Effect of Short-Term High-Temperature Stimuli on the Functional Response of Trichopria drosophilae (Matsumura)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Cultures
2.2. Functional Response at Constant Parasitoid Density and Varying Host Densities
2.3. Mutual Interference Response of Parasitic Wasps
2.4. Data Analysis
3. Results
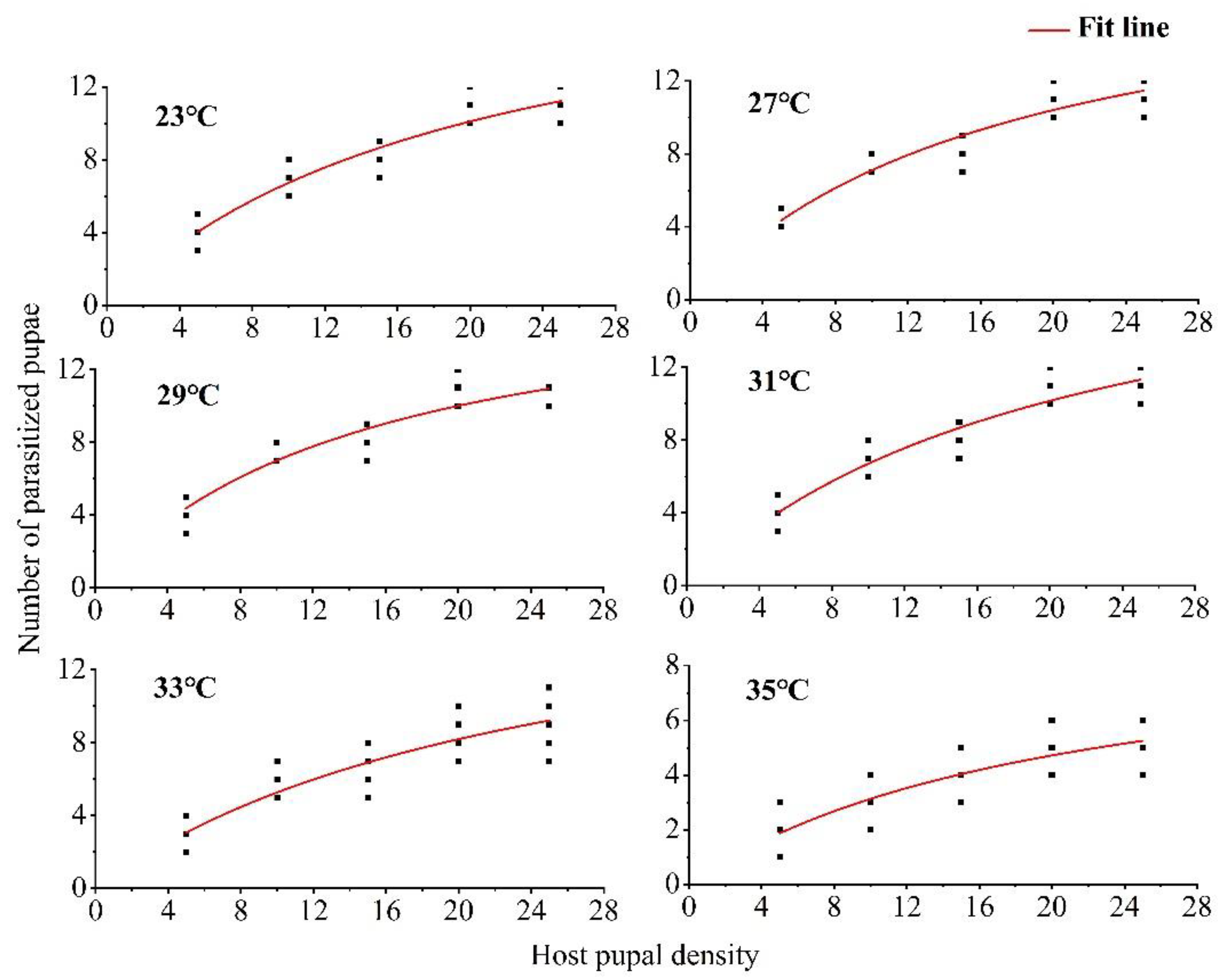
3.1. Functional Response at Constant Parasitoid Density and Varying Host Density after Stimulation at Different Temperatures
3.2. Mutual Interference at Constant Host Density and Varying Parasitoid Densities
4. Discussion
4.1. Functional Response after Stimulation at Different Temperatures
4.2. The Search Effect and Interference Effect of Trichopria drosophilae
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doris, Y.; Zalom, F.G.; Hamby, K.A. Host status and fruit odor response of Drosophila suzukii (Diptera: Drosophilidae) to figs and mulberries. J. Econ. Entomol. 2013, 106, 1932–1937. [Google Scholar]
- Kruit, W.A.; Wertheim, B.; Beukeboom, L.W. Artificial selection for nonreproductive host killing in a native parasitoid on the invasive pest, Drosophila suzukii. Evol. Appl. 2021, 14, 1993–2011. [Google Scholar]
- Elsensohn, J.E.; Schal, C.; Burrack, H.J. Plasticity in oviposition site selection behavior in Drosophila suzukii (Diptera: Drosophilidae) in relation to adult density and host distribution and quality. J. Econ. Entomol. 2021, 114, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Bolda, M.P.; Goodhue, R.E.; Zalom, F.G. Spotted wing drosophila: Potential economic impact of a newly established pest. Agric. Resour. Econ. Update 2010, 13, 5–8. [Google Scholar]
- Wiman, N.G.; Walton, V.; Dalton, D.T.; Gianfranco, A.; Hannah, J.B.; Joanna, C.C.; Kent, M.D.; Alberto, G.; Betsey, M.; Samantha, T.; et al. Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS ONE 2014, 9, e106909. [Google Scholar] [CrossRef] [PubMed]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.S.; Dong, C.; Kent, M.D.; Patricia, G.; Andrew, P.G.; Kim, A.H.; William, D.H.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Swoboda, B.K.A.; Mcphie, D.R.; Burrack, H.J. Reproductive Status of Drosophila suzukii (Diptera: Drosophilidae) females influences attraction to fermentation-based baits and ripe fruits. J. Econ. Entomol. 2017, 110, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.D. Studies Basic Research on the Biology, Rapid Reproduction and Cold Storage of Trichopria drosophilae (Hymenoptera: Diapriidae), one of Parasitoids of Drosophila suzukii Matsumura. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2019. [Google Scholar]
