Integrated Effect of Plastic Mulches and Biorational Insecticides in Managing Tomato Chlorotic Spot Virus (TCSV) and Its Vector Thrips in Tomatoes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Time and Location of the Study, Plant Material, and Field Preparation
2.2. Plastic Mulches, Insecticide Treatments, and Experimental Design
2.3. Evaluation of Biorational Insecticides and Plastic Mulch Treatments
2.3.1. Sample Collection and Processing for Thrips Separation
2.3.2. Marketable Yield
2.4. Statistical Analysis
3. Results
3.1. Correlation of the Abundance of Thrips, Marketable Yield, Number of Marketable Fruits, and Incidence of TCSV in Tomatoes
3.2. The Abundance of Thrips in Tomatoes Based on Leaf Sample
3.3. The Abundance of Thrips in Tomatoes Based on Flower Sample
3.4. Marketable Yield
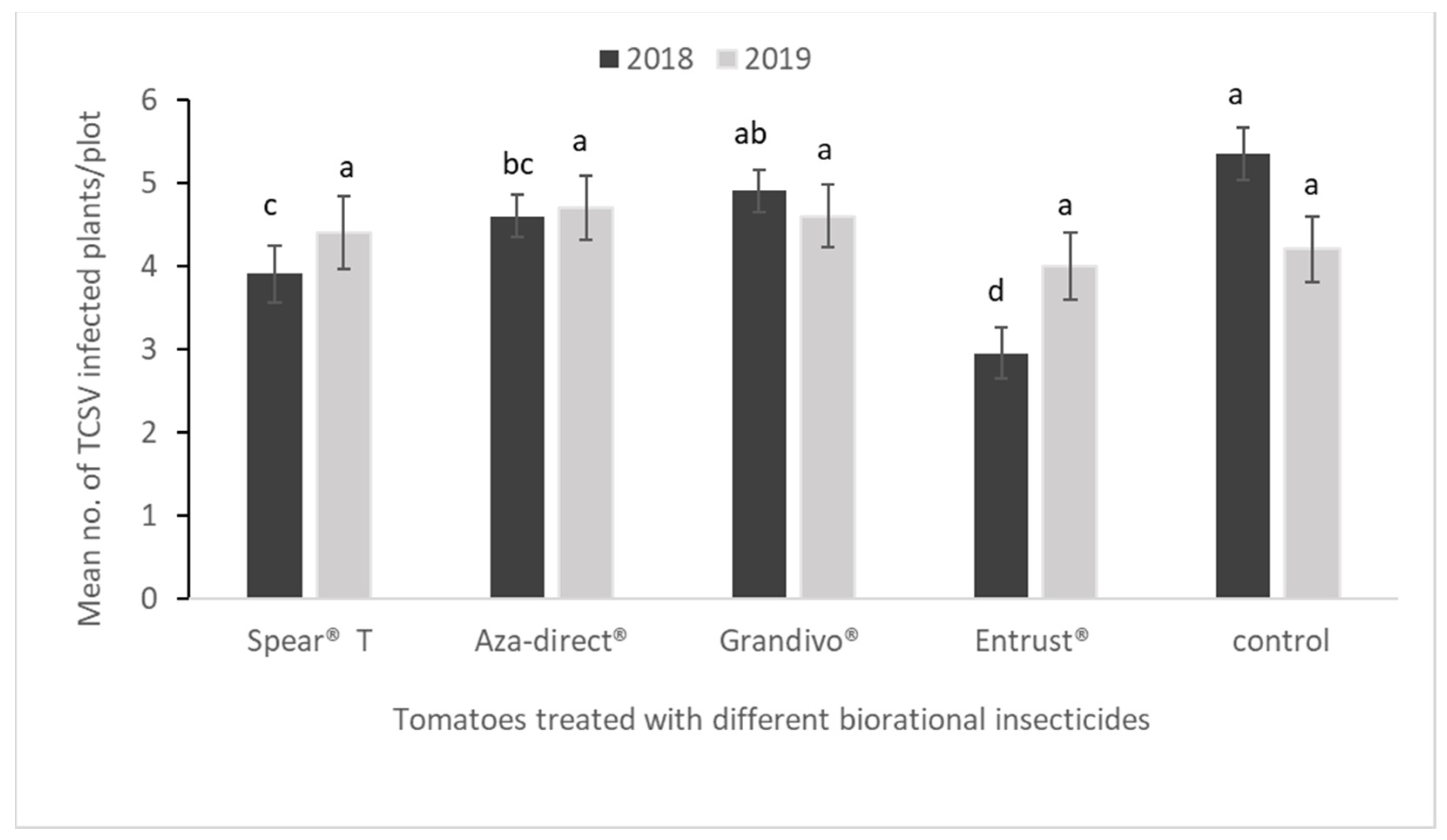
3.5. Incidence of TCSV
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Londoño, A.; Capobianco, H.; Zhang, S.; Polston, J.E. First record of Tomato chlorotic spot virus in the USA. Trop. Plant Pathol. 2012, 37, 333–338. [Google Scholar] [CrossRef]
- Polston, J.E.; Wood, E.; Palmateer, A.J.; Zhang, S. Tomato Chlorotic Spot Virus. In UF/IFAS Cooperative Extension Service Fact Sheet PP306; University of Florida: Gainesville, FL, USA, 2013; Available online: http://edis.ifas.ufl.edu/pp306 (accessed on 1 May 2013).
