Bioclimatic and Landscape Factors drive the Potential Distribution of Philaenus spumarius, Neophilaenus campestris and N. lineatus (Hemiptera, Aphrophoridae) in Southeastern Iberian Peninsula
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
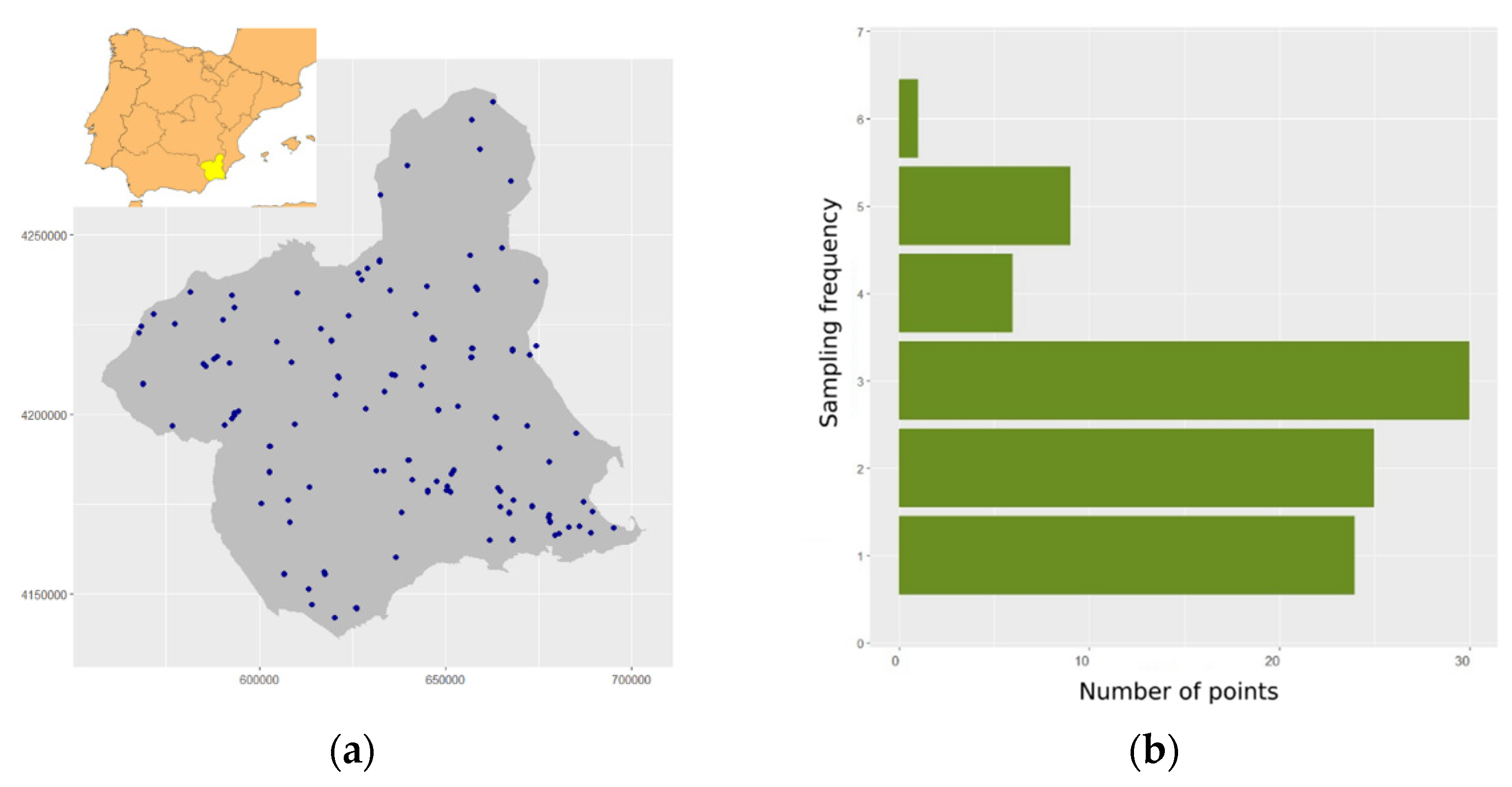
2.1. Study Area
2.2. Sampling Design
2.3. Distribution and Abundance of Species
2.4. Distribution Modeling
3. Results
3.1. Sampling Results, Temporal Catches and Abundance Distribution
3.2. Models of Habitat Suitability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornara, D.; Sicard, A.; Zeilinger, A.R.; Porcelli, F.; Purcell, A.H.; Almeida, R.P.P. Transmission of Xylella fastidiosa to grapevine by the meadow spittlebug. Phytopathology 2016, 106, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Cornara, D.; Saponari, M.; Zeilinger, A.R.; de Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Marteli, G.P.; Almedia, R.P.P.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest. Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies Pauca sequence Type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Cornara, D.; Morente, M.; Markheiser, A.; Bodino, N.; Tsai, C.; Fereres, A.; Redak, R.A.; Perring, T.M.; Lopes, J.R. An overview on the worldwide vectors of Xylella fastidiosa. Entomol. Gen. 2019, 39, 157–181. [Google Scholar] [CrossRef]
- Greco, D.; Aprile, A.; De Bellis, L.; Luvisi, A. Diseases Caused by Xylella fastidiosa in Prunus Genus: An Overview of the Research on an Increasingly Widespread Pathogen. Front. Plant. Sci. 2021, 12, 712452. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant. Pathol. 2013, 95, 668. [Google Scholar] [CrossRef]
- Olmo, D.; Nieto, A.; Adrover, F.; Urbano, A.; Beidas, O.; Juan, A.; Marco-Noales, E.; López, M.M.; Navarro, I.; Monterde, A.; et al. First detection of Xylella fastidiosa infecting cherry (Prunus avium) and Polygala myrtifolia plants, in Mallorca Island, Spain. Plant. Dis. 2017, 101, 1820. [Google Scholar] [CrossRef]
- EPPO. First Report of Xylella fastidiosa in Israel. EPPO Rep. Serv. 2019, 6, 1. [Google Scholar]
- Amanifar, N.; Taghavi, S.M.; Izadpanah, K.; Babaie, G. Isolation and pathogenicity of Xylella fastidiosa from grapevine and almond in Iran. Phytopathol. Mediterr. 2014, 53, 318–327. [Google Scholar] [CrossRef]