- Yi, C.; Cai, P.; Lin, J.; Liu, X.; Ao, G.; Zhang, Q.; Xia, H.; Yang, J.; Ji, Q. Life History and Host Preference of Trichopria drosophilae from Southern China, one of the effective pupal parasitoids on the Drosophila species. Insects 2020, 11, 103. [Google Scholar] [CrossRef]
- Stacconi, M.R.; Grassi, A.; Ioriatti, C.; Anfora, G. Augmentative releases of Trichopria drosophilae for the suppression of early season Drosophila suzukii populations. BioControl 2019, 64, 9–19. [Google Scholar] [CrossRef]
- Wang, X.G.; Nance, A.H.; Jones, J.M.L.; Kim, A.H.; Kent, M.D. Aspects of the biology and reproductive strategy of two Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii. Biol. Control 2018, 121, 58–65. [Google Scholar] [CrossRef]
- Häussling, B.J.M.; Lienenlüke, J.; Stökl, J. The preference of Trichopria drosophilae for pupae of Drosophila suzukii is independent of host size. Sci. Rep. 2021, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.R.P.; Coelho, A., Jr. Insect Rearing Techniques for Biological Control Programs, a Component of Sustainable Agriculture in Brazil. Insects 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, R.N. Functional response of the parasite Cotesia flavipes (Hymenoptera: Braconidae) at low densities of the host Diatraea saccharalis (Lepidoptera: Pyralidae). Environ. Entomol. 1993, 22, 849–858. [Google Scholar] [CrossRef]
- Kaçar, G.; Wang, X.G.; Biondi, A.; Kent, M.D. Linear functional response by two pupal Drosophila parasitoids foraging within single or multiple patch environments. PLoS ONE 2017, 12, e0183525. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef]
- Romo, C.M.; Tylianakis, J.M. Elevated temperature and drought interact to reduce parasitoid effectiveness in suppressing hosts. PLoS ONE 2013, 8, e58136. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Effects of High Temperature Stimulate on the Growth, Development and Parasitic Behavior of Trichogramma chrysalis. Master’s Thesis, Shihezi University, Xinjiang, China, 2008. [Google Scholar]
- Xie, L.; Dong, H.; Qian, H.T.; Yan, J.J.; Cong, B. Parasitic functional response of Trichogramma dendrolimi parthenogenetic strain and hermaphroditic strain to rice moth eggs at different temperatures. Acta Insectae Sin. 2013, 56, 263–269. [Google Scholar]