- Moriones, E.; Aramburu, J.; Riudavets, J.; Arno, J.; Lavina, A. Effect of plant age at time of infection by tomato spotted wilt tospovirus on the yield of field-grown tomato. Eur. J. Plant Pathol. 1998, 104, 295–300. [Google Scholar] [CrossRef]
- Poudel, B.; Abdalla, O.A.; Liu, Q.; Wang, Q.; McAvoy, E.; Seal, D.R.; Ling, K.S.; McGrath, M.; Zhang, S. Field distribution and disease incidence of tomato chlorotic spot virus, an emerging virus threatening tomato production in South Florida. Trop. Plant Pathol. 2019, 44, 430–437. [Google Scholar] [CrossRef]
- Khan, R.A.; Dakshina, D.R.; Zhang, S.; Oscar, O.E.; Srinivasan, R.; Evans, E. Distribution Pattern of Thrips (Thysanoptera: Thripidae) and Tomato Chlorotic Spot Virus in South Florida Tomato Fields. Environ. Entomol. 2020, 49, 73–87. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Li, R.; Ling, K.S.; Miller, S.A. First report of Tomato chlorotic spot virus infecting tomatoes in Ohio. Plant Dis. 2015, 99, 163. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; McGrath, M.T.; Zhang, S.; Wu, Z.; Ling, K.S. First report of tomato chlorotic spot virus infecting tomato in New York. Plant Dis. 2018, 102, 460. [Google Scholar] [CrossRef]
- de Borbón, C.M.; Gracia, O.; Piccolo, R. Relationship between tospovirus incidence and thrips populations on tomato in Mendoza, Argentina. J. Phytopathol. 2006, 154, 93–99. [Google Scholar] [CrossRef]
- Kakkar, G.; Seal, D.R.; Stansly, P.A.; Liburd, O.E.; Kumar, V. Abundance of Frankniella schultzei (Thysnoptera: Thripidae) in flowers on major vegetable crops of South Florida. Fla. Entomol. 2012, 95, 468–475. [Google Scholar] [CrossRef]
- Webster, C.G.; de Jensen, C.E.; Rivera-Vargas, L.I.; Rodrigues, J.C.V.; Mercado, W.; Frantz, G.; Mellinger, H.C.; Adkins, S. First report of Tomato chlorotic spot virus (TCSV) in tomato, pepper, and jimsonweed in Puerto Rico. Plant Health Prog. 2013, 14, 47. [Google Scholar] [CrossRef]
- de Jensen, C.E.; Adkins, S. First report of Tomato chlorotic spot virus in lettuce in Puerto Rico. Plant Dis. 2014, 98, 1015–1016. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, X.; Fu, Y.; Wang, Q.; McAvoy, E.; Seal, D.R. Field Evaluation of Tomato Cultivars for Tolerance to Tomato Chlorotic Spot Tospovirus. Plant Health Prog. 2019, 20, 77–82. [Google Scholar] [CrossRef]
- Robb, K.L.; Newman, J.; Virzi, J.K.; Parrella, M.P. Insecticide resistance in western flower thrips. In Thrips Biology and Management; Springer: Boston, MA, USA, 1995; pp. 341–346. [Google Scholar]
- Dutcher, J.D. A review of resurgence and replacement causing pest outbreaks in IPM. In General Concepts in Integrated Pest and Disease Management; Springer: Dordrecht, The Netherlands, 2007; pp. 27–43. [Google Scholar]
- Regan, K.; Ordosch, D.; Glover, K.D.; Tilmon, K.J.; Szczepaniec, A. Effects of a pyrethroid and two neonicotinoid insecticides on population dynamics of key pests of soybean and abundance of their natural enemies. Crop Prot. 2017, 98, 24–32. [Google Scholar] [CrossRef]
- Serrano, R.; Simal-Julián, Á.; Pitarch, E.; Hernández, F.; Varó, I.; Navarro, J.C. Biomagnification study on organochlorine compounds in marine aquaculture: The sea bass (Dicentrarchus labrax) as a model. Environ. Sci. Technol. 2003, 37, 3375–3381. [Google Scholar] [CrossRef] [PubMed]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef]
