Transcriptomic Analysis of Starvation on the Silkworm Brain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect
2.2. Starvation Treatment and RNA Preparation
2.3. Library Construction and Illumina Hiseq Xten Sequencing
2.4. Read Mapping
2.5. Differential Expression Analysis
2.6. Functional Annotation and Enrichment of DEGs
2.7. Gene Set Enrichment Analysis (GSEA)
2.8. qRT-PCR
2.9. Economic Features Comparison
3. Results
3.1. Overview of the RNA-Seq Data
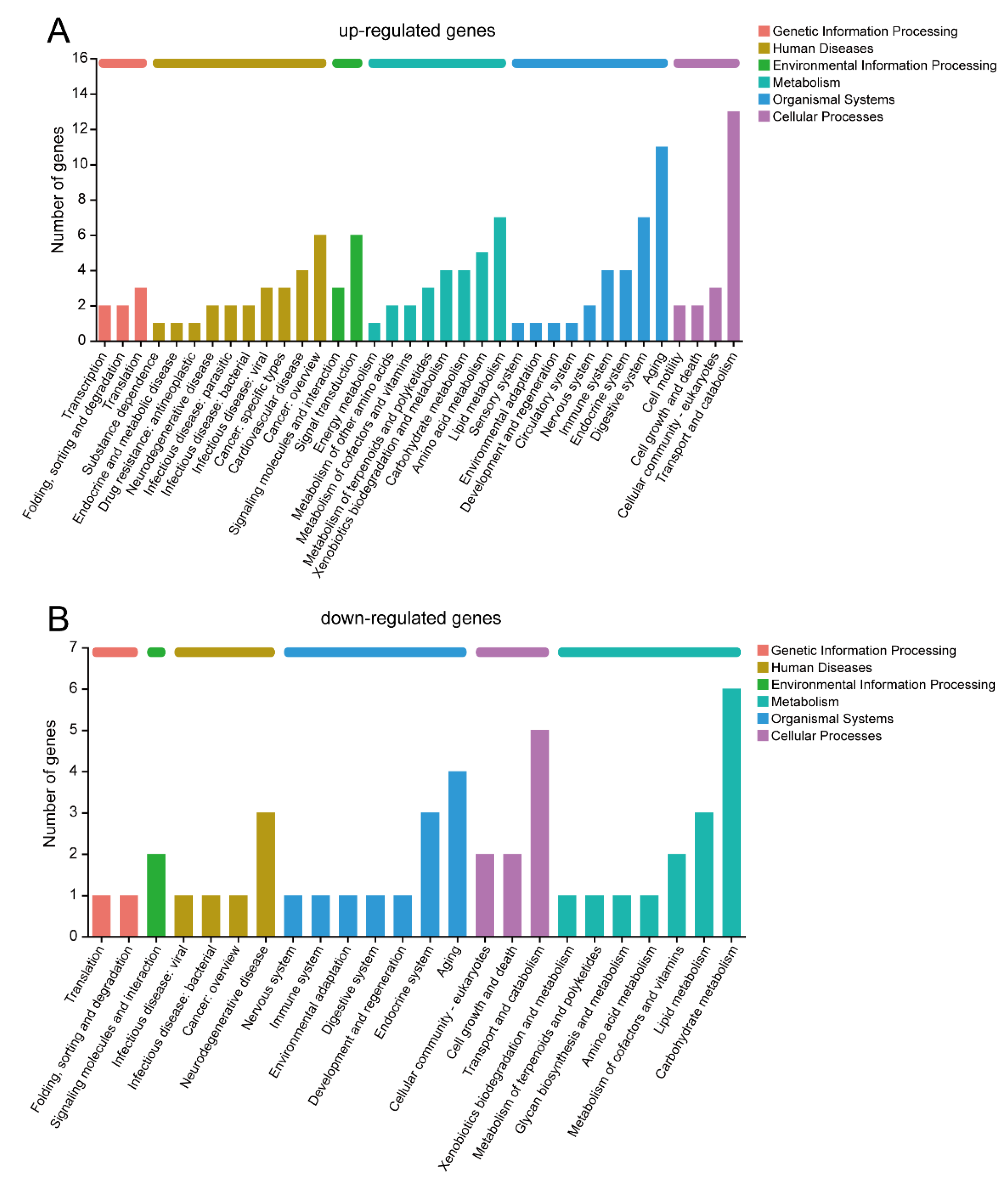
3.2. Functional Annotation of DEGs
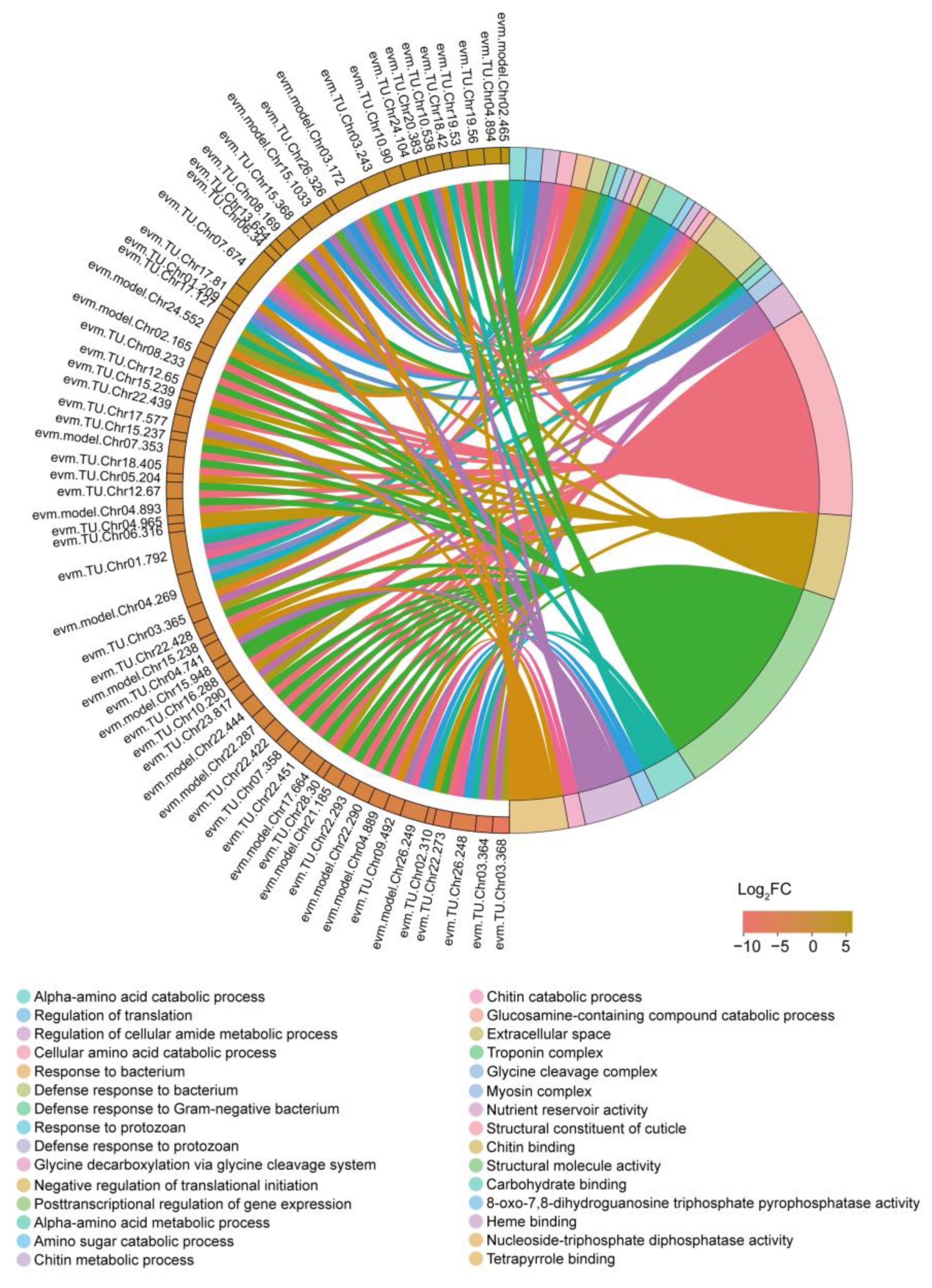
3.3. GO and KEGG Enrichment Analysis
3.4. GSEA
3.5. Results of qRT-PCR
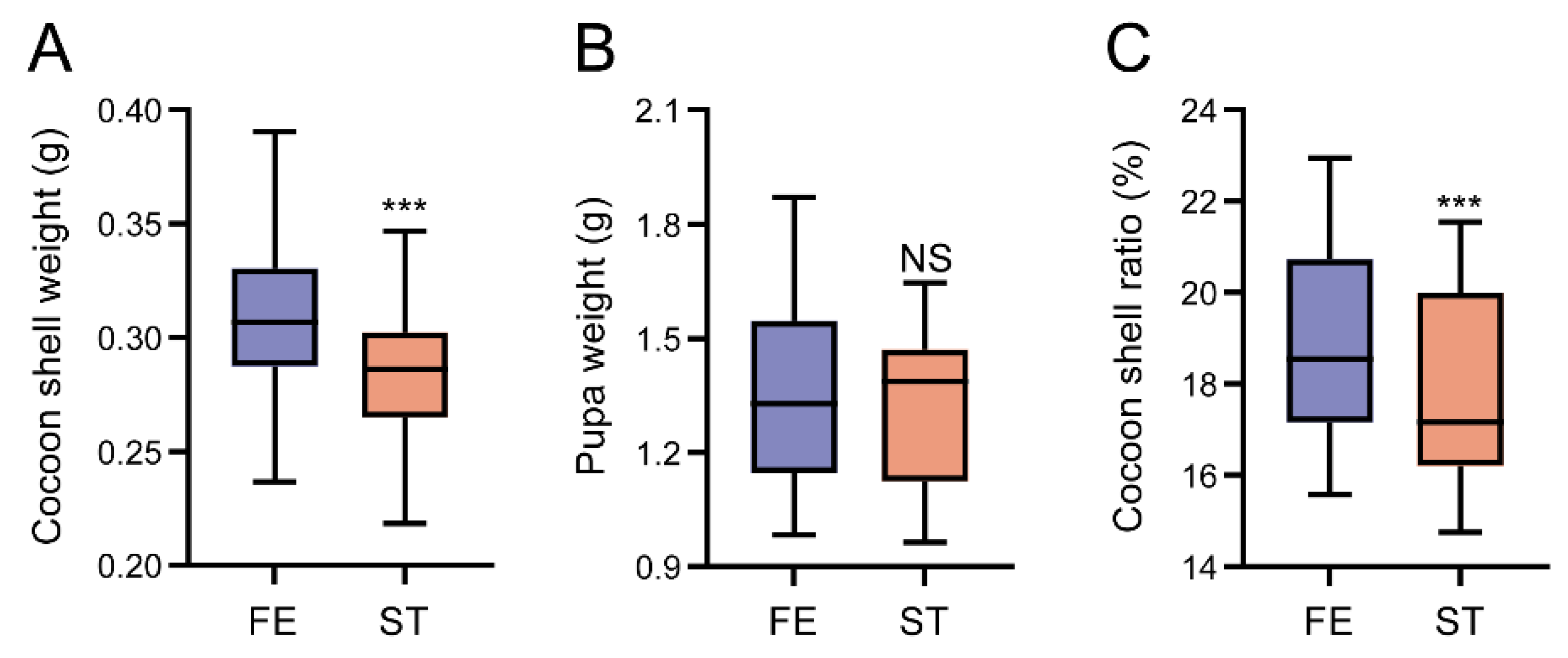
3.6. The Effect of Starvation on Silkworm Economic Features
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef]
- Ochoa-Reparaz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P. Starvation and Its Effects on the Gut. Adv. Nutr. 2021, 12, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Versloot, C.J.; Voskuijl, W.; van Vliet, S.J.; Di Giovanni, V.; Zhang, L.; Richardson, S.; Bourdon, C.; Netea, M.G.; Berkley, J.A.; et al. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: An observational cohort study. Am. J. Clin. Nutr. 2016, 104, 1441–1449. [Google Scholar] [CrossRef]
- Ferraris, R.P.; Carey, H.V. Intestinal transport during fasting and malnutrition. Annu. Rev. Nutr. 2000, 20, 195–219. [Google Scholar] [CrossRef]
- Genton, L.; Cani, P.D.; Schrenzel, J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin. Nutr. 2015, 34, 341–349. [Google Scholar] [CrossRef]
- Seitz, J.; Belheouane, M.; Schulz, N.; Dempfle, A.; Baines, J.F.; Herpertz-Dahlmann, B. The Impact of Starvation on the Microbiome and Gut-Brain Interaction in Anorexia Nervosa. Front. Endocrinol. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, S.G.; Knudsen, G.M.; Jakobsen, J.; Hageman, L.P.; Holm, S.; Paulson, O.B. Brain Metabolism during Short-Term Starvation in Humans. J. Cereb. Blood Flow Metab. 1994, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Avalos, C.B.; Maier, G.L.; Bruggmann, R.; Sprecher, S.G. Single cell transcriptome atlas of the Drosophila larval brain. eLife 2019, 8, e50354. