Insect Biodiversity in a Prealpine Suburban Hilly Area in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area
2.2. Checklist Update
2.2.1. Pitfall Traps
2.2.2. Car-Mounted Net
2.2.3. Cross-Vane Traps
2.2.4. Light-Trap Captures
2.2.5. Visual Sampling and Direct Captures
2.2.6. Gall Collection
2.3. Analysis of the Community Structure of Carabidae and Staphylinidae Habitats
3. Results
3.1. Checklist Update
3.1.1. Coleoptera
3.1.2. Lepidoptera
3.1.3. Hymenoptera
3.1.4. Hemiptera, Diptera, Neuroptera, Orthoptera
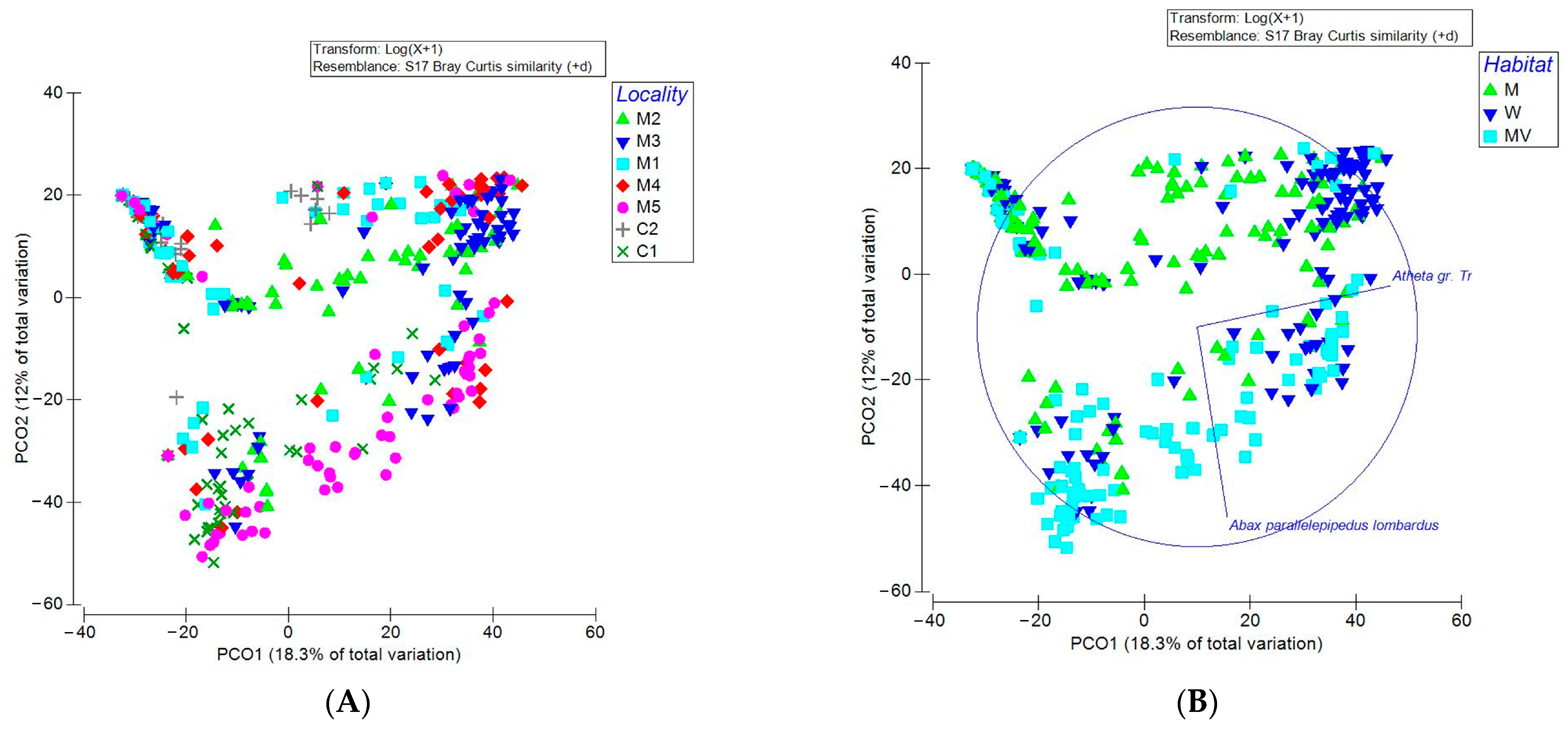
3.2. Analysis of the Community Structure of Carabidae and Staphylinidae
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartel, T.; Dorresteijn, I.; Klein, C.; Máthé, O.; Moga, C.I.; Öllerer, K.; Fischer, J. Wood-pastures in a traditional rural region of Eastern Europe: Characteristics, management and status. Biol. Conserv. 2013, 166, 267–275. [Google Scholar] [CrossRef]
- Plieninger, T.; Hartel, T.; Martín-López, B.; Beaufoy, G.; Bergmeier, E.; Kirby, K.; Montero, M.J.; Moreno, G.; Oteros-Rozas, E.; Van Uytvanck, J. Wood-pastures of Europe: Geographic coverage, social–ecological values, conservation management, and policy implications. Biol. Conserv. 2015, 190, 70–79. [Google Scholar] [CrossRef]
- Carlson, B.Z.; Renaud, J.; Biron, P.E.; Choler, P. Long-term modeling of the forest–grassland ecotone in the French Alps: Implications for land management and conservation. Ecol. Appl. 2014, 24, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.; Kaufmann, R.; Vorhauser, K.; Erschbamer, B. Effects of grazing exclusion on species composition in high-altitude grasslands of the Central Alps. Basic Appl. Ecol. 2009, 10, 447–455. [Google Scholar] [CrossRef]
- Lawrence, J.G.; Colwell, A.; Sexton, O.J. The ecological impact of allelopathy in Ailanthus altissima (Simaroubaceae). Am. J. Bot. 1991, 78, 948–958. [Google Scholar] [CrossRef]
- Kowarik, I.; Säumel, I. Biological flora of central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Kleinbauer, I.; Dullinger, S.; Peterseil, J.; Essl, F. Il cambiamento climatico potrebbe spingere l’albero invasivo Robinia pseudacacia nelle riserve naturali e negli habitat in via di estinzione. Conserv. Biol. 2010, 143, 382–390. [Google Scholar] [CrossRef]
- Mangels, J.; Fiedler, K.; Schneider, F.D.; Blüthgen, N. Diversity and trait composition of moths respond to land-use intensification in grasslands: Generalists replace specialists. Biodivers. Conserv. 2017, 26, 3385–3405. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Fox, R.; Warren, M.S.; Brereton, T.M.; Roy, D.B.; Robinson, A. A new Red List of British butterflies. Insect Conserv. Divers 2011, 4, 159–172. [Google Scholar] [CrossRef]