- Wu, J.; Liao, T.l.; Sun, P.; Shi, Z.H.; Chen, J.H. Bionomics of Drosophila suzukii Matsumura,1931 (Diptera: Drosophilidae). Plant Quar. 2013, 27, 36–41. [Google Scholar]
- Amiresmaeili, N.; Jucker, C.; Savoldelli, S.; Lupi, D. Understanding Trichopria drosophilae performance in laboratory conditions. Bull. Insectology 2018, 71, 251–256. [Google Scholar]
- Wang, C.Q.; He, L.; Hu, X.; Liu, X.L.; Yang, Z.Z.; Gu, X.S. Parasitic behavior observation and artificial breeding conditions of Trichopria drosophilae. Tianjin Agric. Sci. 2022, 28, 59–64+69. [Google Scholar]
- Hu, H.; Meng, L.; Li, B.P. Functional responses of Meteorus pulchricornis (Hymenoptera: Braconidae) to Spodoptera litura larvae at different instars. Chin. J. Biol. Control 2015, 31, 176–180. [Google Scholar]
- Liu, S.; Wang, X.; Shi, Z.; Gebremeskel, F.B. The biology of Diadromus collaris (Hymenoptera: Ichneumonidae), a pupal parasitoid of Plutella xylostella (Lepidoptera: Plutellidae), and its interactions with Oomyzus sokolowskii (Hymenoptera: Eulophidae). Bull. Entomol. Res. 2001, 91, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Barta, R.J.; Hoelmer, K.A.; Hopper, K.R.; Heimpel, G.E. Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 2009, 160, 387–398. [Google Scholar] [CrossRef]
- Hoque, M.F.; Islam, M.W.; Khalequzzaman, M. Functional response of Phytoseiulus persimilis Athias-Henriot to Tetranychus urticae Koch: Effects of prey life stages and temperature. Univ. J. Zool. Rajshahi Univ. 2012, 29, 1–8. [Google Scholar] [CrossRef]
- Enkegaard, A.; Brødsgaard, H.F.; Hansen, D.L. Macrolophus caliginosus: Functional response to whiteflies and preference and switching capacity between whiteflies and spider mites. Entomol. Exp. Appl. 2001, 101, 81–88. [Google Scholar] [CrossRef]
- Arditi, R.; Tyutyunov, Y.; Morgulis, A.; Govorukhin, V. Directed movement of predators and the emergence of density-dependence in predator–prey models. Theor. Popul. Biol. 2001, 59, 207–221. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, H.; Wang, J.X.; Dou, W.J.; Zhang, X.M.; Zhang, F.; Xiao, C.; Chen, G.H. Investigation on the species of parasitic natural enemies of Drosophila variegata in Yunnan Province. J. Environ. Insects 2019, 41, 592–598. [Google Scholar]
- Mondal, R.P.; Chandra, G.; Bandyopadhyay, S.; Ghosh, A. Effect of temperature and search area on the functional response of Anisops sardea (Hemiptera: Notonectidae) against Anopheles stephensi in laboratory bioassay. Acta Trop. 2017, 166, 262–267. [Google Scholar] [CrossRef]
