Ecological Interactions of Predatory Mites, Cheyletus eruditus (Schrank) (Trombidiformes: Cheyletidae) and Cheyletus malaccensis Oudemans, and Prey, Liposcelis decolor (Pearman) (Psocodea: Liposcelididae), under Different Thermo-Hygrometric Regimes
Abstract
:Simple Summary
Abstract
1. Introduction
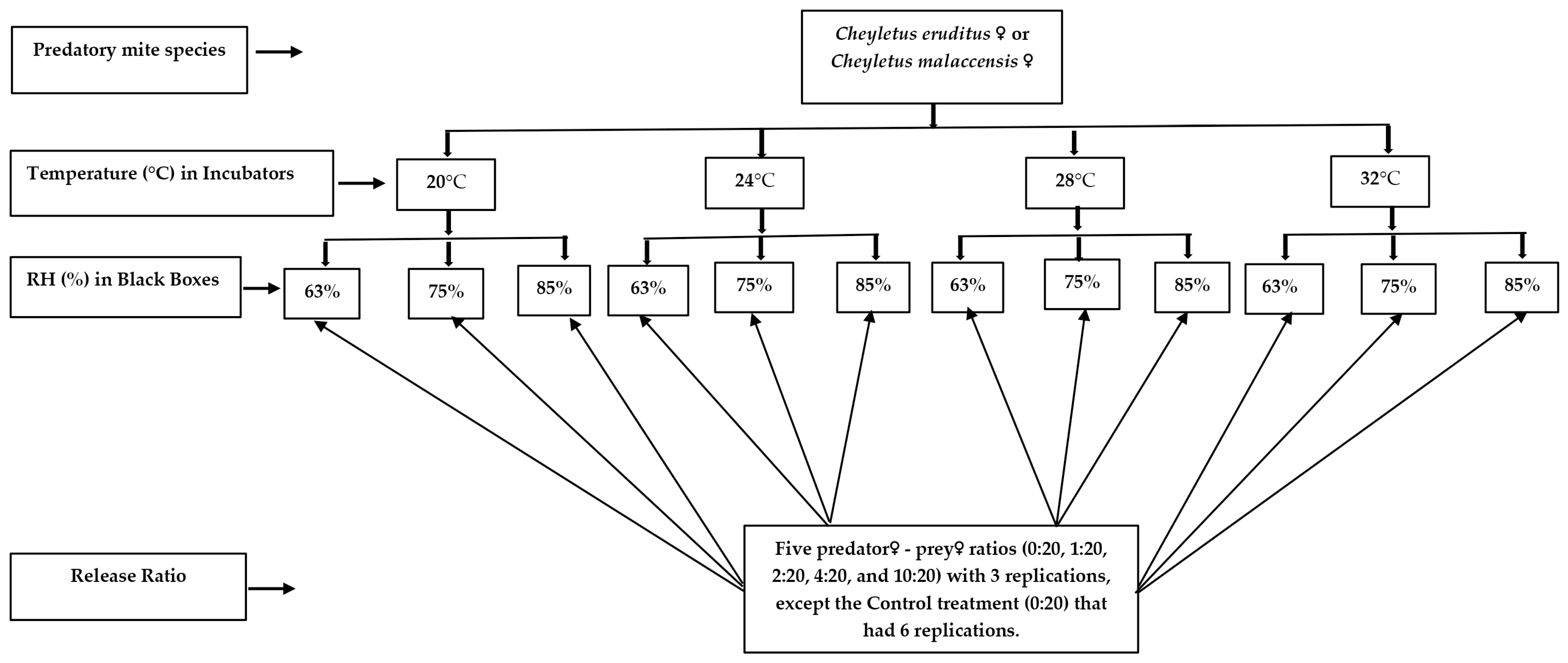
2. Materials and Methods
2.1. Mites Colonies
2.2. Rearing of Predators, Cheyletus eruditus and Cheyletus malaccensis
2.3. Rearing of Prey, Liposcelis decolor
2.4. Experimental Arenas
2.5. Predation and Progeny of Cheyletus eruditus and Cheyletus malaccensis
2.6. Statistical Analysis
3. Results
3.1. Effects of Predator-to-Prey Ratio, Temperature, and RH on Percentage Survival of L. decolor
3.2. Effect of Predator-to-Prey Ratio, Temperature, and RH on C. eruditus and C. malaccensis Progeny Production
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zdarkova, E. Biological control of storage mites by Cheyletus eruditus. Integr. Pest Manag. 1998, 3, 111–116. [Google Scholar] [CrossRef]
- Palyvos, N.E.; Emmanouel, N.G.; Saitanis, C.J. Mites associated with stored products in Greece. Exp. Appl. Acarol. 2008, 44, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Palyvos, N.E. The Cheyletoidea (Prostigmata), with special reference to potential of Cheyletus malaccensis Oudemans as biological control agent of post-harvest pests. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Progress in Biological Control; Springer: Cham, Switzerland, 2015; Volume 19, p. 241. [Google Scholar]
- Mullen, G.R.; OConnor, B.M. Mites (Acari). In Medical and Veterinary Entomology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Sun, W.; Cui, M.; Xia, L.; Yu, Q.; Cao, Y.; Wu, Y. Age-stage, two-sex life tables of the predatory mite Cheyletus malaccensis Oudemans at different temperatures. Insects 2020, 11, 181. [Google Scholar] [CrossRef]
- Danso, J.K.; Opit, G.P.; Goad, C.L.; Noden, B.H.; Giles, K.L. Functional Responses of Predatory Mites, Cheyletus eruditus (Schrank) and Cheyletus malaccensis Oudemans (Trombidiformes: Cheyletidae) to Liposcelis decolor (Pearman) (Psocodea: Liposcelididae). J. Stored Prod. 2023, 103, 102–141. [Google Scholar] [CrossRef]
- Kucerova, Z. Stored product psocids as one of the prey of the predatory mite Cheyletus eruditus (Schrank). IOBC/WPRS Bull. 2004, 27, 191–195. [Google Scholar]