- MAPA. Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/organismos-nocivos/xylella-fastidiosa/ (accessed on 1 November 2022).
- Godefroid, M.; Morente, M.; Schartel, T.; Cornara, D.; Purcell, A.; Gallego, D.; Moreno, A.; Pereira, J.A.; Fereres, A. Climate tolerances of Philaenus spumarius should be considered in risk assessment of disease outbreaks related to Xylella fastidiosa. J. Pest. Sci. 2021, 95, 855–868. [Google Scholar] [CrossRef]
- Lago, C.; Morente, M.; De las Heras-Bravo, D.; Marti-Campoy, A.; Rodriguez-Ballester, F.; Plaza, M.; Moreno, A.; Fereres, A. Dispersal of Neophilaenus campestris, a vector of Xylella fastidiosa, from olive groves to over-summering hosts. J. App. Ent. 2021, 145, 648–659. [Google Scholar] [CrossRef]
- Chartois, M.; Mesmin, X.; Quiquerez, I.; Borgomano, S.; Farigoule, P.; Pierre, E.; Thuilier, J.M.; Streito, J.C.; Casabianca, F.; Hugot, L.; et al. Environmental factors driving the abundance of Philaenus spumarius in mesomediterranean habitats of Corsica (France). Sci. Rep. 2023, 13, 1901. [Google Scholar] [CrossRef]
- Santoiemma, G.; Tamburini, G.; Sanna, F.; Mori, N.; Marini, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest. Sci. 2019, 92, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Esteve-Selma, M.A.; Martínez-Fernández, J.; Hernández, I.; Montávez, J.P.; Lopez, J.J.; Calvo, J.F.; Robledano, F. Effects of climatic change on the distribution and conservation of Mediterranean forests: The case of Tetraclinis articulata in the Iberian Peninsula. Biodivers. Conserv. 2010, 19, 3809–3825. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2022. Available online: http://qgis.osgeo.org (accessed on 1 November 2022).
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.; et al. Distribution and relative abundance of insect vectors of Xylella fastidiosa in olive groves of the Iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J.; van Etten, J. Raster: Geographic Analysis and Modeling with Raster Data. R Package Version 3.4-13. 2021. Available online: http://CRAN.R-project.org/package=raster (accessed on 1 November 2022).
