- Weissteiner, S.; Huetteroth, W.; Kollmann, M.; Wießbecker, B.; Romani, R.; Schachtner, J.; Schütz, S. Cockchafer larvae smell host root scents in soil. PLoS ONE 2012, 7, e45827. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. An investigation on the chemotactic responses of different entomopathogenic nematode strains to mechanically damaged maize root volatile compounds. Exp. Parasitol. 2013, 134, 349–355. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Horvitz, H.R. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 1991, 7, 729–742. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Arnaiz, A.; Celasco-Arroyo, B.; Grbic, V.; Martinez, M. Arabidopsis response to the spider mite Tetranychus urticae depends on the regulation of reactive oxygen species homeostasis. Sci. Rep. 2018, 8, 9432. [Google Scholar] [CrossRef] [Green Version]
- Passaia, G.; Margis-Pinheiro, M. Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 2015, 234, 22–24. [Google Scholar] [CrossRef]
- Kunert, K.J.; Foyer, C.H. The ascorbate/glutathione cycle. Adv. Bot. Res. 2023, in press. [Google Scholar] [CrossRef]
- Hasan, M.S.; Chopra, D.; Damm, A.; Koprivova, A.; Kopriva, S.; Meyer, A.J.; Müller-Schüssele, S.; Grundler, F.M.W.; Siddique, S. Glutathione contributes to plant defence against parasitic cyst nematodes. Mol. Plant Pathol. 2022, 23, 1048–1059. [Google Scholar] [CrossRef]
- Iftikhar, Y.; Mughal, S.M.; Khan, M.M. Some biochemical changes in tristeza infected citrus trees in Pakistan. Int. J. Sci. Nat. 2011, 2, 621–624. [Google Scholar]
- Frankel, E.N. Hydroperoxide decomposition. In Lipid Oxidation, 2nd ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 67–98. [Google Scholar] [CrossRef]
- Granvogl, M.; Schieberle, P. The sensomics approach: A useful tool to unravel the genuine aroma blueprint of foods and aroma changes during food processing. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 41–68. [Google Scholar] [CrossRef]
- Trindler, C.; Kopf-Bolanz, K.A.; Denkel, C. Aroma of peas, its constituents and reduction strategies–Effects from breeding to processing. Food Chem. 2022, 376, 131892. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.H.; Kihwan, S.; Jeong-Gu, K.; Hwan, L.C. Non-targeted metabolomic analysis for the comparative evaluation of volatile organic compounds in 20 globally representative cucumber lines. Front. Plant Sci. 2022, 13, 1028735. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Are medicinal plants polluted with phthalates? Daru 2013, 21, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Wang, Y.; Ruan, J.; Zhang, J.; Sun, C. Recent advances in analysis of phthalate esters in foods. Trends Anal. Chem. 2015, 72, 10–26. [Google Scholar] [CrossRef]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Campbell, J.F.; Lewis, E.E.; Stock, S.P.; Nadler, S.; Kaya, H.K. Evolution of host search strategies in entomopathogenic nematodes. J. Nematol. 2003, 35, 142–145. [Google Scholar]
- Rae, R.G.; Robertson, J.F.; Wilson, M.J. The chemotactic response of Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) to cues of Deroceras reticulatum (Mollusca: Gastropoda). Nematology 2006, 8, 197–200. [Google Scholar]
- Andrus, P.; Rae, R. Natural variation in chemoattraction of Phasmarhabditis hermaphrodita, Phasmarhabditis neopapillosa and Phasmarhabditis californica exposed to slug mucus. Nematology 2019, 21, 479–488. [Google Scholar] [CrossRef]
- Kruitbos, L.; Heritage, S.; Hapca, S.; Wilson, M.J. The influence of habitat quality on the foraging strategies of the entomopathogenic nematodes Steinernema carpocapsae and Heterorhabdits megidis. Parasitology 2009, 137, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, A.S. Heterorhabditis spp. and Steinernema (=Neoaplectana) spp.: Temperature, and aspects of behavior and infectivity. Exp. Parasitol. 1986, 62, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Grewal, P.S.; Selvan, S.; Gaugler, R. Thermal adaptation of entomopathogenic nematodes: Niche breadth for infection, establishment, and reproduction. J. Therm. Biol. 1994, 19, 245–253. [Google Scholar] [CrossRef]
