Potential Global Invasion Risk of Scale Insect Pests Based on a Self-Organizing Map
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
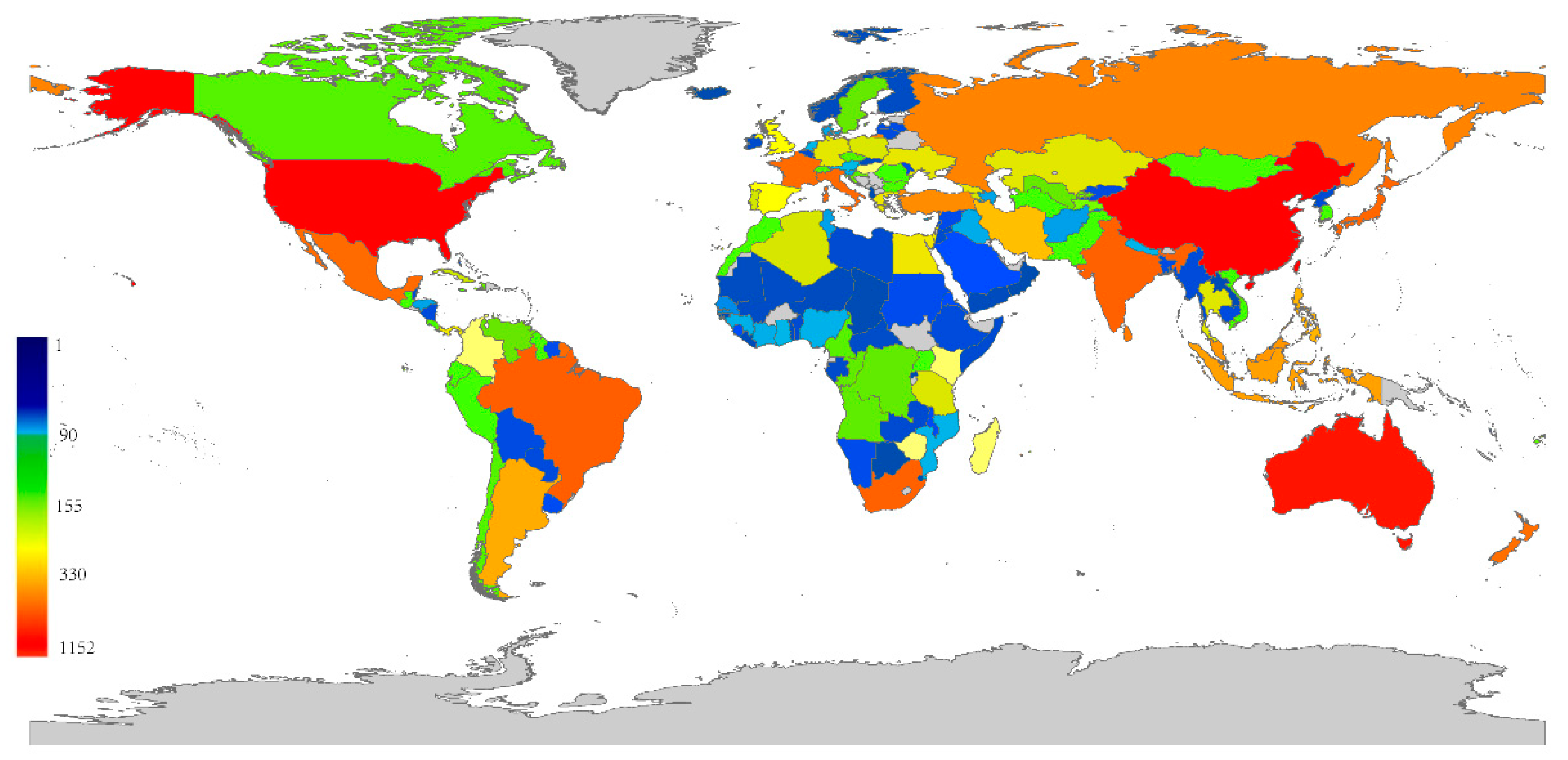
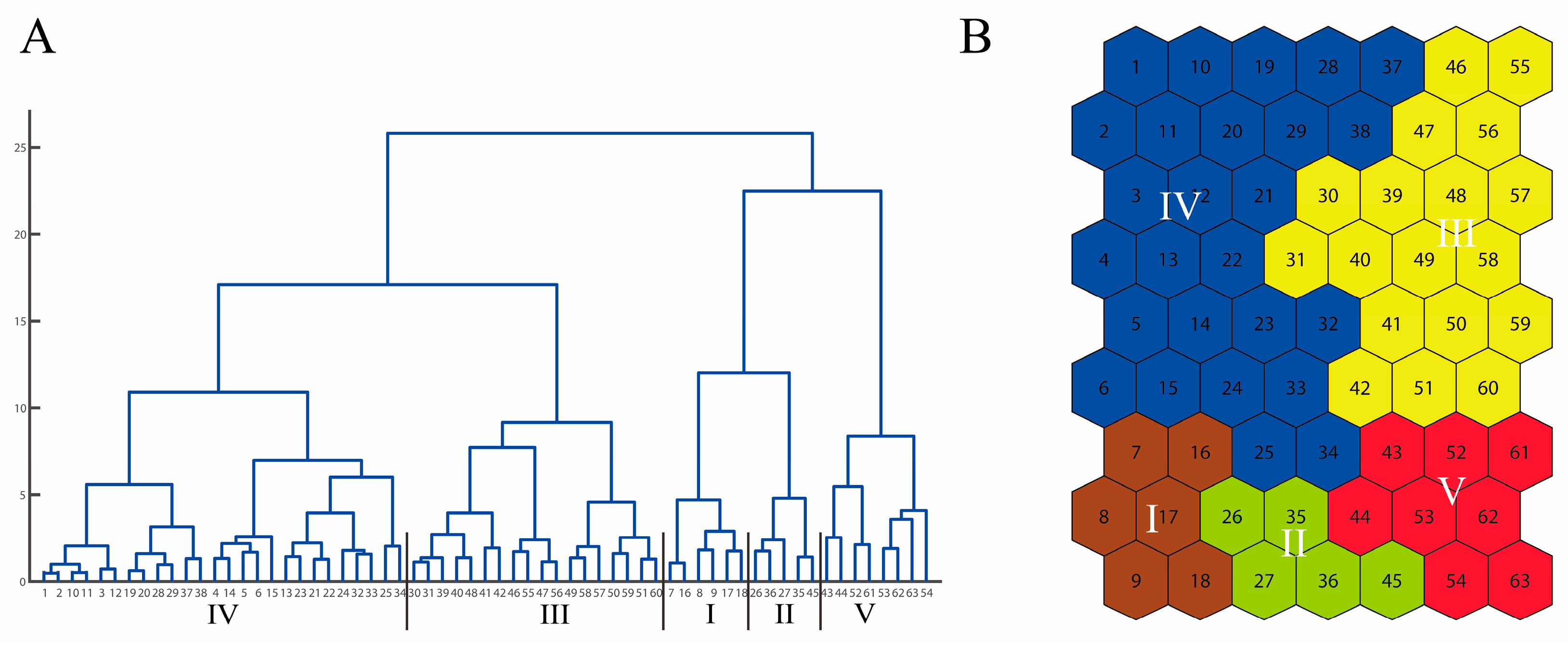
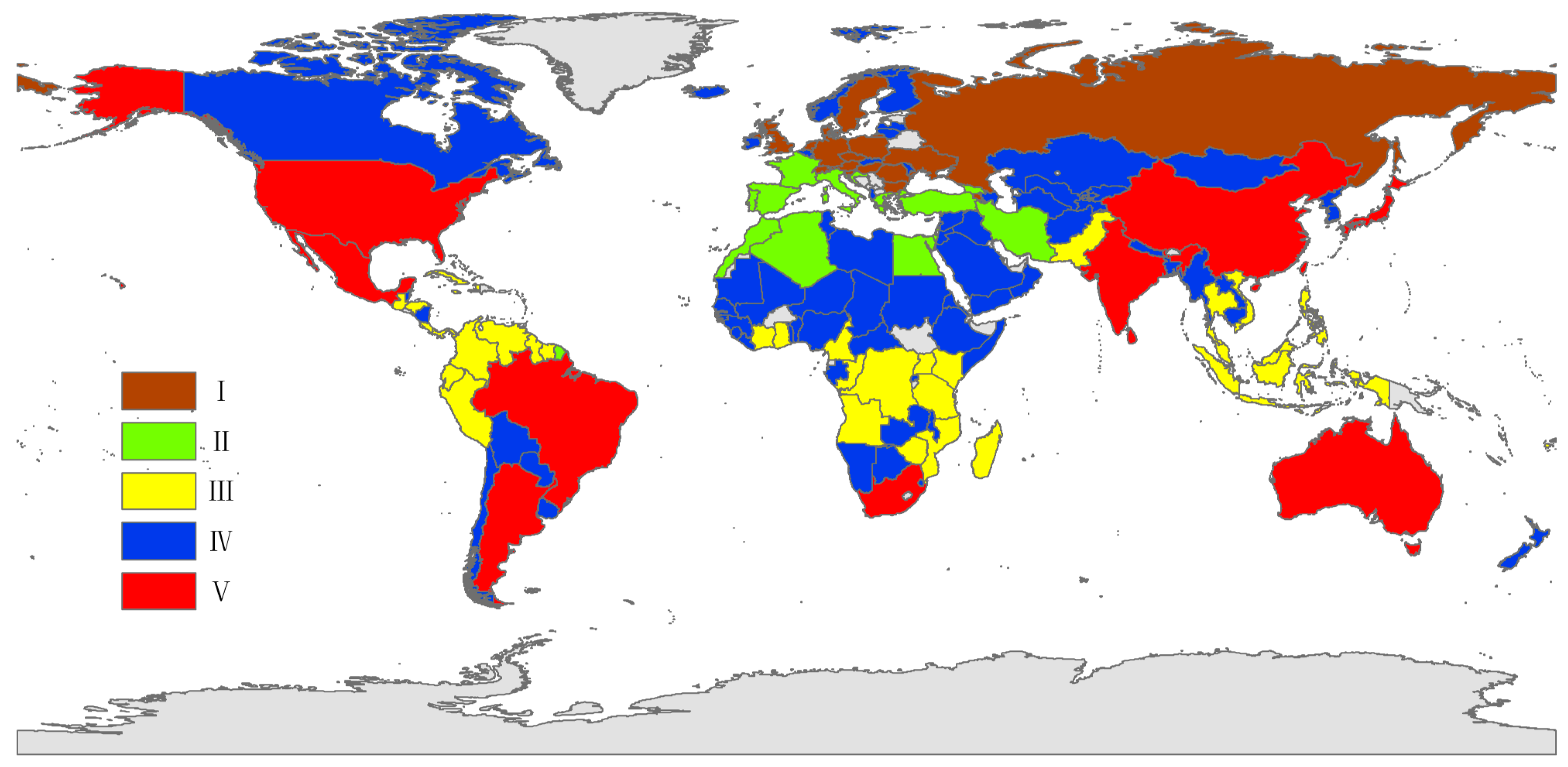
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Worner, S.P.; Gevrey, M. Modelling global insect pest species assemblages to determine risk of invasion. J. Appl. Ecol. 2006, 43, 858–867. [Google Scholar] [CrossRef]
- Pimentel, D. Habitat Factors in New Pest Invasions; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar]
- Watts, M.J.; Worner, S.P. Estimating the risk of insect species invasion: Kohonen self-organising maps versus k-means clustering. Ecol. Model. 2009, 220, 821–829. [Google Scholar]
- Qin, Y.; Paini, D.R.; Wang, C.; Fang, Y.; Li, Z. Global establishment risk of economically important fruit fly species (Tephritidae). PLoS ONE 2015, 10, e0116424. [Google Scholar]
- Stockwell, D.; Peters, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Syst. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G.F.; Yonow, T.; Stevens, P.M. CLIMEX: Predicting the Effects of Climate on Plants and Animals; CSIRO Publishing: Collingwood, Victoria, Australia, 1999. [Google Scholar]