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.J.; Holt, R.A.; Henry, E.; Lyu, Y.; Pletcher, S.D. Effects of hunger on neuronal histone modifications slow aging in Drosophila. Science 2023, 380, 625–632. [Google Scholar] [CrossRef]
- Farahany, N.A.; Greely, H.T.; Hyman, S.; Koch, C.; Grady, C.; Pasca, S.P.; Sestan, N.; Arlotta, P.; Bernat, J.L.; Ting, J.; et al. The ethics of experimenting with human brain tissue. Nature 2018, 556, 429–432. [Google Scholar] [CrossRef]
- Herman, M.M.; Miquel, J.; Johnson, M. Insect brain as a model for the study of aging. Age-related changes in Drosophila melanogaster. Acta Neuropathol. 1971, 19, 167–183. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, F.; Chen, K. Silkworm: A Promising Model Organism in Life Science. J. Insect Sci. 2017, 17, 97. [Google Scholar]
- Panthee, S.; Paudel, A.; Hamamoto, H.; Sekimizu, K. Advantages of the Silkworm As an Animal Model for Developing Novel Antimicrobial Agents. Front. Microbiol. 2017, 8, 373. [Google Scholar] [CrossRef]
- Song, J.B.; Liu, L.; Li, Z.Q.; Mao, T.; Zhang, J.F.; Zhou, L.; Chen, X.; Shang, Y.Z.; Sun, T.; Luo, Y.X.; et al. Lycium barbarum polysaccharide improves dopamine metabolism and symptoms in an MPTP-induced model of Parkinson’s disease. BMC Med. 2022, 20, 412. [Google Scholar] [CrossRef]
- Kim, D.; Landmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szczesniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernandez-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, D.V.; Zhang, L.S.; Pedersen, B.S.; Ramirez, F.; Vesztrocy, A.W.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.Z.; Huang, J.J.; Ding, Y.; Wu, J.M.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L.P. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Borum, P.R. Disease-Related Malnutrition: An Evidence-Based Approach To Treatment: Edited by Rebecca J Stratton, Ceri J Green, and Marinos Elia. Am. J. Clin. Nutr. 2004, 79, 1128–1129. [Google Scholar] [CrossRef]