- Jin, L.H. Functional response and control potential of Orius sauteri (Hemiptera: Anthocoridae) on tea thrips (Dendrothrips minowai Priesner). Insects 2021, 12, 1132. [Google Scholar]
- Latifian, M.; Soleymannejadian, E.; Ghazavy, M.; Mosadegh, M.S.; Rad, B. Effect of the fungus, Beauveria bassiana (Balsamo) (Asc., Hypocreales) on the functional response and host preference of the parasitoid Cephalonomia tarsalis (Ashmead) (Hym., Bethylidae) in larval population of the sawtoothed beetle Oryzaephilus surinamensis L. (Col., Silvanidae). J. Entomol. Res. 2011, 3, 251–264. [Google Scholar]
- Solomon, M.E. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Modesto, D.P.P.; Juan, R.G.G.; Hernandez-Suarez, E.M.; Cabello, T. Effect of temperature on life history and parasitization behavior of Trichogramma achaeae Nagaraja and Nagarkatti (Hym.: Trichogrammatidae). Insects 2020, 11, 482. [Google Scholar]
- Bayoumy, M. Functional response of the aphelinid parasitoid, Aphytis diaspidis: Effect of host scale species, Diaspidiotus perniciosus and Hemiberlesia lataniae. Acta Phytopathol. Entomol. Hung. 2011, 46, 101–113. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhou, X.G.; Xiao, Q.; Tang, P.; Chen, X.X. The potential of Parapanteles hyposidrae and Protapanteles immunis (Hymenoptera: Braconidae) as biocontrol agents for the tea grey geometrid Ectropis grisescens (Lepidoptera). Insects 2022, 13, 937. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhang, F.; Yang, F.X.; Xiao, C.; Zhang, X.M.; Chen, G.H. Study on biological characteristics of Leptopolina japonica. J. Environ. Entomol. 2021, 43, 191–198. [Google Scholar]
- Mills, N.J.; Lacan, I. Ratio dependence in the functional response of insect parasitoids: Evidence from Trichogramma minutum foraging for eggs in small host patches. Ecol. Entomol. 2004, 29, 208–216. [Google Scholar] [CrossRef]
- Li, S.R.; Wu, W.J. Effect of performance on functional response of the parasitoid Spalangiaendius (Hymenoptera: Pharmacolidae) on the pubaeo of the mellonfly, Bactroceraa curbitae (Diptera: Tephritidae). J. Biosaf. 2017, 26, 289–292. [Google Scholar]
- Chen, J.Y.; Chen, T.Y.; Fu, Y.G.; Zhang, F.P.; Han, D.Y.; Niu, L.M. Functional response of Encarsia guadeloupae Viggiani to Aleurodicus dispersus Russell. Chin. J. Biol. Control 2013, 29, 175–180. [Google Scholar]