- Perring, T.M.; Gruenhagen, N.M.; Farrar, C.A. Management of plant viral diseases through chemical control of insect vectors. Annu. Rev. Entomol. 1999, 44, 457–481. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.A.; Todd, J.W.; Weeks, J.R.; Gorbet, D.W.; Culbreath, A.K.; Luke-Morgan, A.S.; Fletcher, S.M.; Brown, S.L. A regional study to evaluate tillage, row patterns, in-furrow insecticide, and planting date on the yield, grade, and tomato spotted wilt virus incidence of the Georgia Green peanut cultivar. Proc. Annu. Southern Conserv. Tillage Conf. Sustain. Agric. 2001, 24, 26–34. [Google Scholar]
- Culbreath, A.K.; Tillman, B.L.; Gorbet, D.W.; Holbrook, C.C.; Nischwitz, C. Response of new field-resistant peanut cultivars to twin-row pattern or in-furrow applications of phorate for management of spotted wilt. Plant Dis. 2008, 92, 1307–1312. [Google Scholar] [CrossRef]
- Gallo-Meagher, M.; Changalrayan, K.; Davis, J.M.; McDonald, G.E. Phorate-induced peanut genes that may condition acquired resistance to tomato spotted wilt. Proc. Am. Peanut Res. Ed. Soc. 2001, 33, 29. [Google Scholar]
- Blacker, A.M.; Kelly, I.D.; Lantz, J.L.; Mihlan, G.J.; Jones, R.L.; Young, B.M. Aldicarb: Toxicity, exposure and risks to humans. In Hayes’ Handbook of Pesticide Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1619–1632. [Google Scholar] [CrossRef]
- Bayer CropScience. Bayer CropScience Announces Decision Not to Resume MIC Production; Bayer CropScience: Leverkusen, Germany, 2011. [Google Scholar]
- Eger, J.E.; Stavisky, J.; Funderburk, J.E. Comparative toxicity of spinosad to Frankliniella spp. (Thysanoptera: Thripidae), with notes on a bioassay technique. Fla. Entomol. 1998, 81, 547–551. [Google Scholar] [CrossRef]
- Cloyd, R.A. Western flower thrips (Frankliniella occidentalis) management on ornamental crops grown in greenhouses: Have we reached an impasse. Pest. Technol. 2009, 3, 1–9. [Google Scholar]
- Bielza, P.; Quinto, V.; Contreras, J.; Torné, M.; Martín, A.; Espinosa, P.J. Resistance to spinosad in the western flower thrips, Frankliniella occidentalis (Pergande), in greenhouses of south-eastern Spain. Pest Manag. Sci. 2007, 63, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Culbreath, A.K.; Todd, T.W.; Brown, S.L. Epidemiology and management of tomato spotted wilt in peanut. Annu. Rev. Phytopathol. 2003, 41, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Momol, M.T.; Olson, S.M.; Funderburk, J.E.; Stavisky, J.; Marois, J.J. Integrated management of tomato spotted wilt on field-grown tomatoes. Plant Dis. 2004, 88, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.; Herron, G.A. Potential new insecticides for the control of western flower thrips (Thysanoptera: Thripidae) on sweet pepper, tomato, and lettuce. J. Econ. Entomol. 2009, 102, 646–651. [Google Scholar] [CrossRef]
- Kay, I.R.; Herron, G.A. Evaluation of existing and new insecticides including spirotetramat and pyridalyl to control Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) on peppers in Queensland. J. Econ. Entomol. 2010, 49, 175–181. [Google Scholar] [CrossRef]
- Kring, J.B.; Schuster, D.J. Management of insects on pepper and tomato with UV-reflective mulches. Fla. Entomol. 1992, 85, 9–14. [Google Scholar] [CrossRef]
- Stavisky, J.; Funderburk, J.; Brodbeck, B.V.; Olson, S.M.; Andersen, P.C. Population dynamics of Frankliniella spp. and tomato spotted wilt incidence as influenced by cultural management tactics in tomato. J. Econ. Entomol. 2002, 95, 1216–1221. [Google Scholar] [CrossRef]