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef]
- Charles, J.P. The regulation of expression of insect cuticle protein genes. Insect Biochem. Mol. Biol. 2010, 40, 205–213. [Google Scholar] [CrossRef]
- Zhong, X.W.; Wang, X.H.; Tan, X.; Xia, Q.Y.; Xiang, Z.H.; Zhao, P. Identification and molecular characterization of a chitin deacetylase from Bombyx mori peritrophic membrane. Int. J. Mol. Sci. 2014, 15, 1946–1961. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Xie, K.; Liu, Q.; Li, Y.; Tan, X.; Dong, H.; Li, X.; Dong, Z.; Xia, Q.; et al. Chitin and cuticle proteins form the cuticular layer in the spinning duct of silkworm. Acta Biomater. 2022, 145, 260–271. [Google Scholar] [CrossRef]
- Ichikawa, M.; Ishizaki, H.J.N. Brain hormone of the silkworm, Bombyx mori. Nature 1961, 191, 933–934. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Rybczynski, R.; Warren, J.T. Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 2002, 47, 883–916. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Gu, S.H. Stage-dependent effects of starvation on the growth, metamorphosis, and ecdysteroidogenesis by the prothoracic glands during the last larval instar of the silkworm, Bombyx mori. J. Insect Physiol. 2006, 52, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Marchionni, S.; Sell, C.; Lorenzini, A. Development and Longevity: Cellular and Molecular Determinants—A Mini-Review. Gerontology 2020, 66, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span-From Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Minchin, S.; Lodge, J. Understanding biochemistry: Structure and function of nucleic acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef]
- Wang, S.Y.; Stickle, W.B. Changes in Nucleic-Acid Concentration with Starvation in the Blue-Crab Callinectes sapidus Rathbun. J. Crustac. Biol. 1986, 6, 49–56. [Google Scholar] [CrossRef]
- Ma, T.Y.; Wang, F.S.; Xu, S.J.; Huang, J.H. Meningeal immunity: Structure, function and a potential therapeutic target of neurodegenerative diseases. Brain Behav. Immun. 2021, 93, 264–276. [Google Scholar] [CrossRef]
