Developmental Indicators of Chrysomya nigripes Aubertin under Different Constant Temperature Conditions and an Application Case for Estimating the PMImin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source
2.2. Laboratory Population Establishment
2.3. Species Identification
2.3.1. Morphological Identification
2.3.2. Molecular Identification
2.4. Observation of the Egg Stage at Different Temperatures
2.5. Changes in Developmental Duration and Body Length at Different Temperatures
2.6. Data Analysis
3. Results
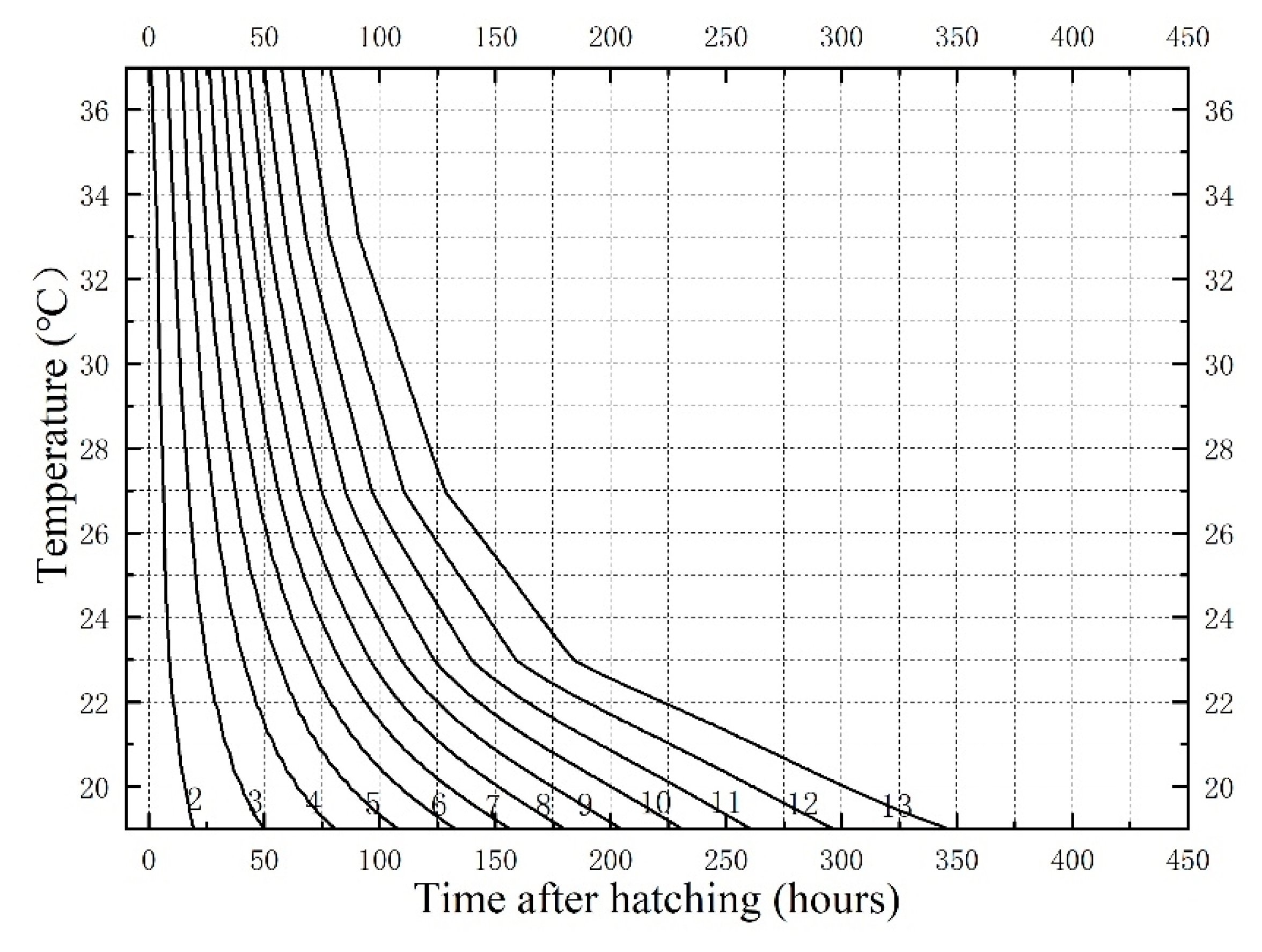
3.1. Developmental Duration and Isomorphen Diagram
3.2. Linear Thermal Summation Model and Optim SSI Model
3.3. Larval Body Length Variation and Isomegalen Diagram
3.4. The Application of C. nigripes in PMImin Estimation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, W.; Adkins, T.; Catts, E. The use of Synthesiomyia nudesita (Van Der Wulp) (Diptera: Muscidae) and Calliphora vicina (Robineau-Desvoidy) (Diptera: Calliphoridae) to estimate the time of death of a body buried under a house. J. Agric. Entomol. 1992, 9, 227–235. [Google Scholar]
- Smith, K.G.V. A Manual of Forensic Entomology; British Museum (Natural History): London, UK, 1986; pp. 1–205. [Google Scholar]
- Hu, C.; Min, J.; Wang, J. Forensic Entomology; Chongqing Press: Chongqing, China, 2000; pp. 1–6. [Google Scholar]
- HB, J.; LC, J. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Zhao, H.; Liu, C. Advanced Forensic Medicine, 3rd ed.; Zhengzhou University Press: Zhengzhou, China, 2021. [Google Scholar]