- Lamont, W.J. Plastics: Modifying the microclimate for the production of vegetable crops. HortTechnology 2005, 15, 477–481. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Borgemeister, C.; Max, J.; Poehling, H.M. Manipulation of ultraviolet light affects immigration behavior of Ceratothripoides claratris (Thysanoptera: Thripidae). J. Econ. Entomol. 2009, 102, 1559–1566. [Google Scholar] [CrossRef]
- Razzak, M.A.; Seal, D.R.; Stansly, P.A.; Liburd, O.E.; Schaffer, B. Host Preference and Plastic Mulches for Managing Melon Thrips (Thysanoptera: Thripidae) on Field-Grown Vegetable Crops. Environ. Entomol. 2019, 48, 434–443. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Distribution, abundance and population dynamics. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, UK, 1997; pp. 217–257. [Google Scholar]
- Iglesias, L.; Havey, M.J.; Nault, B.A. Management of onion thrips (Thrips tabaci) in organic onion production using multiple IPM tactics. Insects 2021, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Schalk, J.M.; Creighton, C.S.; Fery, R.L.; Sitterly, W.R.; Davis, B.W.; McFadden, T.L.; Day, A. Reflective film mulches influences insect control and yield in vegetables. J. Am. Soc. of Hort. Sci. 1979, 104, 759–762. [Google Scholar] [CrossRef]
- Csizinszky, A.A.; Schuster, D.J.; Kring, J.B. Color mulches influence yield and insect pest populations in tomatoes. J. Am. Soc. Hort. Sci. 1995, 120, 778–784. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Q.; Zhang, S. Outbreaks of Tomato Chlorotic Spot Tospovirus in Commercial Tomato Fields and Effectiveness of Different Management Measures in South Florida. Plant Health Prog. 2020, 21, 188–193. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; Batal, K.D.; Granberry, D.; Bertrand, D.; Giddings, D.; Pappu, H. Growth and yield of tomato on plastic film mulches as affected by tomato spotted wilt virus. HortScience 2003, 38, 395–399. [Google Scholar] [CrossRef]
- Adeleye, V.O.; Seal, D.R.; Liburd, O.O.; Martini, X.; Meru, G. Integrated approach using insecticides in combination with reflective plastic mulch for the management of pepper weevil, Anthonomus eugenii (Coleoptera: Curculionidae). Environ. Entomol. 2023, 52, 391–398. [Google Scholar] [CrossRef]
- Summers, C.G.; Newton, A.S.; Mitchell, J.P.; Stapleton, J.J. Population dynamics of arthropods associated with early-season tomato plants as influenced by soil surface microenvironment. Crop Prot. 2010, 29, 249–254. [Google Scholar] [CrossRef]
- Riley, D.G.; Joseph, S.V.; Srinivasan, R. Reflective mulch and acibenzolar-S-methyl treatments relative to thrips (Thysanoptera: Thripidae) and tomato spotted wilt virus incidence in tomato. J. Econ. Entomol. 2012, 105, 1302–1310. [Google Scholar] [CrossRef]
- Noble, C.V.; Drew, R.W.; Slabaugh, V. Soil Survey of Dade County Area, Florida; U.S. Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 1996. [Google Scholar]
- Seal, D.R.; Baranowaski, R.M. Effectiveness of different insecticides for control of Thrips palmi Karny (Thysanoptera: Thripidae) affecting vegetables in south Florida. Proc. Fla. State Hortic. Soc. 1992, 105, 315–319. [Google Scholar]
- Nakahara, S. Annotated list of the Frankliniella species ofthe world (Thysanoptera: Thripidae). Contrib. Entomol. Int. 1997, 2, 355–386. [Google Scholar]
- Cavalleri, A.; Mound, L.A. Toward the identification of Frankliniella species in Brazil (Thysanoptera, Thripidae). Zootaxa 2012, 3270, 1–30. [Google Scholar] [CrossRef]
- USDA. Tomatoes-Shipping Point and Market Inspection Instructions; USDA: Washington, DC, USA, 2005.