- Kohonen, T. Self-organized formation of topologically correct feature maps. Biol. Cybern. 1982, 43, 59–69. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organizing Maps, Second Extended; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Gevrey, M.; Worner, S.; Kasabov, N.; Pitt, J.; Giraudel, J.L. Estimating risk of events using SOM models: A case study on invasive species establishment. Ecol. Model. 2006, 197, 361–372. [Google Scholar]
- Roigé, M.; McGeoch, M.A.; Hui, C.; Worner, S.P. Cluster validity and uncertainty assessment for self-organizing map pest profile analysis. Methods Ecol. Evol. 2016, 8, 349–357. [Google Scholar] [CrossRef]
- Park, Y.S.; Céréghino, R.; Compin, A.; Lek, S. Applications of artificial neural networks for patterning and predicting aquatic insect species richness in running waters. Ecol. Model. 2003, 160, 265–280. [Google Scholar]
- Paini, D.R.; Worner, S.P.; Cook, D.C.; De Barro, P.J.; Thomas, M.B. Using a self-organizing map to predict invasive species: Sensitivity to data errors and a comparison with expert opinion. J. Appl. Ecol. 2010, 47, 290–298. [Google Scholar] [CrossRef]
- Singh, S.K.; Paini, D.R.; Ash, G.J.; Hodda, M. Prioritising plant-parasitic nematode species biosecurity risks using self organising maps. Biol. Invasions 2013, 16, 1515–1530. [Google Scholar]