- Leeuwen, E.V.; Jansen, V.A.A.; Bright, P.W. How population dynamics shape the functional response in a one-predator–two-prey system. Ecology 2007, 88, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Fathipour, Y.; Maleknia, B. Mite Predators. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Elsevier: San Diego, CA, USA, 2016; pp. 329–366. [Google Scholar]
- Elhalawanu, A.S.; Afifi, H.A.; Ayad, E.L. Impact of temperature and prey type on biology and life-table parameters of Cheyletus malaccensis Oudemans (Acari: Cheyletidae). Egypt. J. Basic Appl. Sci. 2022, 9, 452–461. [Google Scholar] [CrossRef]
- Rosenheim, J.A. Intraguild predation of Orius tristicolor by Geocoris spp. and the paradox of irruptive spider mite dynamics in California cotton. Biol. Control 2005, 32, 172–179. [Google Scholar] [CrossRef]
- Pakyari, H.; Fathipour, Y.; Rezapanah, M.; Kamali, K. Temperature-dependent functional response of Scolothrips longicornis (Thysanoptera: Thripidae) preying on Tetranychus urticae. J. Asia-Pac. Entomol. 2009, 12, 23–26. [Google Scholar] [CrossRef]
- Farazmand, A.; Fathipour, Y.; Kamali, K. Intraguild predation among Scolothrips longicornis (Thysanoptera: Thripidae), Neoseiulus californicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae) under laboratory conditions. Insect Sci. 2015, 22, 263–272. [Google Scholar] [CrossRef]
- Zhu, P.; Fan, Y.; Mo, W.; Xin, T.; Xia, B.; Zou, Z. Functional response of adult Cheyletus malaccensis (Acari: Cheyletidae) to different developmental stages of Aleuroglyphus ovatus (Acari: Acaridae). J. Stored Prod. 2019, 84, 101–525. [Google Scholar] [CrossRef]
- Santos, M.A. Functional and numerical responses of the predatory mite, Amblyseius fallacis, to prey density. Environ. Entomol. 1975, 4, 989–992. [Google Scholar] [CrossRef]
- Ryoo, M. Studies on the basic component of the predation of Phytoseiulus persimilis (Acari: Phytoseiidae). Res. Pop. Ecol. 1986, 28, 17–26. [Google Scholar] [CrossRef]
- Xiao, Y.; Fadamiro, H.Y. Functional responses and prey-stage preferences of three species of predacious mites (Acari: Phytoseiidae) on citrus red mite, Panonychus citri (Acari: Tetranychidae). Biol. Control 2010, 53, 345–352. [Google Scholar] [CrossRef]
- Kalinoski, R.M.; DeLong, J.P. Beyond body mass: How prey traits improve predictions of functional response parameters. Oecologia 2016, 180, 543–550. [Google Scholar] [CrossRef]
- Uiterwaal, S.F.; Mares, C.; DeLong, J.P. Body size, body size ratio, and prey type influence the functional response of damselfly nymphs. Oecologia 2017, 185, 339–346. [Google Scholar] [CrossRef]