- Poudel, B.; Huang, Y.; Zhang, Z. First report of Tomato chlorotic spot virus infecting common beans (Phaseolus vulgaris) in the United States. Plant Dis. 2018, 102, 1467. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT 9.3 User’s Guide; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Sakimura, K. Frankliniella fusca, an additional vector for the tomato spotted wilt virus, with notes on Thrips tabaci, another vector. Phytopathology 1963, 53, 412–415. [Google Scholar]
- Cho, J.J.; Mau, R.F.L.; German, T.L.; Hartman, R.W.; Yudin, L.S.; Gonsalves, D.; Provvidenti, R. A multidisciplinary approach to management of tomato spotted wilt virus in Hawaii. Plant Dis. 1989, 73, 375–383. [Google Scholar] [CrossRef]
- Ullman, D.E.; Sherwood, J.L.; German, T.L. Thrips as vectors of plant pathogens. In Thrips as Vectors of Plant Pathogens; Lewis, T.S., Ed.; Thrips as Crop Pests; CAB International: Wallingford, UK, 1997; pp. 539–565. [Google Scholar]
- Zhao, G.; Liu, W.E.I.; Brown, J.M.; Knowles, C.O. Insecticide resistance in field and laboratory strains of western flower thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 1995, 88, 1164–1170. [Google Scholar] [CrossRef]
- Greenough, D.R.; Black, L.L.; Bond, E.P. Aluminum-surfaced mulch: An approach to control of Tomato spotted wilt virus in solanaceous crops. Plant Dis. 1990, 74, 805–808. [Google Scholar] [CrossRef]
- Marrone Bio Innovations. Grandevo. 2012. Available online: http://www.marronebioinnovations.com/products/brand/grandevo/ (accessed on 13 May 2013).
- Mordue, A.J.; Blackwell, A. Azadirachtin: An update. J. Insect Physiol. 1993, 39, 903–924. [Google Scholar] [CrossRef]
- Salgado, V.L. Studies on the mode of action of Spinosad: Insect symptoms and physiological correlates. Pestic. Biochem. Physiol. 1998, 60, 91–102. [Google Scholar] [CrossRef]
- Funderburk, J.; Stavisky, J.; Olson, S. Predation of Frankliniella occidentalis (Thysanoptera: Thripidae) in field peppers by Orius insidiosus (Hemiptera: Anthocoridae). Environ. Entomol. 2000, 29, 376–382. [Google Scholar] [CrossRef]
- Premachandra, D.W.; Borgemeister, C.; Poehling, H.M. Effects of neem and spinosad on Ceratothripoides claratris (Thysanoptera: Thripidae), an important vegetable pest in Thailand, under laboratory and greenhouse conditions. J. Econ. Entomol. 2005, 98, 438–448. [Google Scholar] [CrossRef]
- Broughton, S.; Herron, G.A. Management of western flowers thrips, ’Frankliniella occidentalis’ (Pergande) (Thysanoptera: Thripidae) on strawberries. Gen. Appl. Entomol. J. Entomol. Soc. N. S. Wales 2009, 38, 37–41. [Google Scholar]
- Kivett, J.M.; Cloyd, R.A.; Bello, N.M. Insecticide rotation programs with entomopathogenic organisms for suppression of western flower thrips (Thysanoptera: Thripidae) adult populations under greenhouse conditions. J. Econ. Entomol. 2015, 108, 1936–1946. [Google Scholar] [CrossRef]
- Jones, T.; Scott-Dupree, C.; Harris, R.; Shipp, L.; Harris, B. The efficacy of spinosad against the western flower thrips, Frankliniella occidentalis, and its impact on associated biological control agents on greenhouse cucumbers in southern Ontario. Pest Manag. Sci. 2005, 61, 179–185. [Google Scholar] [CrossRef]
- Vafaie, E.K.; Rydzak, P.M. Insecticidal Control of Western Flower Thrips, 2015. Arthropod Manag. Tests 2017, 42, tsx091. [Google Scholar] [CrossRef]
- Bilbo, T.R.; Schoof, S.C.; Walgenbach, J.F. Foliar insecticide efficacy against western flower thrips in staked tomato, 2019. Arthropod Manag. Tests 2020, 45, tsaa063. [Google Scholar] [CrossRef]
- Brown, S.L.; Brown, J.E. Effect of plastic mulch color and insecticides on thrips populations and damage to tomato. HortTechnol. 1992, 2, 208–211. [Google Scholar] [CrossRef]
- Scott, S.J.; McLeod, P.J.; Montgomery, F.W.; Hander, C.A. Influence of reflective mulch on incidence of thrips (Thysanoptera: Thripidae: Phlaeothripidae) in staked tomatoes. J. Entomol. Sci. 1989, 24, 422–427. [Google Scholar] [CrossRef]
- Terry, L.I. Host selection, communication and reproductive behaviour. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, UK, 1997; pp. 65–118. [Google Scholar]
- Lamont, W.J., Jr. Plastic mulches for the production of vegetable crops. HortTechnology 1993, 3, 35–39. [Google Scholar] [CrossRef]
- Bielza, P. Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag. Sci. 2008, 64, 1131–1138. [Google Scholar] [CrossRef]
- Schalk, J.M.; Robbins, M.L.R. Reflective mulches influence plant survival, production, and insect control in fall tomatoes. HortScience 1987, 22, 30–32. [Google Scholar] [CrossRef]
- Adkins, S.; Zitter, T.; Momol, T. Tospoviruses (Family Bunyaviridae, Genus Tospovirus). University of Florida. EDIS Publ. 2009, PP212. Available online: http://edis,ifas.ufl.edu (accessed on 1 October 2005).