- Paini, D.R.; Bianchi, F.J.; Northfield, T.D.; De Barro, P.J. Predicting invasive fungal pathogens using invasive pest assemblages: Testing model predictions in a virtual world. PLoS ONE 2011, 6, e25695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, L.; Paini, D.R.; Randall, R.P. Can Global Weed Assemblages Be Used to Predict Future Weeds? PLoS ONE 2013, 8, e55547. [Google Scholar]
- García Morales, M.; Denno, B.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: A Literature-Based Model of Scale Insect Biology and Systematics. Available online: http://scalenet.info/flatcat/ (accessed on 2 July 2015).
- Hamon, A.B.; Williams, M.L. The Soft Scale Insects of Florida (Homoptera: Coccoidea: Coccidae); Florida Department of Agriculture & Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 1984. [Google Scholar]
- Gill, R.J. Florida; Analysis and Identification Branch, Division of Plant Industry, California Department of Food and Agriculture: Sacramento, CA, USA, 1988. [Google Scholar]
- Kosztarab, M.; Kozár, F. Scale Insects of Central Europe; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 40. [Google Scholar]
- Miller, D.R.; Miller, G.L.; Hodges, G.S.; Davidson, J.A. Introduced scale insects (Hemiptera: Coccoidea) of the United States and their impact on U.S. agriculture. Proc. Entomol. Soc. Wash. 2005, 107, 123–158. [Google Scholar]
- Herren, H.R.; Neuenschwander, P. Biological Control of Cassava Pests in Africa. Annu. Rev. Entomol. 1991, 36, 257–283. [Google Scholar] [CrossRef]