- Wang, J. Practical Forensic Entomology; Xi’an Jiaotong University Press: Xi’an, China, 2019; pp. 8–24. [Google Scholar]
- Vanin, S.; Gherardi, M.; Bugelli, V.; Di Paolo, M. Insects found on a human cadaver in central Italy including the blowfly Calliphora loewi (Diptera, Calliphoridae), a new species of forensic interest. Forensic Sci. Int. 2011, 207, e30–e33. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Frederickx, C.; Verheggen, F.J.; Boxho, P.; Haubruge, E. Forensic entomology investigations from Doctor Marcel Leclercq (1924–2008): A review of cases from 1969 to 2005. J. Med. Entomol. 2013, 50, 935–954. [Google Scholar] [CrossRef] [PubMed]
- Syamsa, R.; Omar, B.; Zuha, R.; Faridah, M.; Hidayatulfathi, O.; Shahrom, A. Forensic entomology of high-rise buildings in Malaysia: Three case reports. Trop. Biomed. 2015, 32, 291–299. [Google Scholar]
- Lutz, L.; Zehner, R.; Verhoff, M.A.; Bratzke, H.; Amendt, J. It is all about the insects: A retrospective on 20 years of forensic entomology highlights the importance of insects in legal investigations. Int. J. Legal. Med. 2021, 135, 2637–2651. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Sanford, M.R. Is PMI the Hypothesis or the Null Hypothesis? J. Med. Entomol. 2017, 54, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.H.U.O.; Butler, J.F. Effects of temperature on Cochliomyia macellaria (Diptera: Calliphoridae) development. J. Med. Entomol. 1996, 33, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.H.; Butler, J.F. Effects of temperature on Sarcophaga haemorrhoidalis (Diptera: Sarcophagidae) development. J. Med. Entomol. 1998, 35, 694–698. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef]