- Rhodes, C.J. Are insect species imperilled? Critical factors and prevailing evidence for a potential global loss of the entomofauna: A current commentary. Sci. Prog. 2019, 102, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Wagner, D.L.; Van Driesche, R.G. Threats posed to rare or endangered insects by invasions of nonnative species. Annu. Rev. Entomol. 2010, 55, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Jucker, C.; Lupi, D. Exotic insects in Italy: An overview on their environmental impact. In The Importance of Biological Interactions in the Study of Biodiversity; InTech: London, UK, 2011; pp. 51–74. [Google Scholar]
- Waldron, A.; Miller, D.C.; Redding, D.; Mooers, A.; Kuhn, T.S.; Nibbelink, N.; Timmons Roberts, J.; Tobias, J.A.; Gittleman, J.L. Reductions in global biodiversity loss predicted from conservation spending. Nature 2017, 551, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Timonen, J.; Siitonen, J.; Gustafsson, L.; Kotiaho, J.S.; Stokland, J.N.; Sverdrup-Thygeson, A.; Mönkkönen, M. Woodland key habitats in northern Europe: Concepts, inventory and protection. Scand. J. For. Res. 2010, 25, 309–324. [Google Scholar] [CrossRef]
- Miklín, J.; Čížek, L. Erasing a European biodiversity hot-spot: Open woodlands, veteran trees and mature forests succumb to forestry intensification, succession, and logging in a UNESCO Biosphere Reserve. J. Nat. Conserv. 2014, 22, 35–41. [Google Scholar] [CrossRef]
- Giovanetti, M.; Giuliani, C.; Boff, S.; Fico, G.; Lupi, D. A botanic garden as a tool to combine public perception of nature and life-science investigations on native/exotic plants interactions with local pollinators. PLoS ONE 2020, 15, e0228965. [Google Scholar] [CrossRef]
- Duelli, P. Biodiversity evaluation in agricultural landscapes: An approach at two different scales. Agric. Eecosyst. Environ. 1997, 62, 81–91. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Niemelä, J.; Kotze, J.; Ashworth, A.; Brandmayr, P.; Desender, K.; New, T.; Penev, L.; Samways, M.; Spence, J. The search for common anthropogenic impacts on biodiversity: A global network. J. Insect Conserv. 2000, 4, 3–9. [Google Scholar] [CrossRef]
- Elek, Z.; Lövei, G.L. Patterns in ground beetle (Coleoptera: Carabidae) assemblages along an urbanisation gradient in Denmark. Acta Oecologica 2007, 32, 104–111. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Fenoglio, M.S.; Calviño, A.; González, E.; Salvo, A.; Videla, M. Urbanisation drivers and underlying mechanisms of terrestrial insect diversity loss in cities. Ecol. Entomol. 2021, 46, 757–771. [Google Scholar] [CrossRef]