- Lewin-Koh, J.N.; Bivand, R. “Package ‘Maptools’: Tools for Reading and Handling Spatial Objects,” R Package Version 0.8-10; 2011. Available online: http://CRAN.R-project.org/package=maptools (accessed on 1 November 2022).
- Wickhamn, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 1 November 2022).
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 November 2022).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Package ‘Dismo’. 2020. Available online: http://cran.r-project.org/web/packages/dismo/index.html (accessed on 1 November 2022).
- Sanchez-García, F.J.; Galián, J.; Gallego, D. Distribution of Tomicus destruens (Coleoptera: Scolytinae) mitochondrial lineages: Phylogeographic insights and niche modelling. Org. Div. Evol. 2015, 15, 101–113. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Guevara, L.; Gerstner, B.E.; Kass, J.M.; Anderson, R.P. Toward ecologically realistic predictions of species distributions: A cross-time example from tropical montane cloud forests. Glob. Chang. Biol. 2018, 24, 1511–1522. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef] [Green Version]
- Radosavljevic, A.; Anderson, R.P. Making better MAXENT models of species distributions: Complexity, overfitting and evaluation. J. Biogeography. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Plazio, E.; Saladini, M.A.; Volani, S.; Simonetto, A.; Fumarola, G.; Di Carolo, M.; Porcelli, F.; et al. Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy. Sci. Rep. 2019, 9, 17725. [Google Scholar] [CrossRef] [Green Version]
- Beal, D.J.; Cooper, M.; Daugherty, M.P.; Purcell, A.H.; Almeida, R.P.P. Seasonal Abundance and Infectivity of Philaenus spumarius (Hemiptera: Aphrophoridae), a Vector of Xylella fastidiosa in California Vineyards. Environ. Entomol. 2021, 50, 467–476. [Google Scholar] [CrossRef]
- Daugherty, M.P.; Almeida, R.P.P. Understanding how an invasive vector drives Pierce’s disease epidemics: Seasonality and vine-to-vine spread. Phytopathology 2019, 109, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Maryańska-Nadachowska, A.; Kajtoch, L.; Lachowska, D. Genetic diversity of Philaenus spumarius and P. tesselatus (Hemiptera, Aphrophoridae): Implications for evolution and taxonomy. Syst. Entomol. 2011, 37, 55–64. [Google Scholar] [CrossRef]
- Tsagkarakis, A.E.; Afentoulis, D.G.; Matared, M.; Thanou, Z.N.; Stamatakou, G.D.; Kalaitzaki, A.P.; Perdikis, D.C. Identification and seasonal abundance of Auchenorrhyncha with a focus on potential insect vectors of Xylella fastidiosa in olive orchards in three regions of Greece. J. Econ. Entom. 2018, 111, 2536–2545. [Google Scholar] [CrossRef]
- Drosopoulos, S.; Asche, M. Biosystematic studies on the spittlebug genus Philaenus with the description of a new species. Zool. J. Linn. Soc. 2001, 101, 169–177. [Google Scholar] [CrossRef]
- Cruaud, A.; Gonzalez, A.A.; Godefroid, M.; Nidelet, S.; Streito, J.C.; Thuillier, J.M.; Rasplus, J.Y. Using insects to detect, monitor and predict the distribution of Xylella fastidiosa: A case study in Corsica. Sci. Rep. 2018, 8, 15628. [Google Scholar] [CrossRef] [Green Version]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus spumarius: When an old acquaintance becomes a new threat to European agriculture. J. Pest. Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Zajac, M.A.; Hall, F.R.; Wilson, M.C. Heat unit model for the development of meadow spittlebug (Homoptera Cercopidae) on strawberry. Environ. Entomol. 1989, 18, 347–350. [Google Scholar] [CrossRef]
- Godefroid, M.; Durán, J.M. Composition of landscape impacts the distribution of the main vectors of Xylella fastidiosa in southern Spain. J. App. Ent. 2022, 146, 666–675. [Google Scholar] [CrossRef]






| Kind of Data/Units | Layer | Description |
|---|---|---|
| Bioclimatic | ||
| °C, mm | Bio1 | Annual mean temperature |