- Buckley, M.W.; McGavern, D.B. Immune dynamics in the CNS and its barriers during homeostasis and disease. Immunol. Rev. 2022, 306, 58–75. [Google Scholar] [CrossRef]
- Neuvonen, P.T.; Salo, M. Effects of Short-Term Starvation on the Immune-Response. Nutr. Res. 1984, 4, 771–776. [Google Scholar] [CrossRef]
- Song, L.; Bao, X.B.; Liu, Y.; Liu, W.D.; Zhao, S.F.; Liu, S.X. Effect of Heat Starvation Stress on Physiological Immunity and Metabolism of Mizuhopecten yessoensis. Sustainability 2022, 14, 13217. [Google Scholar] [CrossRef]
- Sakyi, M.E.; Cai, J.; Ampofo-Yeboah, A.; Anokyewaa, M.A.; Wang, Z.W.; Jian, J.C. Starvation and re-feeding influence the growth, immune response, and intestinal microbiota of Nile tilapia (Oreochromis niloticus; Linnaeus 1758). Aquaculture 2021, 543, 736959. [Google Scholar] [CrossRef]
- Martin, S.A.; Douglas, A.; Houlihan, D.F.; Secombes, C.J. Starvation alters the liver transcriptome of the innate immune response in Atlantic salmon (Salmo salar). BMC Genom. 2010, 11, 418. [Google Scholar] [CrossRef]
- Beninger, R.J. The role of dopamine in locomotor activity and learning. Brain Res. 1983, 287, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Lu, J.Y.; Sun, Y.M.; Li, L.; Yang, Y.J.; Zhao, J.; Ge, J.J.; Wu, P.; Jiang, J.H.; Wu, J.J.; et al. Dopaminergic Dysfunction and Glucose Metabolism Characteristics in Parkin-Induced Early-Onset Parkinson’s Disease Compared to Genetically Undetermined Early-Onset Parkinson’s Disease. Phenomics 2023, 3, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z.; Feng, W.; Zhao, Y.; Aldanondo, A.; de Brito Sanchez, M.G.; Paoli, M.; Rolland, A.; Li, Z.; Nie, H.; et al. Food wanting is mediated by transient activation of dopaminergic signaling in the honey bee brain. Science 2022, 376, 508–512. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Xie, X.; Liu, Q.; Dong, H.; Hou, Y.; Xia, Q.; Zhao, P. Enhanced locomotor behaviour is mediated by activation of tyrosine hydroxylase in the silkworm brain. Insect Mol. Biol. 2023, 32, 251–262. [Google Scholar] [CrossRef]




| Gene Set | Description | Leading Edge/Size | ES | NES | p-Value | FDR |