- Solomon, M.E. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Schöller, M.E.; Flinn, P.W.; Grieshop, M.J.; Zd’árková, E. Biological control of stored product pests. In Insect Management for Food Storage and Processing, 2nd ed.; Heaps, J.W., Ed.; American Association of Cereal Chemistry International: St. Paul, MN, USA, 2006; pp. 67–87. [Google Scholar]
- Danso, J.K.; Opit, G.P.; Noden, B.H.; Giles, K.L. Estimating discriminating doses of phosphine for adults of eight species of psocids of genera Liposcelis (Psocodea: Liposcelididae) and Lepinotus (Psocodea: Trogiidae). J. Stored. Prod. 2022, 99, 102025. [Google Scholar] [CrossRef]
- Danso, J.K.; Opit, G.P.; Giles, K.L.; Noden, B.H. Numerical responses of the predatory mites, Cheyletus eruditus (Trombidiformes: Cheyletidae) and Cheyletus malaccensis, to Liposcelis decolor (Psocodea: Liposcelididae). J. Econ. Entomol. 2023, 116, 1447–1457. [Google Scholar] [CrossRef]
- Zd’árková, E. Mass rearing of the predator Cheyletus eruditus (Schrank) (Acarina: Cheyletidae) for biological control of acarid mites infesting stored products. Crop Prot. 1986, 5, 122–124. [Google Scholar] [CrossRef]
- Shen, Z. The biology of stored mites—Cheyletus malaccensis. Grain Storage 1997, 5, 50–51. [Google Scholar]
- Opit, G.P.; Throne, J.E. Population growth and development of the psocid Lepinotus reticulatus at constant temperatures and relative humidities. J. Econ. Entomol. 2008, 101, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Opit, G.P.; Roitberg, B.; Gillespie, D.R. The functional response and prey preference of Feltiella acarisuga (Vollot) (Diptera: Cecidomyiidae) for two of its prey: Male and female two-spotted spider mites, Tetranychus urticae Koch (Acari: Tetranychidae). Can. Entomol. 1997, 129, 221–227. [Google Scholar] [CrossRef]
- Gerson, U.; Ochoa, R.; Smiley, R.L. Mites (Acari) for Pest Control, 2nd ed.; Blackwell Science: Oxford, UK, 2003. [Google Scholar]
- Rizk, G.N.; El-Badry, E.; Hafez, S.M. The effectiveness of predacious and parasitic mites in controlling Tribolium confusum Duv. Mesopotamia J. Agric. 1979, 14, 167–182. [Google Scholar]
- Asanov, K. Predators and parasites of the lesser grain borer Rhyzopertha dominica. Zashchita Rastenii 1980, 5, 23–24. [Google Scholar]
- Nangia, N.; ChannaBasavanna, G.P.; Jagadish, P.S. The biology of Cheyletus malaccensis (Acari: Prostigmata)—A predator of primary acariformes in storage. Curr. Res. Univ. Agric. Sci. Bangalore 1995, 24, 13–15. [Google Scholar]