- Lord, W.; Goff, M.; Adkins, T.; Haskell, N. The black soldier fly Hermetia illucens (Diptera: Stratiomyidae) as a potential measure of human postmortem interval: Observations and case histories. J. Forensic Sci. 1994, 39, 215–222. [Google Scholar] [CrossRef]
- Sukontason, K.; Narongchai, P.; Kanchai, C.; Vichairat, K.; Sribanditmongkol, P.; Bhoopat, T.; Kurahashi, H.; Chockjamsai, M.; Piangjai, S.; Bunchu, N.; et al. Forensic entomology cases in Thailand: A review of cases from 2000 to 2006. Parasitol. Res. 2007, 101, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Silahuddin, S.A.; Latif, B.; Kurahashi, H.; Walter, D.E.; Heo, C.C. The importance of habitat in the ecology of decomposition on rabbit carcasses in Malaysia: Implications in forensic entomology. J. Med. Entomol. 2015, 52, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Syamsa, R.A.; Omar, B.; Ahmad, F.M.S.; Hidayatulfathi, O.; Shahrom, A.W. Comparative fly species composition on indoor and outdoor forensic cases in Malaysia. J. Forensic Leg. Med. 2017, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.L.; Kanchai, C.; Piangjai, S.; Boonsriwong, W.; Bunchu, N.; Sripakdee, D.; Chaiwong, T.; Kuntalue, B.; Siriwattanarungsee, S.; Sukontason, K. Morphological observation of puparia of Chrysomya nigripes (Diptera: Calliphoridae) from human corpse. Forensic Sci. Int. 2006, 161, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z. The Identification Keys of Common Flies in China; Science Press: Beijing, China, 1992; pp. 872–877. [Google Scholar]
- O’Flynn, M.A. The succession and rate of development of blowflies in carrion in Southern Queensland and the application of these data to forensic entomology. Aust. J. Entomol. 1983, 22, 12. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Wang, J.; Ma, M.; Lai, Y. Temperature-dependent development and the significance for estimating postmortem interval of Chrysomya nigripes Aubertin, a new forensically important species in China. Int. J. Legal. Med. 2016, 130, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Ikemoto, T.; Takai, K. A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ. Entomol. 2000, 29, 671–682. [Google Scholar] [CrossRef]
- Ikemoto, T.; Kiritani, K. Novel method of specifying low and high threshold temperatures using thermodynamic SSI model of insect development. Environ. Entomol. 2019, 48, 479–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yang, L.; Tao, L.; Wang, J. Development of Chrysomya megacephala at constant temperatures within its colony range in Yangtze River Delta region of China. Forensic Sci. Res. 2018, 3, 74–82. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, G.; Zhang, Y.; Wang, M.; Amendt, J.; Wang, J. Development of Muscina stabulans at constant temperatures with implications for minimum postmortem interval estimation. Forensic Sci. Int. 2019, 298, 71–79. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, M.A. Notes on the Biology of Chrysomya nigripes Aubertin (Diptera: Calliphoridae). Aust. J. Entomol. 1983, 22, 341–342. [Google Scholar] [CrossRef]
- O’Flynn, M.; Moorhouse, D.E. Species of Chrysomya as primary flies in carrion. Aust. J. Entomol. 1979, 18, 31–32. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, A.H. A preliminary study on the decomposition and dipteran associated with exposed carcasses in an oil palm plantation in Bandar Baharu, Kedah, Malaysia. Trop. Biomed. 2009, 26, 1–10. [Google Scholar]
- Sukontason, K.L.; Vogtsberger, R.C.; Boonchu, N.; Chaiwong, T.; Sripakdee, D.; Ngern-klun, R.; Piangjai, S.; Sukontason, K. Larval morphology of Chrysomya nigripes (Diptera: Calliphoridae), a fly species of forensic importance. J. Med. Entomol. 2005, 42, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.L.; Chaiwong, T.; Piangjai, S.; Upakut, S.; Moophayak, K.; Sukontason, K. Ommatidia of blow fly, house fly, and flesh fly: Implication of their vision efficiency. Parasitol. Res. 2008, 103, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Szpila, K.; Wallman, J.F. Morphology and identification of first instar larvae of Australian blowflies of the genus Chrysomya of forensic importance. Acta Trop. 2016, 162, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Ren, L.P.; Leng, J.; Shang, Y.J.; Zhu, G.H. The complete mitochondrial genome of Chrysomya nigripes (Diptera: Calliphoridae). Mitochondrial DNAB 2019, 4, 4012–4013. [Google Scholar] [CrossRef]