- Skovgrd, H.; Nachman, G. Temperature-Dependent Functional Response of Spalangia cameroni (Hymenoptera: Pteromalidae), a Parasitoid of Stomoxys calcitrans (Diptera: Muscidae). Environ. Entomol. 2015, 44, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, D.A.; Mccann, K.S. A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am. Nat. 2005, 166, 184–198. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wan, N.F.; Fan, N.N.; Jiang, J.X. Functional response of Microplitis pallidipes parasitizing Spodoptera frugiperda. Chin. J. Appl. Entomol. 2020, 57, 1319–1325. [Google Scholar]
- Shi, M.; Tang, P.; Wang, Z.Z.; Huang, J.H.; Chen, X.X. Parasitic wasps in China and their application in biological control of pests. Chin. J. Appl. Entomol. 2020, 57, 491–548. [Google Scholar]
- Grether, G.F.; Losin, N.; Anderson, C.N.; Okamoto, K. The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biol. Rev. 2009, 84, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, F.J.F.; van Holland, P.D.; Godfray, H.C.J. Stable coexistence in insect communities due to density-and trait-mediated indirect effects. Ecology 2005, 86, 3182–3189. [Google Scholar] [CrossRef]
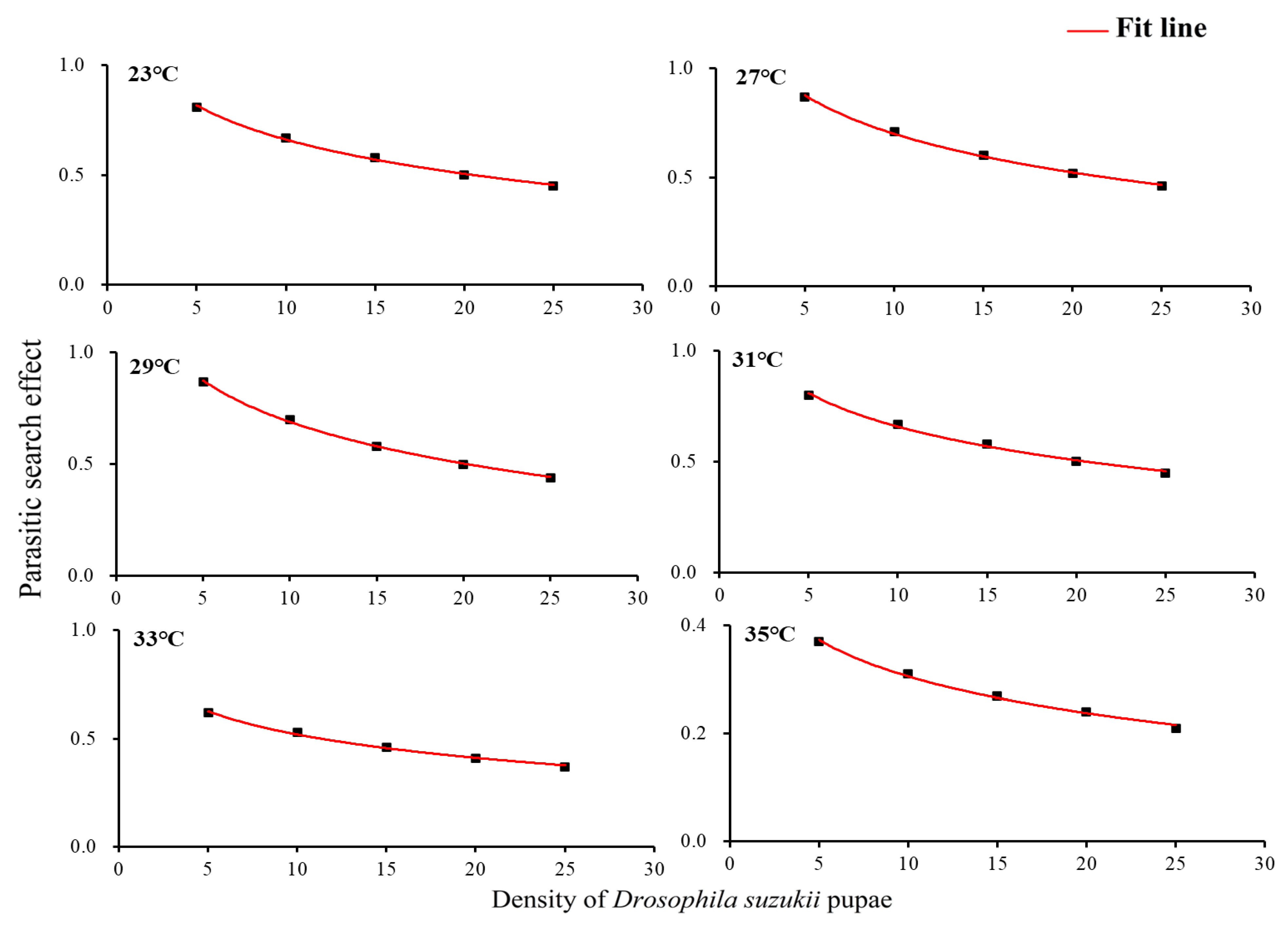
| Temperature (°C) | Coefficient | Estimate | SE | Pseudo-R2 |
|---|---|---|---|---|
| 23 | P1 | −0.89 | 0.40 | 0.90 |
| 27 | P1 | −1.39 | 0.48 | 0.93 |
| 29 | P1 | −1.57 | 0.60 | 0.91 |
| 31 | P1 | −0.48 | 0.29 | 0.91 |
| 33 | P1 | −0.13 | 0.29 | 0.79 |
| 35 | P1 | −0.06 | 0.22 | 0.70 |
| Temperature (°C) | Holling II Disc Equation | Instantaneous Search Rate (a) | Handling Time (Th) | Theoretical Parasitic Maximum (Namax) | Parasitic Efficiency (a/Th) | Pseudo-R2 |
|---|---|---|---|---|---|---|
| 23 | Na = 1.0102Nt/(1 + 0.0500Nt) | 1.0102 ± 0.1118 | 0.0495 ± 0.0062 | 20.20 | 20.41 | 0.89 |
| 27 | Na = 1.1169Nt/(1 + 0.0573Nt) | 1.1169 ± 0.1094 | 0.0514 ± 0.0050 | 19.46 | 21.73 | 0.90 |
| 29 | Na = 1.1611Nt/(1 + 0.0662Nt) | 1.1611 ± 0.1292 | 0.0570 ± 0.0056 | 17.54 | 20.37 | 0.88 |
| 31 | Na = 0.9886Nt/(1 + 0.0474Nt) | 0.9886 ± 0.1001 | 0.0479 ± 0.0057 | 20.88 | 20.64 | 0.91 |
| 33 | Na = 0.7384Nt/(1 + 0.0401Nt) | 0.7384 ± 0.1191 | 0.0543 ± 0.0119 | 18.42 | 13.59 | 0.80 |
| 35 | Na = 0.4652Nt/(1 + 0.0485Nt) | 0.4652 ± 0.0907 | 0.1043 ± 0.0234 | 9.59 | 4.46 | 0.72 |
| Temperature | Number of Trichopria drosophilae (pair) | Total Number of Parasitic | Parasitism Rate | Interference Equation | Pseudo-R2 |
|---|---|---|---|---|---|