- Athanassiou, C.G.; Palyvos, N.E. Laboratory evaluation of two diatomaceous earth formulations against Blattisocius keegani Fox (Mesostigmata, Ascidae) and Cheyletus malaccensis Oudemans (Prostigmata, Cheyletidae). Biol. Control 2006, 38, 350–355. [Google Scholar] [CrossRef]
- Cebolla, R.; Pekar, S.; Hubert, J. Prey range of the predatory mite Cheyletus malaccensis (Acari: Cheyletidae) and its efficacy in the control of seven stored-products pests. Biol. Control 2009, 50, 1–6. [Google Scholar] [CrossRef]
- Žd’árková, E.; Horák, E. Preventive biological control of stored food mites in empty stores using Cheyletus eruditus (Schrank). Crop Protect. 1990, 9, 378–382. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Subramanyam, B. Fundamentals of Stored-Product Entomology; AACC International: St. Paul, MN, USA, 2006. [Google Scholar]
- Athanassiou, C.G.; Kavallieratos, N.G.; Sciarretta, A.; Palyvos, N.; Trematerra, P. Spatial associations of insects and mites in stored wheat. J. Econ. Entomol. 2011, 104, 1752–1764. [Google Scholar] [CrossRef]
- Hubert, J.; Mûnzbergova, Z.; Kučerová, Z.; Stejskal, V. Comparison of communities of stored product mites in grain mass and grain residues in the Czech Republic. Exp. Appl. Acarol. 2006, 39, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zdarkova, E.; Fejt, R. Possibilities of biological control of stored product mites. In Proceedings of the 7th International Working Conference on Stored-Product Protection, Beijing, China, 14–19 October 1998; Jin, Z., Liang, Q., Liang, Y., Tan, X., Guan, L., Eds.; Sichuan Publishing House of Science and Technology: Chengdu, China, 1999; pp. 1243–1245. [Google Scholar]
- Opit, G.P.; Ocran, A.; Shakya, K. Population growth and development of Liposcelis obscura Broadhead (Psocodea: Liposcelididae) at constant temperatures and relative humidities. In Proceedings of the 12th International Working Conference on Stored Product Protection (IWCSPP), Berlin, Germany, 7–11 October 2018; Adler, C.S., Opit, G., Fürstenau, B., Müller-Blenkle, C., Kern, P., Arthur, F.H., Athanassiou, C.G., Bartosik, R., Campbell, J., Carvalho, M.O., et al., Eds.; Julius Kuhn Institute: Berlin, Germany, 2018; pp. 151–159. [Google Scholar]
| Variable | Predator | Source | DF | F | p Value |
|---|---|---|---|---|---|
| Prey survival | CE | T | 3, 91.64 | 6.10 | 0.0008 |
| RH | 2, 91.64 | 30.90 | <0.0001 | ||
| T*RH | 6, 91.64 | 5.41 | <0.0001 | ||