- Hardy, N.B. The status and future of scale insect (Coccoidea) systematics. Syst. Entomol. 2013, 38, 453–458. [Google Scholar]
- Vesanto, J.; Alhoniemi, E. Clustering of the self-organizing map. Trans. Neural Netw. 2000, 11, 586–600. [Google Scholar] [CrossRef]
- Ward Jr, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Li, B.; Watanabe, K.; Kim, D.H.; Lee, S.B.; Heo, M.; Kim, H.S.; Chon, T.S. Identification of Outlier Loci Responding to Anthropogenic and Natural Selection Pressure in Stream Insects Based on a Self-Organizing Map. Water 2016, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Davies, D.L.; Bouldin, D.W. A cluster separation measure. IEEE Trans. Pattern Anal. Mach. Intell. 1979, 1, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Mcgeoch, M.A. Zeta diversity as a concept and metric that unifies incidence-based biodiversity patterns. Am. Nat. 2014, 184, 684–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauber, A. Data Mining with SOMVIS. Available online: http://ifs.tuwien.ac.at/dm/somvis-matlab/index.html (accessed on 15 June 2015).
- Latombe, G.; Mcgeoch, M.A.; Hui, C. Zetadiv: Functions to Compute Compositional Turnover Using Zeta Diversity. Available online: https://rdrr.io/cran/zetadiv/ (accessed on 11 June 2022).
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 28 April 2016).