- Gruner, S.V.; Slone, D.H.; Capinera, J.L.; Turco, M.P. Development of the Oriental latrine Fly, Chrysomya megacephala (Diptera: Calliphoridae), at five constant temperatures. J. Med. Entomol. 2017, 54, 290–298. [Google Scholar] [CrossRef]
- Yanmanee, S.; Husemann, M.; Benbow, M.E.; Suwannapong, G. Larval development rates of Chrysomya rufifacies Macquart, 1842 (Diptera: Calliphoridae) within its native range in South-East Asia. Forensic Sci. Int. 2016, 266, 63–67. [Google Scholar] [CrossRef]
- Marchenko, M.I. Medicolegal relevance of cadaver entomofauna for the determination of the time of death. Forensic Sci. Int. 2001, 120, 89–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Liao, M.; Kang, C.; Hu, G.; Guo, Y.; Wang, Y.; Wang, J. Development of Megaselia scalaris at constant temperatures and its significance in estimating the time of death. Int. J. Leg. Med. 2023, 1–10. [Google Scholar] [CrossRef]
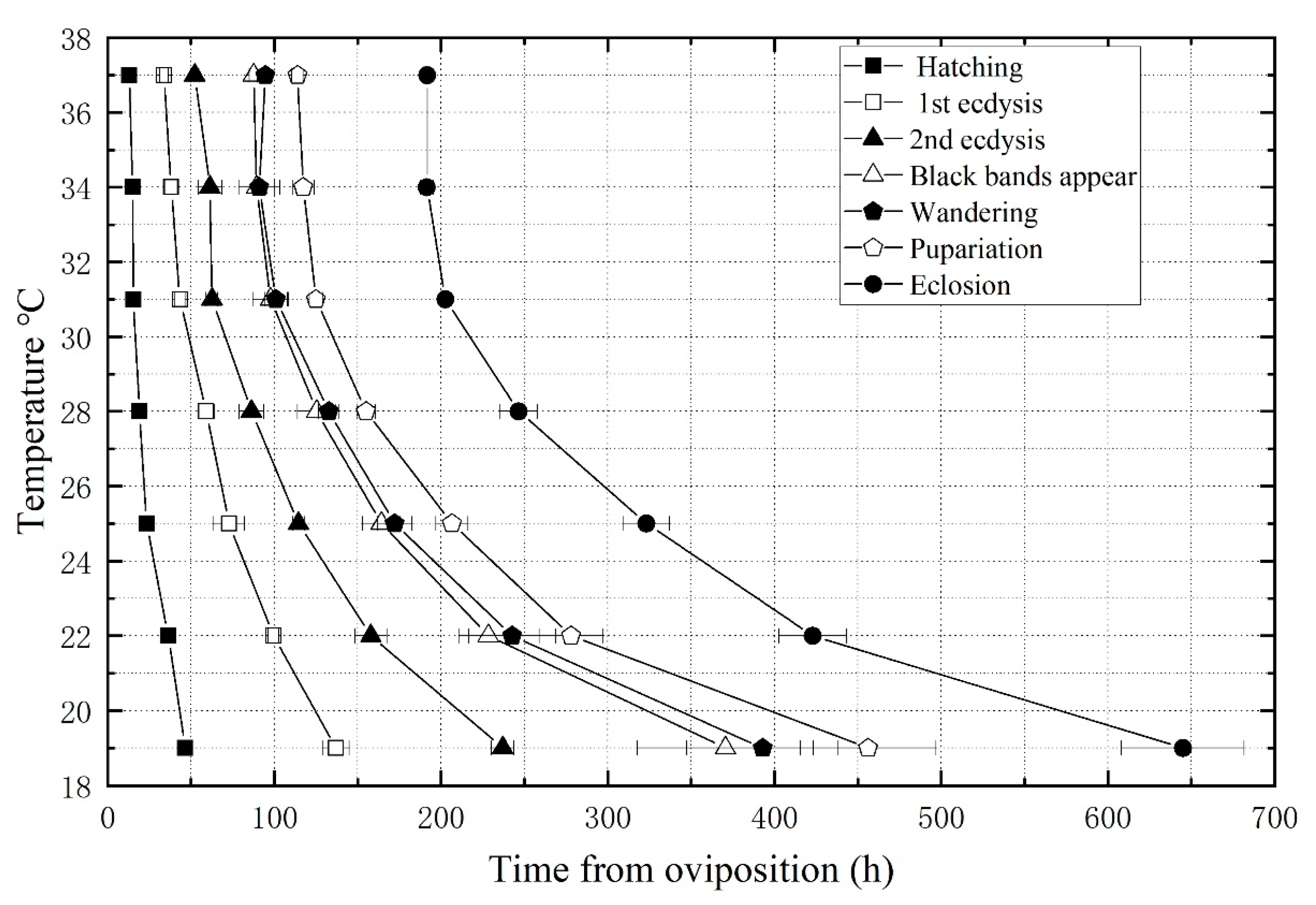
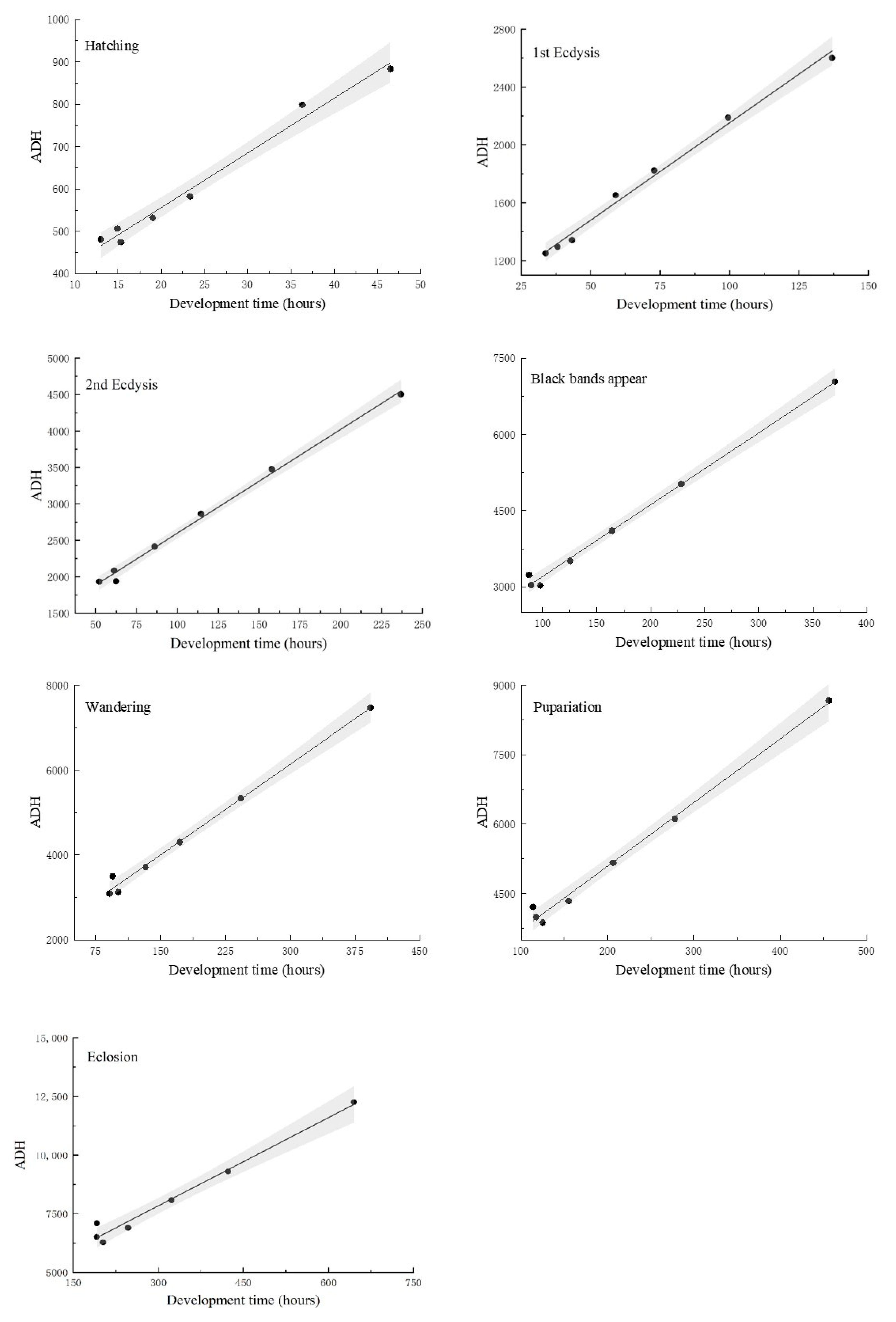
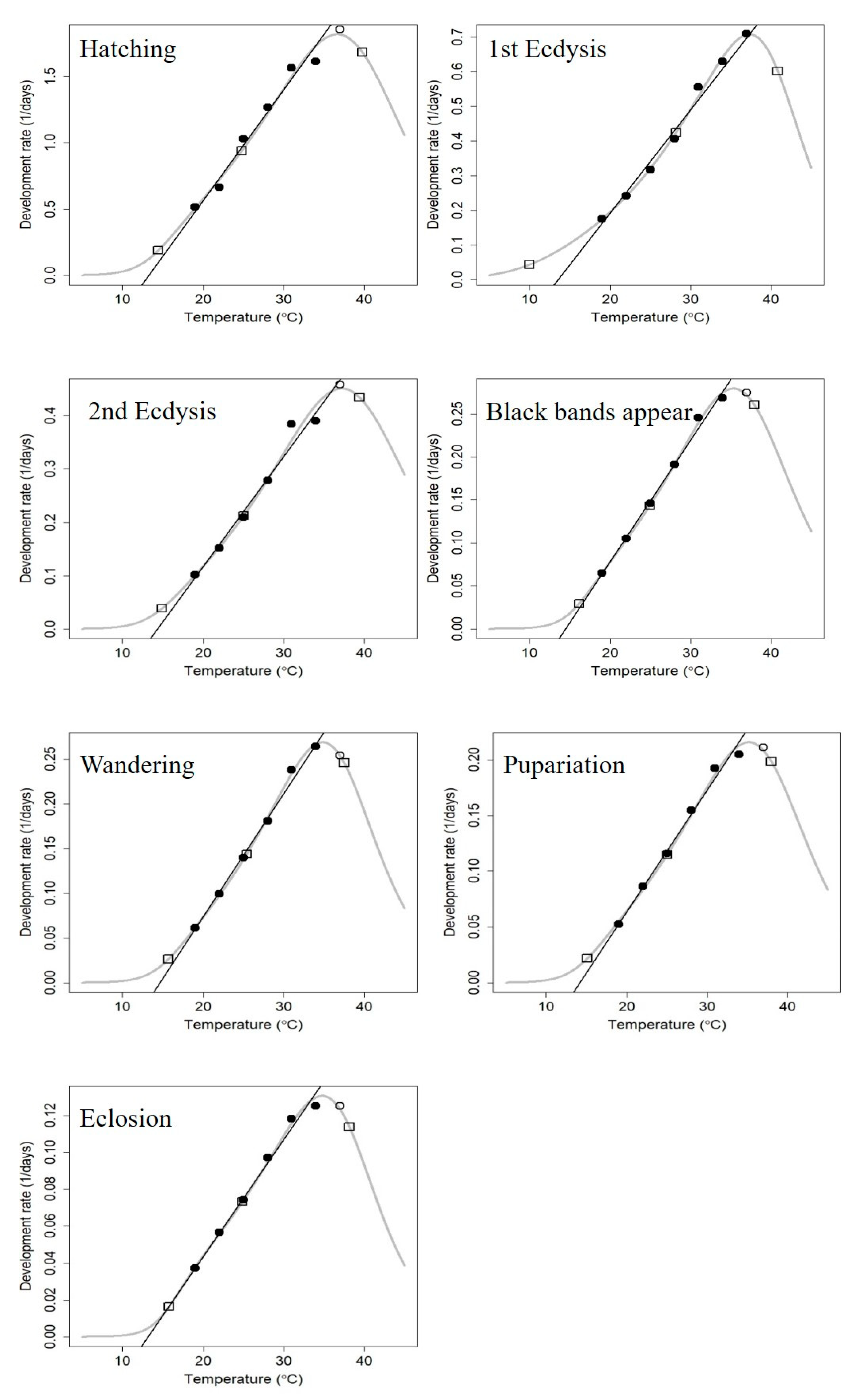
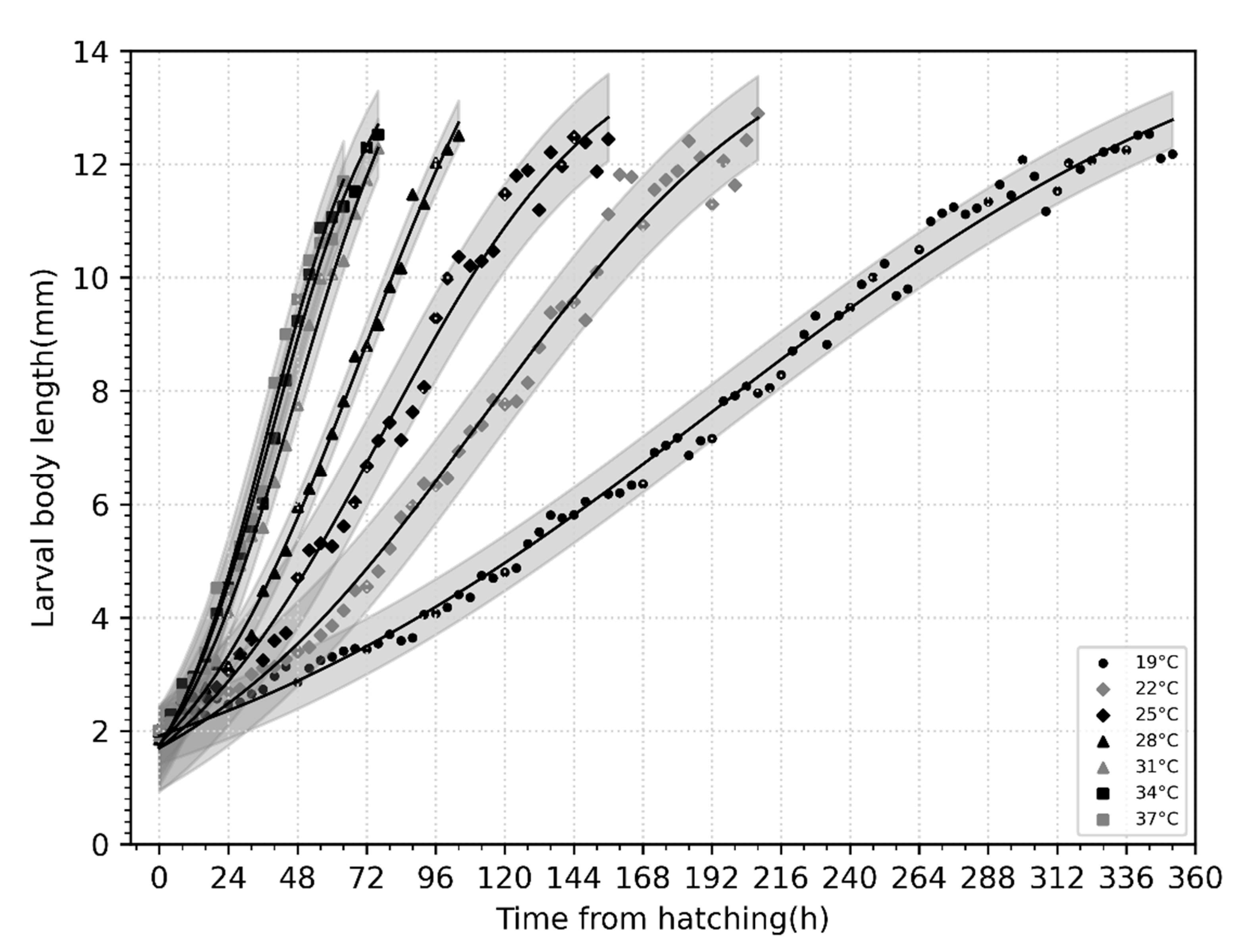


| Developmental Events | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 19 °C | 22 °C | 25 °C | 28 °C | 31 °C | 34 °C | 37 °C | |
| Hatching | 46.5 ± 2.4 | 36.3 ± 0.4 | 23.3 ± 0.2 | 19.0 ± 0.8 | 15.3 ± 1.2 | 14.9 ± 0.7 | 13.0 ± 1.2 |
| First ecdysis | 136.9 ± 8.0 | 99.5 ± 3.3 | 72.9 ± 9.2 | 59.0 ± 4.8 | 43.3 ± 3.2 | 38.1 ± 3.8 | 33.8 ± 2.6 |