| P-P-R | 4, 57.25 | 249.09 | <0.0001 | ||
| T*P-P-R | 12, 80.62 | 7.07 | <0.0001 | ||
| RH*P-P-R | 8, 73.59 | 6.68 | <0.0001 | ||
| T*RH*P-P-R | 24, 87.52 | 1.81 | 0.0250 | ||
| CM | T | 3, 84.59 | 0.42 | 0.7383 | |
| RH | 2, 84.59 | 25.37 | <0.0001 | ||
| T*RH | 6, 84.59 | 1.61 | 0.1539 | ||
| P-P-R | 4, 60.7 | 469.66 | <0.0001 | ||
| T*P-P-R | 12, 80.7 | 12.13 | <0.0001 | ||
| RH*P-P-R | 8, 73.89 | 7.29 | <0.0001 | ||
| T*RH*P-P-R | 24, 87.18 | 1.62 | 0.0539 | ||
| Predator progeny | CE | T | 3, 66.28 | 39.08 | <0.0001 |
| RH | 2, 66.28 | 247.13 | <0.0001 | ||
| T*RH | 6, 66.28 | 8.65 | <0.0001 | ||
| P-P-R | 3, 49.45 | 43.95 | <0.0001 | ||
| T*P-P-R | 9, 62.3 | 6.02 | <0.0001 | ||
| RH*P-P-R | 6, 57.99 | 13.76 | <0.0001 | ||
| T*RH*P-P-R | 18, 66.04 | 3.98 | <0.0001 | ||
| CM | T | 3, 58.69 | 63.85 | <0.0001 | |
| RH | 2, 58.69 | 137.73 | <0.0001 | ||
| T*RH | 6, 58.69 | 8.20 | <0.0001 | ||
| P-P-R | 3, 46.72 | 10.35 | <0.0001 | ||
| T*P-P-R | 9, 58.59 | 3.02 | 0.0050 | ||
| RH*P-P-R | 6, 54.64 | 17.91 | <0.0001 | ||
| T*RH*P-P-R | 18, 61.77 | 4.53 | <0.0001 |
| P | T | RH | P-P-R | ||||
|---|---|---|---|---|---|---|---|
| 0:20 | 1:20 | 2:20 | 4:20 | 10:20 | |||
| CE | 20 | 63 | 35.8 ± 5.23 aH | 21.7 ± 3.33 bDE | 10.0 ± 5.77 bC | 10.0 ± 2.89 bAB | 1.7 ± 1.67 cD |
| 20 | 75 | 59.2 ± 4.90 aDE | 33.3 ± 3.33 bB | 16.7 ± 8.33 cBC | 8.3 ± 4.41 dBC | 5.0 ± 2.89 eBC | |
| 20 | 85 | 74.2 ± 9.08 aCD | 21.7 ± 3.33 bDE | 13.3 ± 4.41 bBC | 8.3 ± 4.41 cBC | 1.7 ± 1.67 dD | |
| 24 | 63 | 42.5 ± 2.14 aGH | 21.7 ± 6.01 bDE | 10.0 ± 5.00 cC | 6.7 ± 4.41 dCD | 6.7 ± 1.67 dBC | |
| 24 | 75 | 63.3 ± 8.82 aDE | 25.0 ± 2.89 bCD | 13.3 ± 1.67 cBC | 6.7 ± 1.67 cB | 3.3 ± 1.67 dCD | |
| 24 | 85 | 65.8 ± 9.87 aDE | 20.0 ± 0.00 bD | 13.3 ± 6.01 bcBC | 5.0 ± 5.00 dD | 6.7 ± 1.67 cB | |
| 28 | 63 | 44.0 ± 3.87 aFG | 23.3 ± 3.33 bD | 13.3 ± 1.67 bBC | 5.0 ± 2.89 cD | 5.0 ± 5.00 cBC | |
| 28 | 75 | 207.5 ± 21.16 aA | 51.7 ± 7.26 bA | 11.7 ± 1.67 cBC | 8.3 ± 1.67 cBC | 8.3 ± 1.67 cAB | |
| 28 | 85 | 95.8 ± 7.90 aBC | 33.3 ± 6.01 bB | 16.7 ± 1.67 cA | 5.0 ± 5.00 dBC | 3.3 ± 3.33 eCD | |
| 32 | 63 | 70.8 ± 10.60 aDE | 16. 7 ± 1.67 bE | 3.3 ± 1.67 cD | 1.7 ± 1.67 dD | 1.7 ± 1.67 dD | |
| 32 | 75 | 234.2 ± 21.07 aA | 58.3 ± 9.28 bA | 16.7 ± 4.41 cA | 13.3 ± 4.41 cA | 10.0 ± 0.00 cA | |
| 32 | 85 | 110.8 ± 8.60 aB | 38.3 ± 4.41 bB | 10.0 ± 5.00 cC | 5.0 ± 5.00 dD | 1.7 ± 1.67 dD | |
| CM | 20 | 63 | 44.2 ± 5.69 aG | 18.3 ± 3.33 bC | 11.7 ± 6.01 cC | 6.7 ± 3.33 cBC | 8.3 ± 3.33 cAB |