- Roigé, M.; Parry, M.; Phillips, C.; Worner, S. Self-organizing maps for analysing pest profiles: Sensitivity analysis of weights and ranks. Ecol. Model. 2016, 342, 113–122. [Google Scholar]
- Deng, J.; Li, K.; Cui, C.; Wu, S.; Huang, X. Discovery pattern and species number of scale insects (Hemiptera: Coccoidea). Peerj 2016, 4, e2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellizzari, G.; Germain, J.F. Scales (Hemiptera, Superfamily Coccoidea). BioRisk 2010, 4, 475. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.B.; Wu, S.A. A new pest, Phenacoccus parvus Morrison (Hemiptera: Coccoidea: Pseudococcidae), in mainland China. Chin. J. Appl. Entomol. 2014, 51, 1098–1103. [Google Scholar]
- Waterhouse, D.F.; Sands, D.P.A. Classical biological control of arthropods in Australia. ACIAR Monogr. 2001, 77, 560. [Google Scholar]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.A.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.F.; Chang, G.B.; Dang, Z.Y. The situations and management strategies of Japanese pine scale in China. For. Sci. Technol. 1990, 12, 1–3. [Google Scholar]
- Ercsey-ravasz, M.; Toroczkai, Z.; Lakner, Z.; Baranyi, J. Complexity of the International Agro-Food Trade Network and Its Impact on Food Safety. PLoS ONE 2012, 7, e37810. [Google Scholar] [CrossRef]
- Miller, D.R.; Davidson, J.A. Armored Scale Insect Pests of Trees and Shrubs (Hemiptera: Diaspididae); Cornell University Press: Ithaca, NY, USA, 2005. [Google Scholar]
- Hodgson, C.J.; Peronti, A.J.B.G. A revision of the wax scale insects (Hemiptera: Sternorrhyncha: Coccoidea: Ceroplastinae) of the Afrotropical Region. Zootaxa 2012, 3372, 1–265. [Google Scholar] [CrossRef] [Green Version]

| Rank | China | USA | UK |
|---|---|---|---|
| 1 | Uhleria araucariae | Nipaecoccus viridis | Physokermes piceae |
| 2 | Odonaspis ruthae | Icerya seychellarum | Phenacoccus piceae |
| 3 | Odonaspis saccharicaulis | Planococcus minor | Sphaerolecanium prunastri |
| 4 | Ferrisia malvastra | Pseudococcus cryptus | Diaspidiotus gigas |
| 5 | Dactylopius ceylonicus | Pulvinaria polygonata | Coccura comari |
| 6 | Dactylopius opuntiae | Pseudaulacaspis eugeniae | Ceroputo pilosellae |
| 7 | Pulvinaria urbicola | Paralecanium expansum | Eulecanium franconicum |