|---|---|---|---|---|---|---|
| GO:0009166 | nucleotide catabolic process | 12/19 | 0.75 | 1.99 | 0 | 0.014 |
| GO:1901292 | nucleoside phosphate catabolic process | 12/19 | 0.75 | 1.96 | 0 | 0.014 |
| GO:0042742 | defense response to bacterium | 10/18 | 0.70 | 1.83 | 0 | 0.020 |
| GO:0006955 | immune response | 15/34 | 0.61 | 1.81 | 0 | 0.020 |
| GO:0009150 | purine ribonucleotide metabolic process | 28/50 | 0.57 | 1.83 | 0 | 0.021 |
| GO:0098542 | defense response to other organism | 10/19 | 0.68 | 1.81 | 0.0016 | 0.022 |
| GO:0009607 | response to biotic stimulus | 10/19 | 0.68 | 1.81 | 0.0032 | 0.022 |
| GO:0006753 | nucleoside phosphate metabolic process | 50/101 | 0.49 | 1.79 | 0 | 0.022 |
| GO:0002376 | immune system process | 16/36 | 0.61 | 1.86 | 0 | 0.023 |
| GO:0009117 | nucleotide metabolic process | 50/101 | 0.49 | 1.79 | 0 | 0.023 |
| GO:1901135 | carbohydrate derivative metabolic process | 75/149 | 0.49 | 1.83 | 0 | 0.023 |
| GO:0045087 | innate immune response | 15/30 | 0.62 | 1.84 | 0.0015 | 0.024 |
| GO:0043207 | response to external biotic stimulus | 10/19 | 0.68 | 1.78 | 0.0016 | 0.025 |
| GO:0006091 | generation of precursor metabolites and energy | 21/33 | 0.63 | 1.87 | 0 | 0.026 |
| GO:0046434 | organophosphate catabolic process | 13/27 | 0.66 | 1.88 | 0 | 0.027 |
| GO:0009617 | response to bacterium | 10/18 | 0.70 | 1.84 | 0 | 0.027 |
| GO:0006720 | isoprenoid metabolic process | 11/16 | 0.71 | 1.76 | 0.0016 | 0.028 |
| GO:0019693 | ribose phosphate metabolic process | 35/60 | 0.57 | 1.89 | 0.0014 | 0.030 |
| GO:0051707 | response to other organism | 10/19 | 0.68 | 1.76 | 0.0032 | 0.030 |
| GO:0009152 | purine ribonucleotide biosynthetic process | 24/45 | 0.55 | 1.74 | 0.0044 | 0.035 |
| GO:0044455 | mitochondrial membrane part | 27/42 | 0.60 | 1.88 | 0 | 0.011 |
| GO:0098800 | inner mitochondrial membrane protein complex | 19/30 | 0.64 | 1.90 | 0 | 0.014 |
| GO:0098798 | mitochondrial protein complex | 30/50 | 0.56 | 1.79 | 0.0015 | 0.026 |
| GO:0044429 | mitochondrial part | 70/135 | 0.45 | 1.71 | 0 | 0.069 |