- Cetin, E.; Odabasi, M.; Seyfioglu, R. Ambient volatile organic compound (VOC) concentration around a petrochemical complex and a petroleum refinery. Sci. Total Environ. 2003, 312, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hiltpold, I.; Bernklau, E.; Bjostad, L.B.; Alvarez, N.; Miller-Struttmann, N.E.; Lundgren, J.G.; Hibbard, B.E. Nature, evolution and characterisation of rhizospheric chemical exudates affecting root herbivores. In Behaviour and Physiology of Root Herbivores; Johnson, S.N., Hiltpold, I., Turlings, T.C.J., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 97–157. [Google Scholar]



| Factor | Sum of Squares | Df | F | p |
|---|---|---|---|---|
| Species (S) | 23,268.30 | 4 | 78.13 | <0.01 |
| VOCs (V) | 55,759.40 | 3 | 249.63 | <0.01 |
| Temperature (T) | 5203.51 | 1 | 69.89 | <0.01 |
| Replication (R) | 1130.76 | 9 | 1.69 | 0.0881 |
| S × V | 18,577.40 | 12 | 20.79 | <0.01 |
| S × T | 3746.41 | 4 | 12.58 | <0.01 |
| V × T | 2673.59 | 3 | 11.97 | <0.01 |
| S × V × T | 7424.32 | 12 | 8.31 | <0.01 |
| Residual | 55,916.30 | 751 | ||
| Total (Corrected) | 157,331.00 | 799 |
| Factor | Sum of Squares | Df | F | p |
|---|---|---|---|---|
| Species (S) | 1.41 | 4 | 88.90 | <0.01 |
| VOCs (V) | 2.41 | 3 | 203.33 | <0.01 |
| Temperature (T) | 0.07 | 1 | 17.63 | <0.01 |
| Replication (R) | 0.03 | 9 | 0.77 | 0.65 |
| S × V | 1.66 | 12 | 35.06 | <0.01 |
| S × T | 0.30 | 4 | 19.15 | <0.01 |
| V × T | 0.08 | 3 | 6.93 | <0.01 |
| S × V × T | 0.28 | 12 | 5.83 | <0.01 |
| Residual | 2.97 | 751 | ||
| Total (Corrected) | 8.24 | 799 |
| A | 18 °C | ||||
|---|---|---|---|---|---|
| Hb | Sc | Sf | Om | Pp | |
| 2,4-ND | −0.20 ± 0.02 b | −0.07 ± 0.04 cd | −0.26 ± 0.02 a | −0.07 ± 0.02 c | −0.02 ± 0.01 d |
| GSH | 0.09 ± 0.03 c | −0.02 ± 0.03 b | −0.14 ± 0.02 a | −0.02 ± 0.00 b | 0.00 ± 0.02 b |
| ASC | 0.06 ± 0.03 b | 0.05 ± 0.03 b | −0.02 ± 0.02 a | 0.03 ± 0.02 b | 0.07 ± 0.03 b |
| C | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.01 ± 0.02 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| B | 20 °C | ||||
| Hb | Sc | Sf | Om | Pp | |
| 2,4-ND | −0.19 ± 0.02 b | −0.12 ± 0.03 c | −0.39 ± 0.02 a | −0.11 ± 0.02 c | −0.11 ± 0.02 c |
| GSH | 0.14 ± 0.03 c | −0.03 ± 0.04 b | 0.19 ± 0.04 c | −0.03 ± 0.01 b | −0.11 ± 0.02 a |
| ASC | 0.27 ± 0.03 d | −0.06 ± 0.06 ab | −0.08 ± 0.06 a | 0.01 ± 0.02 bc | 0.02 ± 0.01 c |
| C | 0.03 ± 0.02 c | 0.00 ± 0.00 b | −0.02 ± 0.00 a | 0.03 ± 0.02 c | 0.00 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laznik, Ž.; Križman, M.; Zekič, J.; Roškarič, M.; Trdan, S.; Urbanek Krajnc, A. The Role of Ascorbate–Glutathione System and Volatiles Emitted by Insect-Damaged Lettuce Roots as Navigation Signals for Insect and Slug Parasitic Nematodes. Insects 2023, 14, 559. https://doi.org/10.3390/insects14060559
Laznik Ž, Križman M, Zekič J, Roškarič M, Trdan S, Urbanek Krajnc A. The Role of Ascorbate–Glutathione System and Volatiles Emitted by Insect-Damaged Lettuce Roots as Navigation Signals for Insect and Slug Parasitic Nematodes. Insects. 2023; 14(6):559. https://doi.org/10.3390/insects14060559
Chicago/Turabian StyleLaznik, Žiga, Mitja Križman, Jure Zekič, Mihaela Roškarič, Stanislav Trdan, and Andreja Urbanek Krajnc. 2023. "The Role of Ascorbate–Glutathione System and Volatiles Emitted by Insect-Damaged Lettuce Roots as Navigation Signals for Insect and Slug Parasitic Nematodes" Insects 14, no. 6: 559. https://doi.org/10.3390/insects14060559
APA StyleLaznik, Ž., Križman, M., Zekič, J., Roškarič, M., Trdan, S., & Urbanek Krajnc, A. (2023). The Role of Ascorbate–Glutathione System and Volatiles Emitted by Insect-Damaged Lettuce Roots as Navigation Signals for Insect and Slug Parasitic Nematodes. Insects, 14(6), 559. https://doi.org/10.3390/insects14060559