| Second ecdysis | 236.9 ± 6.7 | 157.9 ± 9.5 | 114.5 ± 3.5 | 86.2 ± 7.3 | 62.5 ± 3.3 | 61.3 ± 7.1 | 52.2 ± 2.6 |
| Black bands appear | 370.5 ± 52.7 | 228.3 ± 17.6 | 164.1 ± 11.2 | 125.4 ± 11.5 | 97.7 ± 10.6 | 89.3 ± 10.6 | 87.4 ± 2.5 |
| Wandering | 392.9 ± 45.4 | 242.7 ± 25.9 | 172.1 ± 10.3 | 132.6 ± 6.0 | 100.9 ± 6.9 | 90.9 ± 12.3 | 94.6 ± 2.2 |
| Pupariation | 456.1 ± 40.8 | 277.9 ± 19.0 | 206.5 ± 9.7 | 155.0 ± 5.6 | 124.9 ± 3.9 | 117.3 ± 6.5 | 113.8 ± 3.4 |
| Eclosion | 644.9 ± 36.8 a | 422.9 ± 20.1 b | 323.1 ± 13.9 c | 246.6 ± 11.2 d | 202.5 ± 1.8 e | 191.5 ± 3.8 e | 191.8 ± 2.0 e |
| Developmental Stage | K ± SE (Degree Hours) | TL ± SE (°C) | R2 |
|---|---|---|---|
| Hatching | 298.02 ± 19.58 | 12.91 ± 0.73 ab | 0.98 |
| First ecdysis | 806.38 ± 39.21 | 13.46 ± 0.51 bc | 0.99 |
| First ecdysis | 1174.29 ± 55.43 | 14.24 ± 0.44 c | 0.99 |
| Black bands appear | 1792.52 ± 87.52 | 14.13 ± 0.46 c | 0.99 |
| Wandering | 1868.06 ± 116.42 | 14.24 ± 0.57 c | 0.99 |
| Pupariation | 2340.76 ± 135.44 | 13.76 ± 0.57 c | 0.99 |
| Eclosion | 4083.00 ± 293.39 | 12.52 ± 0.83 a | 0.97 |
| Parameter (Unit) | Hatching | First Ecdysis | First Ecdysis | Black Bands Appear | Wandering | Pupariation | Eclosion |
|---|---|---|---|---|---|---|---|
| TΦ (°C) | 24.78 | 28.17 | 25.03 | 24.93 | 25.40 | 25.04 | 24.88 |
| ρΦ (day−1) | 0.97 | 0.44 | 0.22 | 0.15 | 0.15 | 0.12 | 0.75 |
| ∆HA (cal/mol) | 1.48 × 104 | 1.45 × 104 | 1.70 × 104 | 1.72 × 104 | 1.77 × 104 | 1.66 × 104 | 1.49 × 104 |