| GO:1990204 | oxidoreductase complex | 11/16 | 0.66 | 1.64 | 0.0047 | 0.12 |
| GO:0044815 | DNA packaging complex | 6/24 | 0.57 | 1.59 | 0.024 | 0.17 |
| GO:0005615 | extracellular space | 21/96 | 0.42 | 1.52 | 0.0094 | 0.17 |
| GO:0098796 | membrane protein complex | 86/163 | 0.39 | 1.51 | 0.0053 | 0.18 |
| GO:0031090 | organelle membrane | 63/126 | 0.41 | 1.52 | 0.0053 | 0.19 |
| GO:0000786 | nucleosome | 6/22 | 0.58 | 1.56 | 0.025 | 0.19 |
| GO:0044421 | extracellular region part | 22/103 | 0.42 | 1.53 | 0.0080 | 0.20 |
| GO:0031966 | mitochondrial membrane | 28/56 | 0.47 | 1.54 | 0.0030 | 0.20 |
| GO:0019866 | organelle inner membrane | 15/38 | 0.48 | 1.47 | 0.036 | 0.21 |
| GO:0005743 | mitochondrial inner membrane | 15/38 | 0.48 | 1.46 | 0.042 | 0.21 |
| GO:0005623 | cell | 27/50 | 0.45 | 1.47 | 0.032 | 0.22 |
| GO:0032993 | protein-DNA complex | 6/23 | 0.53 | 1.44 | 0.043 | 0.23 |
| GO:0042302 | structural constituent of cuticle | 35/139 | 0.64 | 2.42 | 0 | 0 |
| GO:0005198 | structural molecule activity | 64/414 | 0.44 | 1.82 | 0 | 0.06 |
| GO:0005179 | hormone activity | 27/49 | −0.51 | −1.81 | 0 | 0.13 |
| GO:0019200 | carbohydrate kinase activity | 12/32 | 0.57 | 1.68 | 0.0046 | 0.21 |
| MAP04540 | Gap junction | 27/57 | −0.52 | −1.66 | 0.0015 | 0.042 |
| MAP04215 | Apoptosis—multiple species | 7/19 | −0.60 | −1.57 | 0.024 | 0.067 |
| MAP04144 | Endocytosis | 70/149 | −0.38 | −1.44 | 0.019 | 0.14 |
| MAP04310 | Wnt signaling pathway | 49/87 | −0.42 | −1.48 | 0.0069 | 0.10 |
| MAP04012 | ErbB signaling pathway | 24/58 | −0.45 | −1.50 | 0.018 | 0.11 |
| MAP04024 | cAMP signaling pathway | 50/149 | −0.36 | −1.38 | 0.013 | 0.16 |
| MAP04013 | MAPK signaling pathway—fly | 47/129 | −0.37 | −1.40 | 0.022 | 0.17 |
| MAP04071 | Sphingolipid signaling pathway | 37/77 | −0.44 | −1.50 | 0.014 | 0.17 |
| MAP03050 | Proteasome | 28/38 | −0.64 | −1.98 | 0 | 0.0011 |
| MAP04141 | Protein processing in endoplasmic reticulum | 72/131 | −0.50 | −1.87 | 0 | 0.0015 |
| MAP03060 | Protein export | 13/23 | −0.65 | −1.76 | 0.0078 | 0.0095 |
| MAP03010 | Ribosome | 85/121 | 0.32 | 1.40 | 0.016 | 0.12 |