| 20 | 75 | 64.2 ± 5.97 aF | 35.0 ± 5.00 bA | 21.7 ± 3.33 bA | 10.0 ± 2.89 cA | 5.00 ± 2.89 dBC | |
| 20 | 85 | 73.3 ± 5.87 aEF | 33.3 ± 1.67 bA | 28.3 ± 10.14 bA | 8.3 ± 3.33 cAB | 6.7 ± 1.67 cAB | |
| 24 | 63 | 58.3 ± 4.41 aFG | 28.3 ± 3.33 bAB | 15.0 ± 2.89 cBC | 6.7 ± 1.67 dAB | 1.7 ± 1.67 dC | |
| 24 | 75 | 95.0 ± 8.06 aDE | 28.3 ± 6.01 bAB | 23.3 ± 6.67 bA | 10.0 ± 5.77 cAB | 1.7 ± 1.67 dC | |
| 24 | 85 | 90.8 ± 7.35 aDE | 36.7 ± 3.33 bA | 28.3 ± 4.41 bA | 10.0 ± 5.77 cAB | 1.7 ± 1.67 dC | |
| 28 | 63 | 60.0 ± 4.08 aFG | 16.7 ± 4.41 bC | 5.0 ± 5.00 cD | 1.7 ± 1.67 cD | 0.0 ± 0.00 dD | |
| 28 | 75 | 165.8 ± 12.07 aB | 38.3 ± 6.01 bA | 20.0 ± 5.00 bAB | 5.0 ± 5.00 cC | 0.0 ± 0.00 dD | |
| 28 | 85 | 115.0 ± 12.78 aCD | 38.3 ± 8.82 bA | 26.7 ± 4.41 bA | 8.3 ± 3.33 cAB | 1.7 ± 1.67 dC | |
| 32 | 63 | 75.8 ± 4.36 aEF | 16.7 ± 4.41 bC | 10.0 ± 2.89 bC | 5.0 ± 5.00 cC | 0.0 ± 0.00 dD | |
| 32 | 75 | 220.8 ± 15.24 aA | 38.3 ± 3.33 bA | 13.3 ± 10.91 cC | 5.00 ± 5.00 dC | 3.3 ± 1.67 dBC | |
| 32 | 85 | 135.0 ± 13.10 aC | 30.0 ± 8.66 bAB | 10.0 ± 5.77 cC | 6.7 ± 3.33 dBC | 10.0 ± 2.896 cdA | |
| P | T | RH | P-P-R | |||
|---|---|---|---|---|---|---|
| 1:20 | 2:20 | 4:20 | 10:20 | |||
| CE | 20 | 63 | 266.7 ± 120.19 aE | 66.7 ± 16.67 bE | 25.0 ± 14.43 bD | 53.3 ± 14.53 bD |
| 20 | 75 | 1366.7 ± 375.65 aBC | 816.7 ± 142.40 bB | 333.3 ± 22.05 cBC | 326.7 ± 48.07 cAB | |
| 20 | 85 | 1200.0 ± 321.46 aBC | 766.7 ± 92.80 bB | 308.3 ± 16.7 cBC | 133.3 ± 14.53 dCD | |
| 24 | 63 | 33.3 ± 33.33 aF | 16.7 ± 16.67 aE | 50.0 ± 28.87 aD | 36.7 ± 8.82 aD | |
| 24 | 75 | 1233.3 ± 392.99 aBC | 1416.7 ± 365.53 aA | 1066.7 ± 130.97 aA | 190.0 ± 60.83 bBC | |
| 24 | 85 | 2800.0 ± 763.76 aA | 1733.3 ± 337.06 bA | 775.0 ± 202.07 cA | 316.7 ± 76.23 dAB | |
| 28 | 63 | 0.0 ± 0.00 aF | 0.0 ± 0.00 aE | 0.0 ± 0.00 aD | 0.0 ± 0.00 aD | |
| 28 | 75 | 1100.0 ± 264.58 aBC | 433.3 ± 60.09 bC | 208.3 ± 50.69 cC | 146.7 ± 37.12 cCD | |
| 28 | 85 | 366.7 ± 66.67 aDE | 366.7 ± 72.65 aCD | 250.0 ± 50.00 aBC | 116.7 ± 17.64 bCD | |
| 32 | 63 | 0.0 ± 0.00 aF | 0.0 ± 0.00 aE | 0.0 ± 0.00 aD | 16.7 ± 16.67 aD | |
| 32 | 75 | 500.0 ± 173.21 aCD | 116.7 ± 72.65 bD | 116.7 ± 104.42 bCD | 386.7 ± 108.37 aA | |
| 32 | 85 | 1266.7 ± 218.58 aBC | 516.7 ± 120.19 bB | 375.0 ± 90.14 bB | 143.3 ± 31.80 cCD | |
| CM | 20 | 63 | 33.3 ± 33.33 cE | 233.3 ± 130.17 aC | 16.7 ± 16.67 cCD | 116.7 ± 14.53 bBC |
| 20 | 75 | 533.3 ± 145.30 aBC | 283.3 ± 33.33 abB | 241.7 ± 44.10 bcB | 180.0 ± 17.32 cAB | |