| 23 °C | 1 | 18.6 ± 0.51 Aa | 37.2 ± 1.02% | A = 19.78P−0.6921 | 0.76 |
| 2 | 15.4 ± 0.51 ABb | 30.8 ± 1.02% | |||
| 3 | 13.4 ± 0.68 Ab | 26.8 ± 1.36% | |||
| 4 | 7.5 ± 0.68 Ac | 15.0 ± 1.36% | |||
| 5 | 5.2 ± 0.37 Ad | 10.4 ± 0.75% | |||
| 27 °C | 1 | 17.6 ± 0.51 Aa | 35.2 ± 1.02% | A = 18.76P−0.6115 | 0.76 |
| 2 | 14.4 ± 0.51 ABb | 28.8 ± 1.02% | |||
| 3 | 12.4 ± 0.68 ABb | 24.8 ± 1.36% | |||
| 4 | 6.6 ± 0.68 Ac | 13.2 ± 1.36% | |||
| 5 | 4.2 ± 0.37 Ac | 8.4 ± 0.75% | |||
| 29 °C | 1 | 16.4 ± 0.51 Aa | 32.8 ± 1.02% | A = 17.28P−0.6406 | 0.83 |
| 2 | 13.0 ± 0.71 Bb | 26.0 ± 1.41% | |||
| 3 | 10.0 ± 0.32 Bb | 20.0 ± 0.65% | |||
| 4 | 6.2 ± 0.58 ABc | 12.4 ± 1.17% | |||
| 5 | 4.2 ± 0.37 Ad | 8.4 ± 0.75% | |||
| 31 °C | 1 | 18.6 ± 0.51 Aa | 37.2 ± 1.02% | A = 19.88P−0.5622 | 0.75 |
| 2 | 15.8 ± 0.37 Ab | 31.6 ± 0.75% | |||
| 3 | 13.4 ± 0.68 Ab | 26.8 ± 1.36% | |||
| 4 | 7.8 ± 0.86 Ac | 15.64 ± 1.72% | |||
| 5 | 5.2 ± 0.37 Ad | 10.4 ± 0.75% | |||
| 33 °C | 1 | 17.2 ± 0.37 Aa | 34.4 ± 0.75% | A = 18.37P−0.6878 | 0.80 |
| 2 | 14.4 ± 0.25 ABb | 28.8 ± 0.51% | |||
| 3 | 9.8 ± 0.37 Bc | 19.6 ± 0.75% | |||
| 4 | 6.0 ± 0.84 ABd | 12.0 ± 1.68% | |||
| 5 | 3.6 ± 0.81 ABd | 7.2 ± 1.62% | |||
| 35 °C | 1 | 9.0 ± 0.31 Ba | 18.0 ± 0.62% | A = 9.048P−0.8923 | 0.91 |
| 2 | 4.8 ± 0.20 Cb | 9.6 ± 0.40% | |||
| 3 | 4.0 ± 0.55 Cbc | 8.0 ± 1.10% | |||
| 4 | 2.6 ± 0.25 Bcd | 5.2 ± 0.51% | |||
| 5 | 1.6 ± 0.25 Bd | 3.2 ± 0.51% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhang, J.; Tian, Y.; Chen, G.; Zhang, X. Effect of Short-Term High-Temperature Stimuli on the Functional Response of Trichopria drosophilae (Matsumura). Insects 2023, 14, 748. https://doi.org/10.3390/insects14090748
Chen Q, Zhang J, Tian Y, Chen G, Zhang X. Effect of Short-Term High-Temperature Stimuli on the Functional Response of Trichopria drosophilae (Matsumura). Insects. 2023; 14(9):748. https://doi.org/10.3390/insects14090748
Chicago/Turabian StyleChen, Qiang, Jinlong Zhang, Ye Tian, Guohua Chen, and Xiaoming Zhang. 2023. "Effect of Short-Term High-Temperature Stimuli on the Functional Response of Trichopria drosophilae (Matsumura)" Insects 14, no. 9: 748. https://doi.org/10.3390/insects14090748
APA StyleChen, Q., Zhang, J., Tian, Y., Chen, G., & Zhang, X. (2023). Effect of Short-Term High-Temperature Stimuli on the Functional Response of Trichopria drosophilae (Matsumura). Insects, 14(9), 748. https://doi.org/10.3390/insects14090748