| Bio2 | Mean diurnal range (mean of monthly (max. temp–min. temp)) | |
| Bio3 | Isothermality (BIO2/BIO7) (×100) | |
| Bio4 | Temperature seasonality (standard deviation ×100) | |
| Bio5 | Max. temperature of warmest month | |
| Bio6 | Min. temperature of coldest month | |
| Bio7 | Temperature annual range (BIO5-BIO6) | |
| Bio8 | Mean temperature of wettest quarter | |
| Bio9 | Mean temperature of driest quarter | |
| Bio10 | Mean temperature of warmest quarter | |
| Bio11 | Mean temperature of coldest quarter | |
| Bio12 | Annual precipitation | |
| Bio13 | Precipitation of wettest month | |
| Bio14 | Precipitation of driest month | |
| Bio15 | Precipitation seasonality (coefficient of variation) | |
| Bio16 | Precipitation of wettest quarter | |
| Bio17 | Precipitation of driest quarter | |
| Bio18 | Precipitation of warmest quarter | |
| Bio19 | Precipitation of coldest quarter | |
| Landscape, tree crops | ||
| 0.06 ha/km2 | Almond_irr | Density of irrigated almond tree crops |
| Almond_dr | Density of rainfed almond tree crops | |
| Olive_irr | Density of irrigated olive tree crops | |
| Olive_dr | Density of rainfed olive tree crops | |
| Vineyard_irr | Density of irrigated vineyards | |
| Vineyard_dr | Density of rainfed vineyards | |
| High_vineyard | Density of table grape crops | |
| Citric_d | Density of citric crops | |
| Stone_fr_irr | Density of irrigated stone fruit tree crops | |
| Stone_fr_dr | Density of rainfed stone fruit tree crops | |
| Landscape, natural vegetation | ||
| ha/km2 | Forest_d | Density of forests |
| Forest_mos | Density of forest mosaic | |
| Bush_d | Density of Mediterranean shrubs | |
| Bush_mos | Density of Mediterranean shrub mosaic | |
| Pasture | Density of grass formations | |
| Wveg | Density of areas without vegetation | |
| Landscape, vegetation geoseries | ||
| presence/absence (binary) | Oro | Presence of oromediterranean zone |
| Supra | Presence of supramediterranean zone | |
| Meso_cd | Presence of cold mesomediterranean zone | |
| Meso_he | Presence of warm mesomediterranean zone | |
| Termo_hi | Presence of upper thermomediterranean zone | |
| Topography | ||
| m, degrees | Altitud | Mean altitude |
| Abrupt | Mean ruggedness index | |
| Slope | Mean slope |
| Species | AUCtest | AUCdiff | RM | FC 1 | Explaining Variables |
|---|---|---|---|---|---|
| P. spumarius | 0.839 | 0.107 | 4 | L | 11 |
| N. campestris | 0.799 | 0.1577 | 0.5 | LQH | 11 |
| N. lineatus | 0.853 | 0.096 | 4 | L | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego, D.; Sabah, S.C.; Lencina, J.L.; Carrillo, A.F. Bioclimatic and Landscape Factors drive the Potential Distribution of Philaenus spumarius, Neophilaenus campestris and N. lineatus (Hemiptera, Aphrophoridae) in Southeastern Iberian Peninsula. Insects 2023, 14, 592. https://doi.org/10.3390/insects14070592
Gallego D, Sabah SC, Lencina JL, Carrillo AF. Bioclimatic and Landscape Factors drive the Potential Distribution of Philaenus spumarius, Neophilaenus campestris and N. lineatus (Hemiptera, Aphrophoridae) in Southeastern Iberian Peninsula. Insects. 2023; 14(7):592. https://doi.org/10.3390/insects14070592
Chicago/Turabian StyleGallego, Diego, Sandra Carol Sabah, José Luísis Lencina, and Antonio Félix Carrillo. 2023. "Bioclimatic and Landscape Factors drive the Potential Distribution of Philaenus spumarius, Neophilaenus campestris and N. lineatus (Hemiptera, Aphrophoridae) in Southeastern Iberian Peninsula" Insects 14, no. 7: 592. https://doi.org/10.3390/insects14070592
APA StyleGallego, D., Sabah, S. C., Lencina, J. L., & Carrillo, A. F. (2023). Bioclimatic and Landscape Factors drive the Potential Distribution of Philaenus spumarius, Neophilaenus campestris and N. lineatus (Hemiptera, Aphrophoridae) in Southeastern Iberian Peninsula. Insects, 14(7), 592. https://doi.org/10.3390/insects14070592