| ∆HL (cal/mol) | −7.30 × 104 | −3.69 × 104 | −6.95 × 104 | −8.63 × 104 | −7.55 × 104 | −7.37 × 104 | −9.29 × 104 |
| ∆HH (cal/mol) | 5.03 × 104 | 6.77 × 104 | 4.85 × 104 | 5.67 × 104 | 6.25 × 104 | 5.75 × 104 | 6.34 × 104 |
| TL (°C) | 14.42 | 9.98 | 14.89 | 16.15 | 15.67 | 15.09 | 15.76 |
| TH (°C) | 39.75 | 40.85 | 39.42 | 37.93 | 37.50 | 38.00 | 38.15 |
| χ2 | 2.43 × 10−2 | 1.89 × 10−3 | 4.81 × 10−3 | 3.51 × 10−4 | 2.29 × 10−4 | 8.81 × 10−4 | 3.32 × 10−4 |
| R2 | 0.980 | 0.996 | 0.983 | 0.998 | 0.999 | 0.994 | 0.995 |
| Model | |
|---|---|
| (1) | |
| (2a) | |
| (2b) | |
| (2c) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Hu, G.; Li, L.; Liao, M.; Wang, J.; Wang, Y.; Tao, L. Developmental Indicators of Chrysomya nigripes Aubertin under Different Constant Temperature Conditions and an Application Case for Estimating the PMImin. Insects 2023, 14, 729. https://doi.org/10.3390/insects14090729
Guo Y, Hu G, Li L, Liao M, Wang J, Wang Y, Tao L. Developmental Indicators of Chrysomya nigripes Aubertin under Different Constant Temperature Conditions and an Application Case for Estimating the PMImin. Insects. 2023; 14(9):729. https://doi.org/10.3390/insects14090729
Chicago/Turabian StyleGuo, Yi, Gengwang Hu, Liangliang Li, Mingqing Liao, Jiangfeng Wang, Yu Wang, and Luyang Tao. 2023. "Developmental Indicators of Chrysomya nigripes Aubertin under Different Constant Temperature Conditions and an Application Case for Estimating the PMImin" Insects 14, no. 9: 729. https://doi.org/10.3390/insects14090729