- Ruffo, S.; Stoch, S. Checklist e distribuzione delle specie della fauna Italiana. Mem. Mus. Civ. Stor. Nat. Verona Second. Ser. Sez. Sci. Vita 2005, 16, 1–309. [Google Scholar]
- Grootaert, P.; Pollet, M.; Dekoninck, W.; van Achterberg, C. Sampling insects: General techniques, strategies and remarks. In Manual on Field Recording Techniques and Protocols for All Taxa Biodiversity Inventories and Monitoring; Eymann, J., Degreef, J., Hāuser, J., Monje, C., Samyn, Y., Vanden Spiegel, D., Eds.; Abc Taxa: Bruxelles, Belgium, 2010; pp. 337–399. [Google Scholar]
- Formenti, S. La flora del Monte Maddalena. Comment. Dell’ateneo Brescia 2002, 64, 327–349. [Google Scholar]
- Minelli, S.; Ruffo, S.; Laposta, S. Check List Della Fauna Italiana; Calderini ed.: Bologna, Verona, Italy, 2005; pp. 1–309. [Google Scholar]
- Trematerra, P. Catalogo dei Lepidoptera Tortricidae della fauna italiana. Geonemia, Distribuzione in Italia, note biologiche, identificazione. Boll. Zool. Agrar. Bachic. 2003, 35 (Suppl. S1), 1–270. [Google Scholar]
- de Jong, Y.; Verbeek, M.; Michelsen, V.; Bjørn, P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E.; et al. Fauna Europaea—all European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef]
- Bertaccini, E.; Fiumi, G.; Provera, P. Bombici e Sfingi d’Italia (Lepidoptera); Natura-Giuliano Russo ed.: Bologna, Italy, 1995; Volume 1, pp. 1–248. [Google Scholar]
- Leraut, P. Papillons de Nuit d’Europe, Noctuelles 2; NAP Editeur: Washington, DC, USA, 2019; Volume 6, pp. 1–576. [Google Scholar]
- Mason, F.; Cerretti, P.; Tagliapietra, A.; Speight, M.C.D.; Zapparoli, M. Materiali e metodi. In Invertebrati di Una Foresta della Pianura Padana. Primo Contributo. Conservazione Habitat Invertebrati I; Arcari, G., Ed.; Centro per lo studio e la conservazione della biodiversità forestale, Servizio conservazione della Natura—Corpo Forestale della Stato: Mantova, Italy, 2002; pp. 28–33. [Google Scholar]
- Peck, S.B.; Cook, J. Use of “car-nets” to sample flying micro-Coleoptera. Can. Entomol. 1992, 124, 745–749. [Google Scholar] [CrossRef]
- Ferracini, C.; Bertolino, S.; Bernardo, U.; Bonsignore, C.P.; Faccoli, M.; Ferrari, E.; Lupi, D.; Maini, S.; Mazzon, L.; Nugnes, F.; et al. Do Torymus sinensis (Hymenoptera: Torymidae) and agroforestry system affect native parasitoids associated with the Asian chestnut gall wasp? Biol. Control. 2018, 121, 36–43. [Google Scholar] [CrossRef]