| MAP03020 | RNA polymerase | 16/76 | 0.37 | 1.43 | 0.022 | 0.15 |
| MAP03410 | Base excision repair | 5/22 | 0.49 | 1.48 | 0.044 | 0.21 |
| MAP05012 | Parkinson disease | 67/128 | −0.53 | −1.96 | 0 | 0.00078 |
| MAP05322 | Systemic lupus erythematosus | 14/22 | −0.69 | −1.84 | 0.0016 | 0.0046 |
| MAP05034 | Alcoholism | 35/74 | −0.50 | −1.72 | 0 | 0.015 |
| MAP05410 | Hypertrophic cardiomyopathy (HCM) | 22/62 | 0.48 | 1.77 | 0 | 0.046 |
| MAP04934 | Cushing syndrome | 36/84 | −0.44 | −1.58 | 0.0044 | 0.054 |
| MAP05414 | Dilated cardiomyopathy (DCM) | 14/60 | 0.44 | 1.65 | 0.0031 | 0.066 |
| MAP04932 | Non-alcoholic fatty liver disease (NAFLD) | 65/155 | −0.41 | −1.54 | 0.0038 | 0.069 |
| MAP05203 | Viral carcinogenesis | 65/123 | −0.40 | −1.49 | 0.010 | 0.081 |
| MAP05030 | Cocaine addiction | 16/29 | −0.53 | −1.50 | 0.034 | 0.082 |
| MAP05226 | Gastric cancer | 29/89 | −0.39 | −1.36 | 0.049 | 0.17 |
| MAP05016 | Huntington disease | 98/291 | −0.32 | −1.29 | 0.030 | 0.19 |
| MAP00900 | Terpenoid backbone biosynthesis | 14/28 | 0.67 | 1.92 | 0 | 0.0071 |
| MAP00220 | Arginine biosynthesis | 8/25 | −0.62 | −1.88 | 0 | 0.0073 |
| MAP00030 | Pentose phosphate pathway | 14/26 | 0.67 | 1.84 | 0 | 0.012 |
| MAP00190 | Oxidative phosphorylation | 57/126 | 0.47 | 1.76 | 0 | 0.022 |
| MAP00640 | Propanoate metabolism | 18/28 | 0.59 | 1.71 | 0.0046 | 0.037 |
| MAP00981 | Insect hormone biosynthesis | 5/36 | −0.49 | −1.66 | 0.0027 | 0.039 |
| MAP00051 | Fructose and mannose metabolism | 16/31 | 0.56 | 1.61 | 0.011 | 0.092 |
| MAP00052 | Galactose metabolism | 12/38 | 0.51 | 1.55 | 0.0094 | 0.12 |
| MAP00790 | Folate biosynthesis | 23/44 | 0.49 | 1.56 | 0.016 | 0.13 |
| MAP00380 | Tryptophan metabolism | 12/32 | 0.51 | 1.49 | 0.030 | 0.14 |
| MAP00010 | Glycolysis/Gluconeogenesis | 24/50 | 0.46 | 1.49 | 0.015 | 0.15 |
| MAP00040 | Pentose and glucuronate interconversions | 22/68 | 0.44 | 1.50 | 0.022 | 0.16 |
| MAP00280 | Valine, leucine and isoleucine degradation | 12/39 | 0.47 | 1.44 | 0.048 | 0.17 |
| MAP00062 | Fatty acid elongation | 9/28 | 0.51 | 1.45 | 0.037 | 0.17 |