- Ham, J.M.; Kluitenberg, G.J.; Lamont, W.J. Optical properties of plastic mulches affect the field temperature regime. J. Amer. Soc. Hort. Sci. 1993, 118, 188–193. [Google Scholar] [CrossRef]
- Riley, D.G.; Pappu, H.R. Evaluation of tactics for management of thrips-vectored tomato spotted wilt virus in tomato. Plant Dis. 2000, 84, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.M.; Stall, W.M.; Vallad, G.E.; Webb, S.E.; Smith, S.A.; Simonne, E.H.; McAvoy, E.J.; Santos, B.M.; Ozores-Hampton, M. Tomato Production in Florida; EDIS. University of Florida Extension Circular; HS739; University of Florida/IFAS: Gainesville, FL, USA, 2012. [Google Scholar]
- Kumar Krishna, N.K.; Ullman, D.E.; Cho, J.J. Evaluation of Lycopersicon germplasm for tomato spotted wilt tospovirus resistance by mechanical and thrips transmission. Plant Dis. 1993, 77, 938–941. [Google Scholar] [CrossRef]
- Saidi, M.; Warade, S.D. Tomato breeding for resistance to Tomato spotted wilt virus (TSWV): An overview of conventional and molecular approaches. Czech. J. Genet. Plant Breed. 2008, 44, 83–92. [Google Scholar] [CrossRef]
- Riley, D.G.; Joseph, S.V.; Kelley, W.T.; Olson, S.; Scott, J. Host plant resistance to Tomato spotted wilt virus (Bunyaviridae: Tospovirus) in tomato. HortScience 2011, 46, 1626–1633. [Google Scholar] [CrossRef]
- Thomas-Carroll, M.L.; Jones, R.A.C. Selection, biological properties and fitness of resistance-breaking strains of Tomato spotted wilt virus in pepper. Ann. Appl. Biol. 2003, 142, 235–243. [Google Scholar] [CrossRef]
- Aramburu, J.; Marti, M. The occurrence in north-east Spain of a variant of tomato spotted wilt virus (TSWV) that breaks resistance in tomato (Lycopersicon esculentum) containing the Sw-5 gene. Plant Pathol. 2003, 52, 407. [Google Scholar] [CrossRef]
- Ciuffo, M.; Finetti-Sialer, M.M.; Gallitelli, D.; Turina, M. First report in Italy of a resistance-breaking strain of Tomato spotted wilt virus infecting tomato cultivars carrying the Sw5 resistance gene. Plant Pathol. 2005, 54, 564. [Google Scholar] [CrossRef]
- Bauske, E.M.; Zehnder, G.M.; Sikora, E.J.; Kemble, J. Southeastern tomato growers adopt integrated pest management. HortTechnol 1998, 8, 40–44. [Google Scholar] [CrossRef]
- Reitz, S.R.; Yearby, E.L.; Funderburk, J.E.; Stavisky, J.; Momol, M.T.; Olson, S.M. Integrated management tactics for Frankliniella thrips (Thysanoptera: Thripidae) in field-grown peppers. J. Econ. Entomol. 2003, 96, 1201–1214. [Google Scholar] [CrossRef]
- Riley, D.G.; Pappu, H.R. Tactics for management of thrips (Thysanoptera: Thripidae and tomato spotted wilt virus in tomato. J. Econ. Entomol. 2004, 97, 1648–1658. [Google Scholar] [CrossRef] [PubMed]

| Variables | Adult Thrips in Leaves | Thrips Larvae in Leaves | Adult Thrips in Flowers | Thrips Larvae in Flowers | TCSV | Marketable Yield | No of Marketable Fruits | |
|---|---|---|---|---|---|---|---|---|