| 8 | Diaspidiotus ancylus | Fiorinia turpiniae | Lepidosaphes conchiformis |
| 9 | Phenacoccus parvus | Icerya aegyptiaca | Heliococcus bohemicus |
| 10 | Furchadaspis zamiae | Aulacaspis madiunensis | Acanthococcus aceris |
| Rank | Australia | France | Argentina |
| 1 | Russellaspis pustulans | Oceanaspidiotus spinosus | Chrysomphalus aonidum |
| 2 | Hemiberlesia cyanophylli | Chrysomphalus aonidum | Ceroplastes floridensis |
| 3 | Parlatoria cinerea | Parlatoria parlatoriae | Aulacaspis tubercularis |
| 4 | Oceanaspidiotus spinosus | Anophococcus formicicola | Aspidiotus destructor |
| 5 | Mycetaspis personata | Peliococcus turanicus | Pinnaspis strachani |
| 6 | Protopulvinaria pyriformis | Lepidosaphes granati | Pulvinaria psidii |
| 7 | Aonidomytilus albus | Nipaecoccus nipae | Coccus longulus |
| 8 | Planococcus ficus | Peliococcopsis priesneri | Ceroplastes sinensis |
| 9 | Kilifia acuminata | Physokermes hemicryphus | Coccus viridis |
| 10 | Nipaecoccus nipae | Asterodiaspis minor | Russellaspis pustulans |
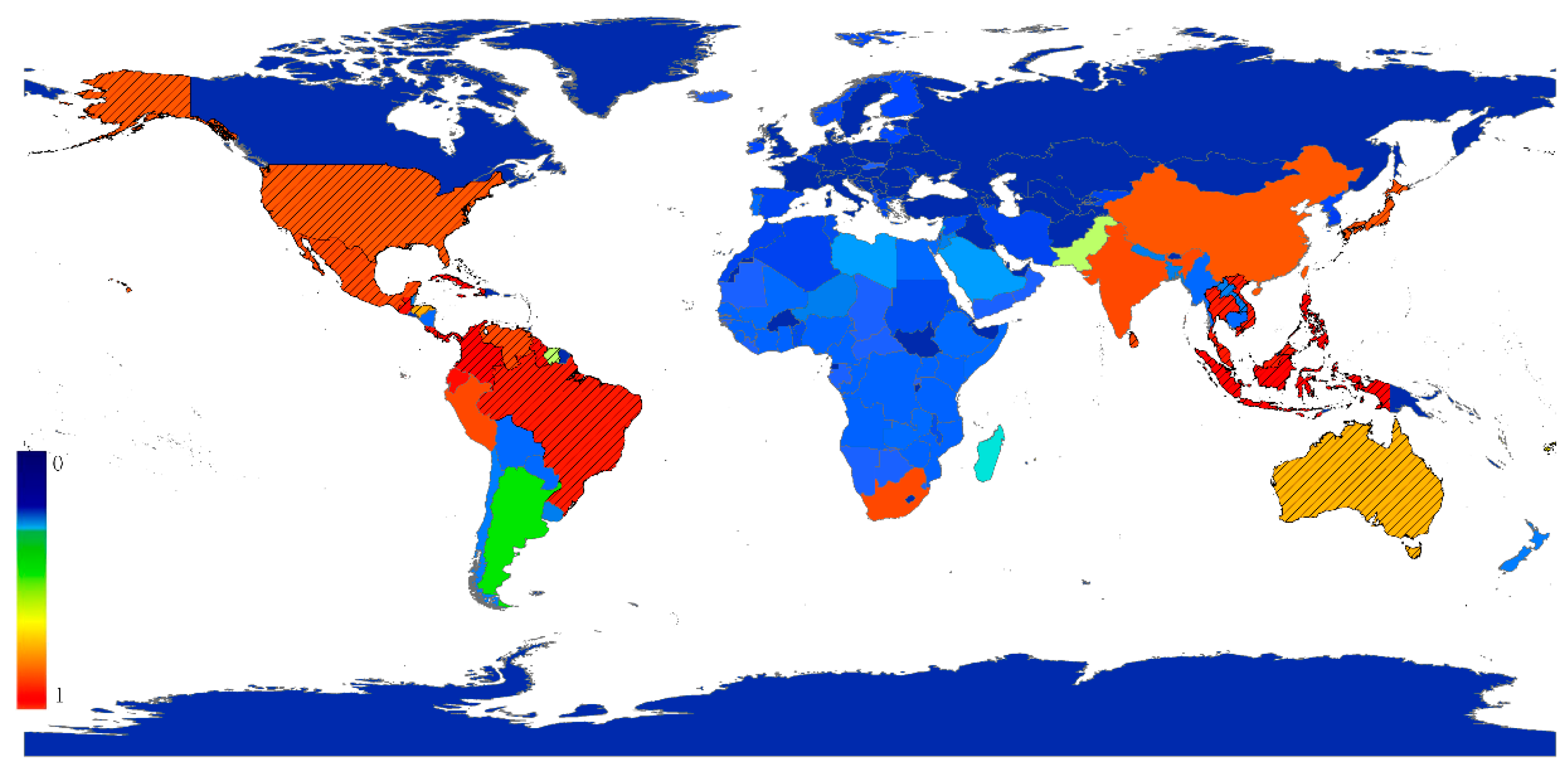
| Uncertainty | Neuron | ζ2 | ζ3 | ζ4 | ζ5 | Asia | Africa | North America | South America | Europe | Oceania |
|---|---|---|---|---|---|---|---|---|---|---|---|
| low | 8 | 0.52 | 0.37 | 0.29 | 0.24 | 0 | 0 | 0 | 0 | 6 | 0 |
| low | 60 | 0.43 | 0.29 | 0.23 | 0.19 | 2 | 0 | 3 | 1 | 0 | 0 |
| low | 36 | 0.45 | 0.31 | 0.25 | 0.21 | 1 | 2 | 0 | 0 | 2 | 0 |
| high | 1 | 0.05 | 0.00 | 0.00 | 0.00 | 2 | 11 | 0 | 0 | 3 | 1 |
| high | 2 | 0.20 | NA | NA | NA | 0 | 0 | 0 | 0 | 2 | 0 |
| high | 3 | 0.20 | 0.06 | 0.02 | 0.00 | 1 | 0 | 0 | 0 | 6 | 0 |
| high | 4 | 0.13 | NA | NA | NA | 1 | 0 | 0 | 0 | 1 | 0 |
| high | 7 | 0.19 | NA | NA | NA | 1 | 0 | 0 | 0 | 1 | 0 |
| high | 10 | 0.06 | 0.01 | 0.00 | 0.00 | 3 | 1 | 1 | 0 | 0 | 0 |
| high | 19 | 0.15 | 0.05 | 0.02 | 0.01 | 2 | 4 | 1 | 1 | 0 | 0 |
| high | 21 | 0.15 | NA | NA | NA | 1 | 0 | 0 | 1 | 0 | 0 |
| high | 24 | 0.18 | 0.07 | NA | NA | 2 | 0 | 0 | 0 | 1 | 0 |
| China † | Australia ‡ | France § | |||
|---|---|---|---|---|---|
| Species | Rank | Species | Rank | Species | Ranks |
| Ischnaspis longirostris | 22p | Saissetia oleae | 2p | Comstockaspis perniciosa | 60p |
| Selenaspidus articulatus | 40p | Coccus hesperidum | 5p | Maconellicoccus hirsutus | 76p |