| 20 | 85 | 766.7 ± 317.98 aB | 666.7 ± 158.99 abAB | 341.7 ± 65.09 bAB | 150.0 ± 36.06 cAB | |
| 24 | 63 | 0.0 ± 00.00 bF | 0.0 ± 0.00 bE | 0.0 ± 0.00 bD | 83.3 ± 23.33 aC | |
| 24 | 75 | 400.0 ± 173.21 aBC | 500.0 ± 144.34 aAB | 416.7 ± 65.09 aA | 196.7 ± 23.33 bAB | |
| 24 | 85 | 1500.0 ± 416.33 aA | 783.3 ± 109.29 bA | 575.00 ± 86.60 bA | 200.0 ± 45.83 cA | |
| 28 | 63 | 0.0 ± 0.00 aF | 0.0 ± 0.00 aE | 0.0 ± 0.00 aD | 0.0 ± 0.00 aD | |
| 28 | 75 | 33.3 ± 33.33 bE | 33.3 ± 33.33 bD | 50.0 ± 25.00 bC | 120.0 ± 17.32 aBC | |
| 28 | 85 | 500.0 ± 152.75 aBC | 200.0 ± 125.83 bC | 166.7 ± 33.33 bBC | 73.3 ± 18.56 cCD | |
| 32 | 63 | 0.0 ± 0.00 aF | 0.0 ± 0.00 aE | 0.0 ± 0.00 aD | 0.0 ± 0.00 aD | |
| 32 | 75 | 200.0 ± 115.47 aCD | 0.0 ± 0.00 cE | 16.7 ± 16.67 bC | 23.3 ± 8.82 bCD | |
| 32 | 85 | 666.7 ± 145.30 aBC | 16.7 ± 16.67 bE | 0.0 ± 0.00 cD | 16.7 ± 12.02 bCD | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danso, J.K.; Opit, G.P.; Giles, K.L.; Noden, B.H. Ecological Interactions of Predatory Mites, Cheyletus eruditus (Schrank) (Trombidiformes: Cheyletidae) and Cheyletus malaccensis Oudemans, and Prey, Liposcelis decolor (Pearman) (Psocodea: Liposcelididae), under Different Thermo-Hygrometric Regimes. Insects 2023, 14, 717. https://doi.org/10.3390/insects14090717
Danso JK, Opit GP, Giles KL, Noden BH. Ecological Interactions of Predatory Mites, Cheyletus eruditus (Schrank) (Trombidiformes: Cheyletidae) and Cheyletus malaccensis Oudemans, and Prey, Liposcelis decolor (Pearman) (Psocodea: Liposcelididae), under Different Thermo-Hygrometric Regimes. Insects. 2023; 14(9):717. https://doi.org/10.3390/insects14090717
Chicago/Turabian StyleDanso, James K., George P. Opit, Kristopher L. Giles, and Bruce H. Noden. 2023. "Ecological Interactions of Predatory Mites, Cheyletus eruditus (Schrank) (Trombidiformes: Cheyletidae) and Cheyletus malaccensis Oudemans, and Prey, Liposcelis decolor (Pearman) (Psocodea: Liposcelididae), under Different Thermo-Hygrometric Regimes" Insects 14, no. 9: 717. https://doi.org/10.3390/insects14090717
APA StyleDanso, J. K., Opit, G. P., Giles, K. L., & Noden, B. H. (2023). Ecological Interactions of Predatory Mites, Cheyletus eruditus (Schrank) (Trombidiformes: Cheyletidae) and Cheyletus malaccensis Oudemans, and Prey, Liposcelis decolor (Pearman) (Psocodea: Liposcelididae), under Different Thermo-Hygrometric Regimes. Insects, 14(9), 717. https://doi.org/10.3390/insects14090717