- Pellizzari, G. Guida al Riconoscimento delle Più Comuni Galle della Flora Italiana; Patron Editore: Bologna, Italy, 1998; pp. 9–24. [Google Scholar]
- Rossi, E.; Antichi, D.; Loni, A.; Canovai, R.; Sbrana, M.; Mazzoncini, M. Ground Beetle (Coleoptera: Carabidae) Assemblages and Slug Abundance in Agricultural Fields Under Organic and Low-Input Conventional Management Within a Long-Term Agronomic Trial in Central Italy. Environ. Entomol. 2019, 48, 1377–1387. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial. Plymouth Routine in Multivariate Ecological Research; Plymouth Marine Laboratory: Plymouth, UK, 2006; pp. 1–182. [Google Scholar]
- Lupi, D.; Zanetti, A.; Triberti, P.; Facchini, S.; Rigato, F.; Jucker, C.; Savoldelli, S.; Malabusini, S.; Cortesi, P.; Loni, A. “Check List Parco Colline Lupi et al-1.tab”, Check List Parco Colline Lupi et al. Available Online at UNIMI Dataverse, V3. Available online: https://doi.org/10.13130/RD_UNIMI/S8MW9P/SUWN7Z (accessed on 12 July 2023).
- Zanetti, A.; Sette, A.; Poggi, R.; Tagliapietra, A. Biodiversity of Staphylinidae (Coleoptera) in the Province of Verona (Veneto, Northern Italy). Mem. Soc. Entomol. Ital. 2016, 93, 3–237. [Google Scholar] [CrossRef]
- Facchini, S. I Coleotteri Carabidi negli agroecosistemi della Pianura Padana. Ann. Mus. Civ. St. Nat. Ferrara. 2001, 4, 65–104. [Google Scholar]
- Zanetti, A.; Tagliapietra, A. Studi sulle taxocenosi a Staphylinidae in boschi di latifoglie italiani (Coleoptera, Staphylinidae). Studi Trent. Sci. Nat. Acta Biol. 2004, 81, 207–231. [Google Scholar]
- Lupi, D.; Eördegh, F.R.; Corsi, N.; Rebecchi, M.; Zanetti, A.; Facchini, S.; Colombo, M. Effect of management strategies on rove and ground beetles in a hilly area in Northern Italy. In Landscape Management for Functional Biodiversity, IOBC/WPRS Bulletin; IOBC/WPRS: Gelderland, The Netherlands, 2012; Volume 75, pp. 133–136. [Google Scholar]
- Sutter, R. Neue Arten der Gattung Oegoconia (Autostichidae). Nota Lepid 2007, 30, 189–201. [Google Scholar]
- Fattorini, S.; Mantoni, C.; De Simoni, L.; Galassi, D.M. Island biogeography of insect conservation in urban green spaces. Environ. Conserv. 2018, 45, 1–10. [Google Scholar] [CrossRef]
- Peng, M.H.; Hung, Y.C.; Liu, K.L.; Neoh, K.B. Landscape configuration and habitat complexity shape arthropod assemblage in urban parks. Scient. Rep. 2020, 10, 16043. [Google Scholar] [CrossRef]
- Loreau, M. Le regime alimentaire de Abax ater. Acta Oecol. 1983, 4, 253–263. [Google Scholar]