| Adult thrips in leaves | 0.7657, <0.0001 | 0.8791, <0.0001 | 0.6682, <0.0001 | 0.3851, <0.0001 | −0.2689, 0.0002 | −0.2753, 0.0002 | ||
| Thrips larvae in leaves | 0.7657, <0.0001 | 0.7802, <0.0001 | 0.6847, <0.0001 | 0.4252, <0.0001 | −0.3840, <0.0001 | −0.3959, 0.0001 | ||
| Adult thrips in flowers | 0.8791, <0.0001 | 0.7802, <0.0001 | 0.7284, <0.0001 | 0.3358, <0.0001 | −0.3079, <0.0001 | −0.3012, <0.0001 | ||
| Thrips larvae in flowers | 0.6682, <0.0001 | 0.6847, <0.0001 | 0.7284, <0.0001 | 0.2977, <0.0001 | 0.2406, 0.0006 | −0.2677, 0.0001 | ||
| TCSV | 0.3851, <0.0001 | 0.4252, <0.0001 | 0.3358, <0.0001 | 0.2977, <0.0001 | −0.4836, <0.0001 | −0.4532, <0.0001 | ||
| Marketable yield | −0.2589, 0.0002 | −0.3840, <0.0001 | −0.3079, <0.0001 | −0.2406, 0.0006 | −0.4836, <0.0001 | 0.9571, <0.0001 | ||
| No. of marketable fruits | −0.2753, 0.0002 | −0.3959, 0.0001 | −0.3012, <0.0001 | −0.2677, 0.0001 | −0.4532, <0.0001 | 0.9571, <0.0001 | ||
| Treatments | Mean ± Standard Error (SE) Number of Thrips per Five Tomato Leaves | |||
|---|---|---|---|---|
| 2018 | 2019 | |||
| Insecticides | Adult | Larva | Adult | Larva |
| Spear® T | 2.17 ± 0.21ab z | 1.75 ± 0.15b | 0.66 ± 0.09 | 0.87 ± 0.15ab |
| Aza-direct® | 1.77 ± 0.18b | 1.93 ± 0.21b | 0.45 ± 0.05 | 0.56 ± 0.09bc |
| Grandevo® | 2.15 ± 0.21ab | 2.18 ± 0.15b | 0.06 ± 0.08 | 0.99 ± 0.20ab |
| Entrust®SC | 0.66 ± 0.08c | 0.76 ± 0.07c | 0.50 ± 0.08 | 0.26 ± 0.04c |
| Untreated control | 2.39 ± 0.22a | 3.40 ± 0.19a | 0.58 ± 0.08 | 1.12 ± 0.17a |
| Statistics | F4,60 = 58.04 | F4,60 = 56.28 | F4,60 = 1.95 | F4,60 = 9.19 |
| p < 0.0001 | p < 0.0001 | p = 0.1140 | p < 0.0001 | |
| Mulches | ||||
| S/B | 0.45 ± 0.06c | 1.15 ± 0.21c | 0.29 ± 0.05c | 0.42 ± 0.08b |
| S/W | 0.62 ± 0.08c | 1.14 ± 0.11c | 0.28 ± 0.04c | 0.65 ± 0.11ab |
| B/B | 2.39 ± 0.20b | 2.02 ± 0.12b | 0.49 ± 0.06abc | 0.66 ± 0.10ab |
| W/B | 2.43 ± 0.20ab | 2.42 ± 0.15b | 0.96 ± 0.11a | 1.11 ± 0.22a |
| 0/0 | 3.25 ± 0.25a | 3.30 ± 0.18a | 0.78 ± 0.09ab | 0.95 ± 0.16ab |
| Statistics | F4,12 = 83.88 | F4,12 = 43.86 | F4,15 = 6.41 | F4,15 = 3.31 |
| p < 0.0001 | p < 0.0001 | p = 0.0032 | p = 0.0394 | |
| Treatments | Mean ± Standard Error (SE) Number of Thrips per Ten Tomato Flowers | |||
|---|---|---|---|---|
| 2018 | 2019 | |||
| Insecticides | Adult | Larva | Adult | Larva |
| Spear® T | 4.10 ± 0.23b z | 0.83 ± 0.10b | 1.87 ± 0.16ab | 0.27 ± 0.06a |
| Aza-direct® | 4.49 ± 0.28b | 0.79 ± 0.11b | 2.18 ± 0.22a | 0.20 ± 0.06a |
| Grandevo® | 4.84 ± 0.30b | 1.08 ± 0.14ab | 2.49 ± 0.25a | 0.37 ± 0.17a |
| Entrust®SC | 1.96 ± 0.15c | 0.80 ± 0.11b | 1.36 ± 0.14b | 0.05 ± 0.02a |
| Untreated control | 5.98 ± 0.31a | 1.36 ± 0.15a | 2.40 ± 0.20a | 0.15 ± 0.04a |
| Statistics | F4,72 = 82.00 | F4,60 = 5.83 | F4,60 = 8.91 | F4,72 = 2.57 |
| p < 0.0001 | p = 0.0005 | p < 0.0001 | p = 0.0448 | |
| Mulches | 2018 | 2019 | ||
| S/B | 2.07 ± 0.15c | 0.45 ± 0.07cd | 1.69 ± 0.17a | 0.20 ± 0.06a |