| MAP04723 | Retrograde endocannabinoid signaling | 43/85 | −0.52 | −1.83 | 0 | 0.022 |
| MAP04916 | Melanogenesis | 35/50 | −0.52 | −1.66 | 0.0059 | 0.057 |
| MAP04714 | Thermogenesis | 86/198 | −0.43 | −1.69 | 0 | 0.067 |
| MAP04912 | GnRH signaling pathway | 28/54 | −0.46 | −1.51 | 0.028 | 0.13 |
| MAP04728 | Dopaminergic synapse | 33/66 | −0.46 | −1.53 | 0.0059 | 0.13 |
| MAP04927 | Cortisol synthesis and secretion | 22/38 | −0.48 | −1.48 | 0.028 | 0.14 |
| MAP04928 | Parathyroid hormone synthesis, secretion and action | 25/47 | −0.47 | −1.49 | 0.026 | 0.14 |
| MAP04721 | Synaptic vesicle cycle | 39/72 | −0.42 | −1.44 | 0.018 | 0.14 |
| MAP04970 | Salivary secretion | 25/55 | −0.43 | −1.43 | 0.038 | 0.15 |
| MAP04740 | Olfactory transduction | 18/28 | −0.51 | −1.46 | 0.044 | 0.15 |
| MAP04926 | Relaxin signaling pathway | 34/77 | −0.44 | −1.54 | 0.016 | 0.16 |
| Gene ID | qPCR FC | RNA-Seq FC | Gene ID | qPCR FC | RNA-Seq FC |
|---|---|---|---|---|---|
| evm.TU.Chr26.279 | 0.91 | 1.36 | evm.TU.Chr01.298 | 0.38 | 0.52 |
| evm.TU.Chr24.417 | 0.42 | 0.93 | evm.TU.Chr04.602 | 0.58 | 0.65 |
| evm.TU.Chr06.225 | 0.74 | 0.66 | evm.model.Chr10.490 | 0.68 | 0.95 |
| evm.TU.Chr11.555 | 2.35 | 1.16 | evm.TU.Chr02.21 | 0.67 | 0.92 |
| evm.TU.Chr22.484 | 0.99 | 1.13 | evm.TU.Chr12.759 | 8.13 | 11.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, X.; Dong, H.; Xia, Q.; Zhao, P. Transcriptomic Analysis of Starvation on the Silkworm Brain. Insects 2023, 14, 658. https://doi.org/10.3390/insects14070658
Li Y, Wang X, Dong H, Xia Q, Zhao P. Transcriptomic Analysis of Starvation on the Silkworm Brain. Insects. 2023; 14(7):658. https://doi.org/10.3390/insects14070658
Chicago/Turabian StyleLi, Yi, Xin Wang, Haonan Dong, Qingyou Xia, and Ping Zhao. 2023. "Transcriptomic Analysis of Starvation on the Silkworm Brain" Insects 14, no. 7: 658. https://doi.org/10.3390/insects14070658
APA StyleLi, Y., Wang, X., Dong, H., Xia, Q., & Zhao, P. (2023). Transcriptomic Analysis of Starvation on the Silkworm Brain. Insects, 14(7), 658. https://doi.org/10.3390/insects14070658