- Lorreau, M. Annual activity and life cycles of carabid beetles in two forest communities. Holarct. Ecol. 1985, 8, 228–235. [Google Scholar] [CrossRef]
- Forbes, V.; Dugmore, A.G.; Òlafsson, E. The life and death of barn beetles: Faunas from manure and stored hay inside farm buildings in northern Iceland. Ecol. Entomol. 2016, 41, 480–499. [Google Scholar] [CrossRef]
| Locality | Altitude | Habitat Classification | Dominant Species | |
|---|---|---|---|---|
| Maddalena (M) | M1 | 750–800 | Meadow | Bromus erectus Hudson |
| Carex humilis Leyser | ||||
| Dactylis glomerata L. | ||||
| Rubus ulmifolius Schott. | ||||
| M2 | 700 | Meadow | Bromus erectus Hudson | |
| Rubus ulmifolius Schott. | ||||
| M3 | 700 | Wood | Castanea sativa Mill. | |
| M4 | 450 | Wood | Castanea sativa Mill. | |
| Carpinus betulus L. | ||||
| Quercus petraea (Matt.) | ||||
| Rubus ulmifolius Schott. | ||||
| M5 | 450 | Mixed Vegetation | Rosa canina L. | |
| Fraxinus ornus L. | ||||
| Betula pendula L. | ||||
| Populus nigra L. | ||||
| Collebeato (C) | C1 | 335 | Mixed Vegetation | Quercus pubescens Willd. |
| Fraxinus ornus L. | ||||
| Rosa canina L. | ||||
| Rubus ulmifolius Schott. | ||||
| C2 | 320 | Meadow | Bromus sterilis L. | |
| Phleum phleoides (L.) Karsten | ||||
| Bromus erectus Hudson | ||||
| Carex caryophyllea La Tourr. | ||||
| Rubus ulmifolius Schott. | ||||
| Order | Families | No. of Species | Number of Times That the Species Was Detected |
|---|---|---|---|
| Orthoptera | 1 | 1 | 1 |
| Neuroptera | 1 | 1 | 1 |
| Hemiptera | 7 | 9 | 13 |
| Lepidoptera | 30 | 150 | 165 |
| Coleoptera | 26 | 202 | 970 |
| Diptera | 4 | 4 | 4 |
| Hymenoptera | 9 | 42 | 120 |
| Total | 78 | 409 | 1274 |
| SOURCE | DF | SS | MS | PSEUDO-F | P (PERM) | PERMS |
|---|---|---|---|---|---|---|
| HA | 2 | 1.0122E5 | 50611 | 1.3698 | 0.0354 | 9916 |
| CONTRAST (W) V (MV, M) | 1 | 38392 | 38392 | 11.244 | 0.0001 *** | 9909 |
| DI | 1 | 50422 | 50422 | 16.789 | 0.0001 *** | 9930 |
| LO (DI) | 5 | 1.1536E5 | 23071 | 7.522 | 0.0001 *** | 9943 |
| SI (LO (DI)) | 7 | 20864 | 2980.5 | 0.92968 | 0.6649 | 9815 |
| HA × SI (LO (DI)) | 1 | 4935.5 | 4935.5 | 1.5395 | 0.096 | 9936 |
| RES | 410 | 1.3145E6 | 3206 | |||
| TOTAL | 426 | 1.6073E6 |
| GROUPS | T | P (PERM) | UNIQUE PERMS |
|---|---|---|---|
| MV, W | 3.2345 | 0.001 *** | 9999 |
| MV, M | 1.266 | 0.04 | 9966 |
| W, M | 3.816 | 0.001 *** | 9998 |
| S | N | J’ | H (Log e) | ||
|---|---|---|---|---|---|
| Locality | M1 | 30 | 388 | 0.61 | 2.10 |
| M2 | 45 | 1309 | 0.44 | 1.67 | |
| M3 | 58 | 3754 | 0.24 | 0.90 | |
| M4 | 35 | 614 | 0.40 | 1.44 | |