| S/W | 2.11 ± 0.17c | 0.35 ± 0.06d | 1.65 ± 0.21a | 0.16 ± 0.05a |
| B/B | 5.03 ± 0.28b | 0.82 ± 0.11bc | 2.52 ± 0.22a | 0.18 ± 0.05a |
| W/B | 5.12 ± 0.24b | 1.22 ± 0.13b | 2.23 ± 0.22a | 0.15 ± 0.04a |
| 0/0 | 7.05 ± 0.27a | 2.01 ± 0.16a | 2.22 ± 0.17a | 0.35 ± 0.16a |
| Statistics | F4,72 = 161.18 | F4,12 = 33.08 | F4,12 = 3.25 | F4,72 = 0.37 |
| p < 0.0001 | p < 0.0001 | p = 0.0503 | p = 0.8263 | |
| Treatments | Mean ± Standard Error (SE) Number of Marketable Fruit Weight in kg and Fruits/Four Tomato Plants | |||
|---|---|---|---|---|
| 2018 | 2019 | |||
| Insecticides | Marketable fruit weight | No. of fruit | Marketable fruit weight | No. of fruit |
| Spear® T | 4.18 ± 0.48b z | 18.90 ± 2.06b | 3.42 ± 0.61abc | 15.05 ± 2.40ab |
| Aza-direct® | 4.26 ± 0.52b | 19.65 ± 2.32b | 4.67 ± 0.75ab | 22.00 ± 3.17a |
| Grandevo® | 2.94 ± 0.38c | 13.30 ± 1.82c | 2.87 ± 0.59bc | 12.90 ± 2.25b |
| Entrust®SC | 7.27 ± 0.70a | 33.35 ± 3.34a | 5.57 ± 0.72a | 24.30 ± 3.38a |
| Untreated control | 2.16 ± 0.28c | 10.70 ± 0.48c | 2.44 ± 0.47c | 12.70 ± 2.34b |
| Statistics | F4,60 = 42.94 | F4,60 = 36.20 | F4,60 = 6.42 | F4,60 = 5.40 |
| p < 0.0001 | p < 0.0001 | p = 0.0002 | p = 0.0009 | |
| Mulch | ||||
| S/B | 6.22 ± 0.79a | 28.45 ± 3.49a | 5.84 ± 0.95a | 25.35 ± 3.98a |
| S/W | 5.12 ± 0.59ab | 23.50 ± 2.93ab | 3.95 ± 0.64ab | 18.65 ± 3.10ab |
| B/B | 4.25 ± 0.49ab | 19.90 ± 2.21ab | 4.25 ± 0.57ab | 19.35 ± 2.31ab |
| W/B | 3.28 ± 0.38bc | 15.10 ± 1.89bc | 2.56 ± 0.40ab | 12.00 ± 1.68ab |
| 0/0 | 1.94 ± 0.30c | 8.95 ± 1.30c | 2.38 ± 0.41b | 11.60 ± 1.84b |
| Statistics | F4,15 = 12.89 | F4,15 = 11.73 | F4,15 = 3.32 | F4,15 = 3.41 |
| p < 0.0001 | p = 0.0002 | p = 0.0390 | p = 0.0358 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.A.; Seal, D.R.; Zhang, S.; Liburd, O.E.; Colee, J. Integrated Effect of Plastic Mulches and Biorational Insecticides in Managing Tomato Chlorotic Spot Virus (TCSV) and Its Vector Thrips in Tomatoes. Insects 2023, 14, 740. https://doi.org/10.3390/insects14090740
Khan RA, Seal DR, Zhang S, Liburd OE, Colee J. Integrated Effect of Plastic Mulches and Biorational Insecticides in Managing Tomato Chlorotic Spot Virus (TCSV) and Its Vector Thrips in Tomatoes. Insects. 2023; 14(9):740. https://doi.org/10.3390/insects14090740
Chicago/Turabian StyleKhan, Rafia A., Dakshina R. Seal, Shouan Zhang, Oscar E. Liburd, and James Colee. 2023. "Integrated Effect of Plastic Mulches and Biorational Insecticides in Managing Tomato Chlorotic Spot Virus (TCSV) and Its Vector Thrips in Tomatoes" Insects 14, no. 9: 740. https://doi.org/10.3390/insects14090740
APA StyleKhan, R. A., Seal, D. R., Zhang, S., Liburd, O. E., & Colee, J. (2023). Integrated Effect of Plastic Mulches and Biorational Insecticides in Managing Tomato Chlorotic Spot Virus (TCSV) and Its Vector Thrips in Tomatoes. Insects, 14(9), 740. https://doi.org/10.3390/insects14090740