| Lepidosaphes ulmi | 49p | Parasaissetia nigra | 6p | Lopholeucaspis japonica | 120p |
| Phenacoccus solenopsis | 63p | Saissetia coffeae | 7p | Lepidosaphes ussuriensis | 405a |
| Lepidosaphes tokionis | 68p | Lepidosaphes gloverii | 16p | ||
| Ceroplastes stellifer | 108p | Lepidosaphes beckii | 17p | ||
| Planococcus minor | 112p | Aonidiella aurantii | 22p | ||
| Dysmicoccus neobrevipes | 143a | Planococcus citri | 24p | ||
| Planococcus lilacius | 154p | Chrysomphalus aonidum | 29p | ||
| Ceroplastes rusci | 171a | Coccus longulus | 41p | ||
| Epidiaspis leperii | 177p | Diaspis bromeliae | 44p | ||
| Aonidiella comperei | 208p | Ceroplastes floridensis | 46p | ||
| Carulaspis juniperi | 240a | Unaspis citri | 47p | ||
| Hemiberlesia pitysophila | 332p | Pseudococcus viburni | 59p | ||
| Eulecanium gigantea | 354p | Aonidiella orientalis | 64p | ||
| Dysmicoccus grassi | 438a | Ceroplastes ceriferus | 69p | ||
| Lepidosaphes tapleyi | 455p | Aonidiella citrina | 75p | ||
| Chionaspis pinifoliae | 491a | Coccus viridis | 79p | ||
| Mercetaspis halli | 688a | Ceroplastes rubens | 85p | ||
| Parlatoria crypta | 1238a | Ceroplastes sinensis | 137p | ||
| Phenacoccus manihoti | 1458a | Coccus pseudomagnoliarum | 196p | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Li, J.; Zhang, X.; Zeng, L.; Guo, Y.; Wang, X.; Chen, Z.; Zhou, J.; Huang, X. Potential Global Invasion Risk of Scale Insect Pests Based on a Self-Organizing Map. Insects 2023, 14, 572. https://doi.org/10.3390/insects14070572
Deng J, Li J, Zhang X, Zeng L, Guo Y, Wang X, Chen Z, Zhou J, Huang X. Potential Global Invasion Risk of Scale Insect Pests Based on a Self-Organizing Map. Insects. 2023; 14(7):572. https://doi.org/10.3390/insects14070572
Chicago/Turabian StyleDeng, Jun, Junjie Li, Xinrui Zhang, Lingda Zeng, Yanqing Guo, Xu Wang, Zijing Chen, Jiali Zhou, and Xiaolei Huang. 2023. "Potential Global Invasion Risk of Scale Insect Pests Based on a Self-Organizing Map" Insects 14, no. 7: 572. https://doi.org/10.3390/insects14070572
APA StyleDeng, J., Li, J., Zhang, X., Zeng, L., Guo, Y., Wang, X., Chen, Z., Zhou, J., & Huang, X. (2023). Potential Global Invasion Risk of Scale Insect Pests Based on a Self-Organizing Map. Insects, 14(7), 572. https://doi.org/10.3390/insects14070572