| M5 | 54 | 1060 | 0.54 | 2.20 | |
| C1 | 47 | 437 | 0.71 | 2.73 | |
| C2 | 22 | 110 | 0.82 | 2.53 | |
| Habitat | Mixed Vegetation | 70 | 1807 | 0.51 | 2.16 |
| Wood | 68 | 4368 | 0.26 | 1.10 | |
| Meadow | 75 | 1497 | 0.60 | 2.58 |
| Habitat | Species | Average Abundance | Average Similarity | Similarity /SD | Contribution % | Cumulative % |
|---|---|---|---|---|---|---|
| Meadow | Atheta gr. trinotata | 0.66 | 3.44 | 0.37 | 32.28 | 32.28 |
| Silpha carinata | 0.55 | 2.88 | 0.26 | 26.99 | 59.28 | |
| Atheta oblita | 0.19 | 0.92 | 0.17 | 8.58 | 67.86 | |
| Hister quadrimaculatus | 0.13 | 0.73 | 0.11 | 6.80 | 74.66 | |
| Carabus (Tomocarabus) convexus convexus | 0.18 | 0.58 | 0.14 | 5.45 | 80.11 | |
| Abax parallelepipedus lombardus | 0.15 | 0.47 | 0.15 | 4.45 | 84.56 | |
| Liparus dirus | 0.08 | 0.38 | 0.08 | 3.56 | 88.11 | |
| Omalium rivulare | 0.26 | 0.30 | 0.12 | 2.81 | 90.92 | |
| Woods | Atheta gr. trinotata | 1.70 | 10.59 | 0.60 | 59.70 | 59.70 |
| Silpha carinata | 0.58 | 2.93 | 0.30 | 16.51 | 76.22 | |
| Abax parallelepipedus lombardus | 0.26 | 1.04 | 0.22 | 5.86 | 82.07 | |
| Omalium rivulare | 0.43 | 0.75 | 0.22 | 4.25 | 86.32 | |
| Echinodera capiomonti | 0.08 | 0.39 | 0.08 | 2.21 | 88.53 | |
| Kyklioacalles aubei | 0.07 | 0.37 | 0.08 | 2.08 | 90.61 | |
| Mixed Vegetation | Abax parallelepipedus lombardus | 1.07 | 10.21 | 0.73 | 60.41 | 60.41 |
| Atheta gr. trinotata | 0.67 | 3.37 | 0.38 | 19.94 | 80.35 | |
| Abax baenningeri | 0.24 | 0.63 | 0.20 | 3.72 | 84.07 | |
| Drusilla canaliculata | 0.23 | 0.51 | 0.16 | 3.03 | 87.10 | |
| Omalium rivulare | 0.25 | 0.35 | 0.14 | 2.07 | 89.18 | |
| Protaetia morio | 0.04 | 0.22 | 0.05 | 1.31 | 90.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupi, D.; Zanetti, A.; Triberti, P.; Facchini, S.; Rigato, F.; Jucker, C.; Malabusini, S.; Savoldelli, S.; Cortesi, P.; Loni, A. Insect Biodiversity in a Prealpine Suburban Hilly Area in Italy. Insects 2023, 14, 727. https://doi.org/10.3390/insects14090727
Lupi D, Zanetti A, Triberti P, Facchini S, Rigato F, Jucker C, Malabusini S, Savoldelli S, Cortesi P, Loni A. Insect Biodiversity in a Prealpine Suburban Hilly Area in Italy. Insects. 2023; 14(9):727. https://doi.org/10.3390/insects14090727
Chicago/Turabian StyleLupi, Daniela, Adriano Zanetti, Paolo Triberti, Sergio Facchini, Fabrizio Rigato, Costanza Jucker, Serena Malabusini, Sara Savoldelli, Paolo Cortesi, and Augusto Loni. 2023. "Insect Biodiversity in a Prealpine Suburban Hilly Area in Italy" Insects 14, no. 9: 727. https://doi.org/10.3390/insects14090727







