Seasonal Patterns in the Frequency of Candidatus Liberibacter Asiaticus in Populations of Diaphorina citri (Hemiptera: Psyllidae) in Florida
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Simplified Overview
2.2. Detailed Methods
2.2.1. Field Sites
2.2.2. Psyllid Collection
2.2.3. Analysis for CLas
2.2.4. Weather
2.2.5. Data Analysis
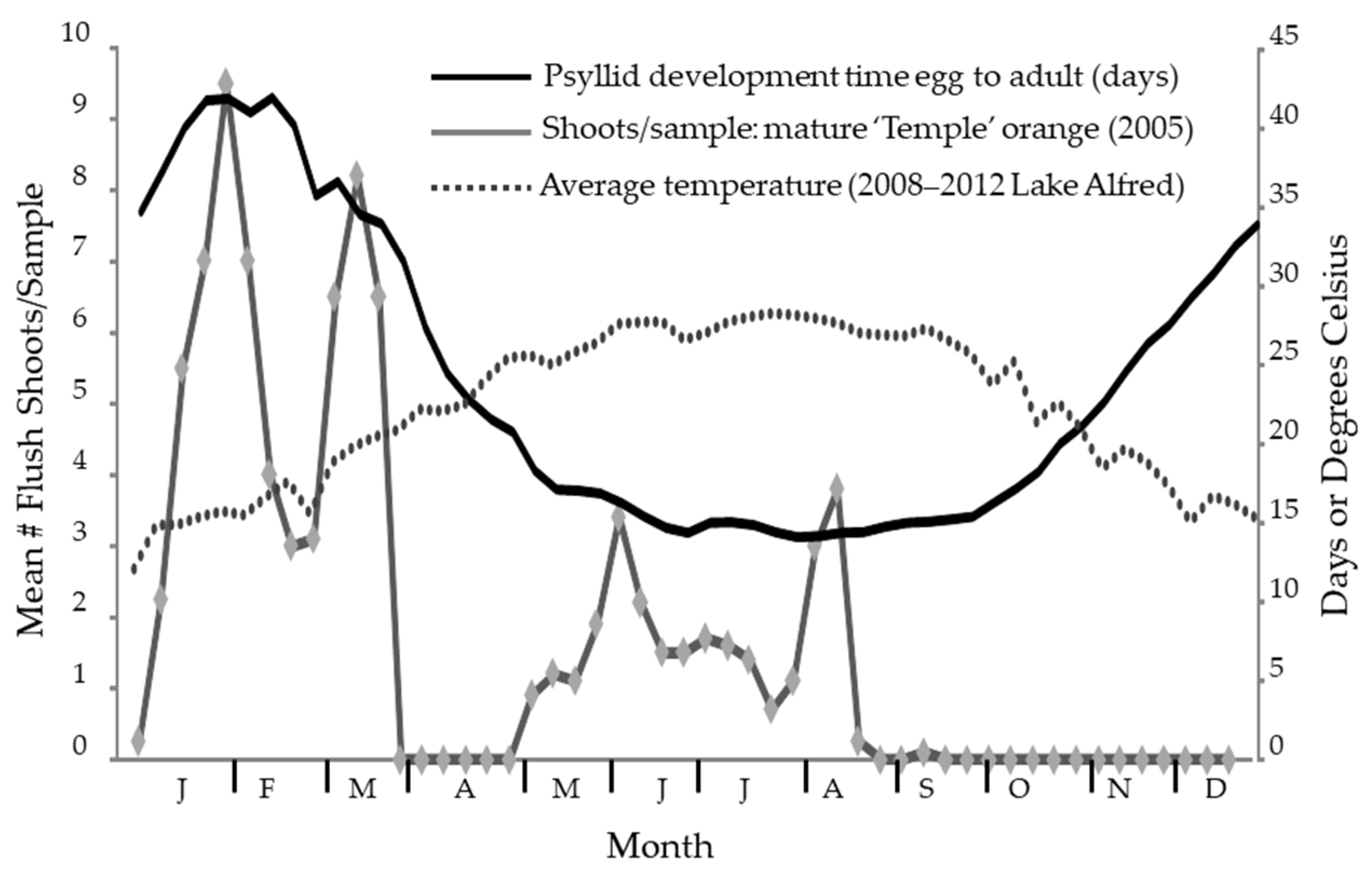
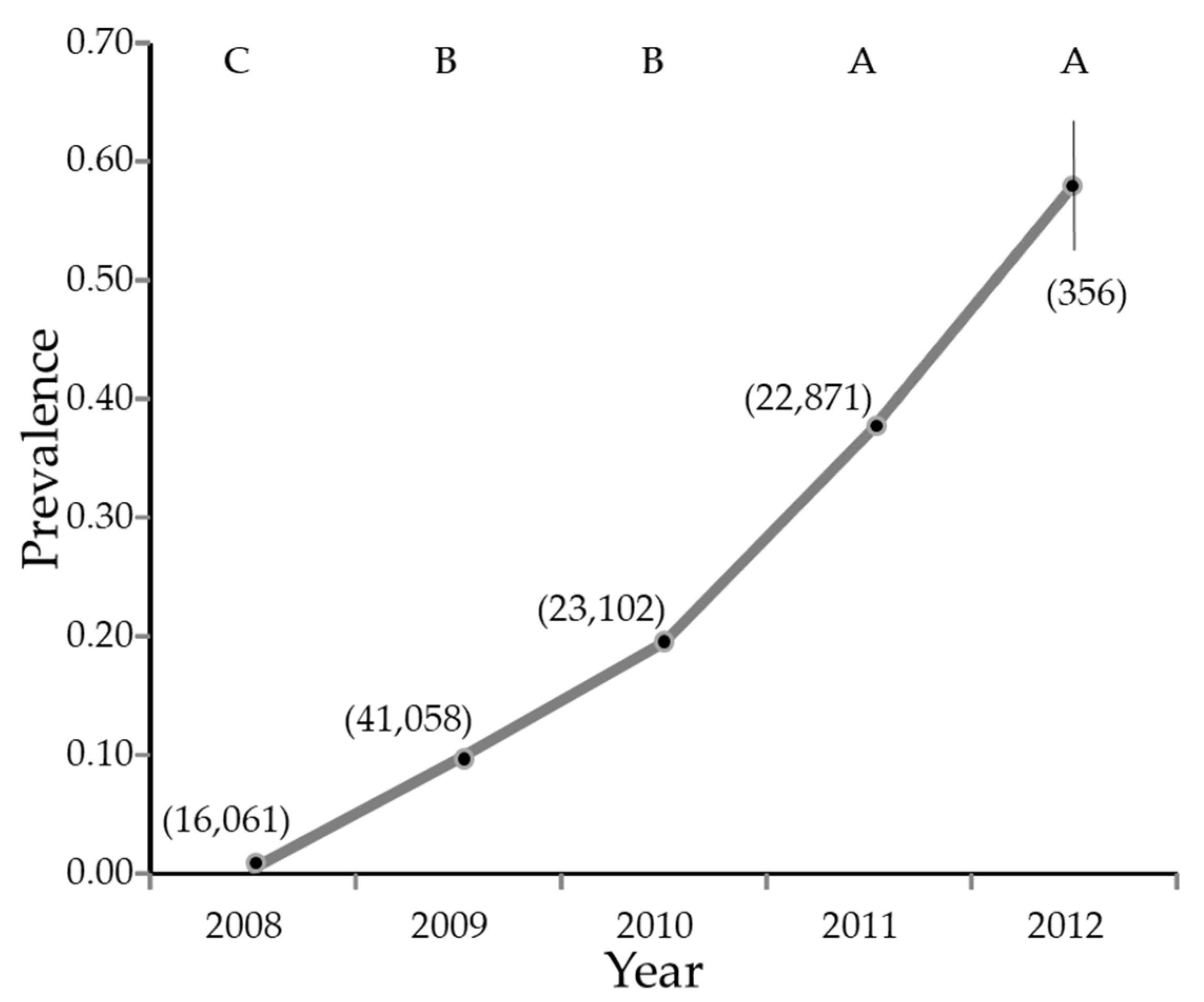
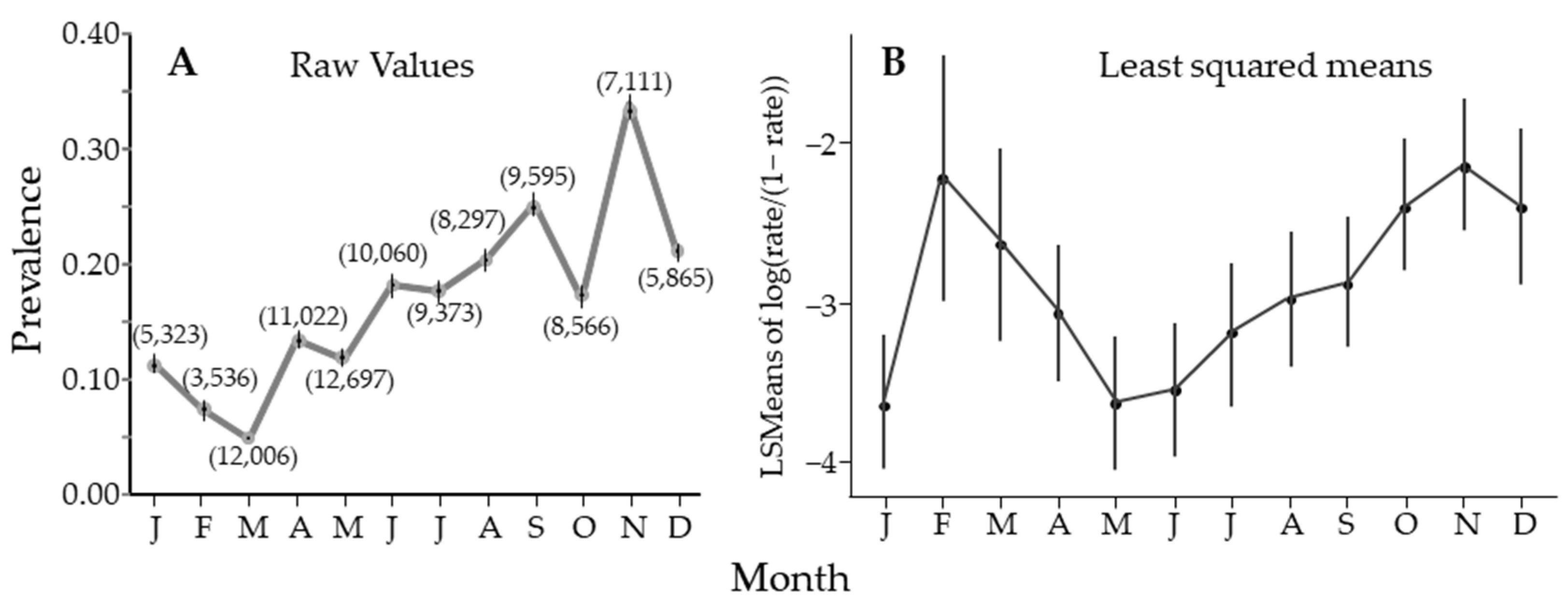
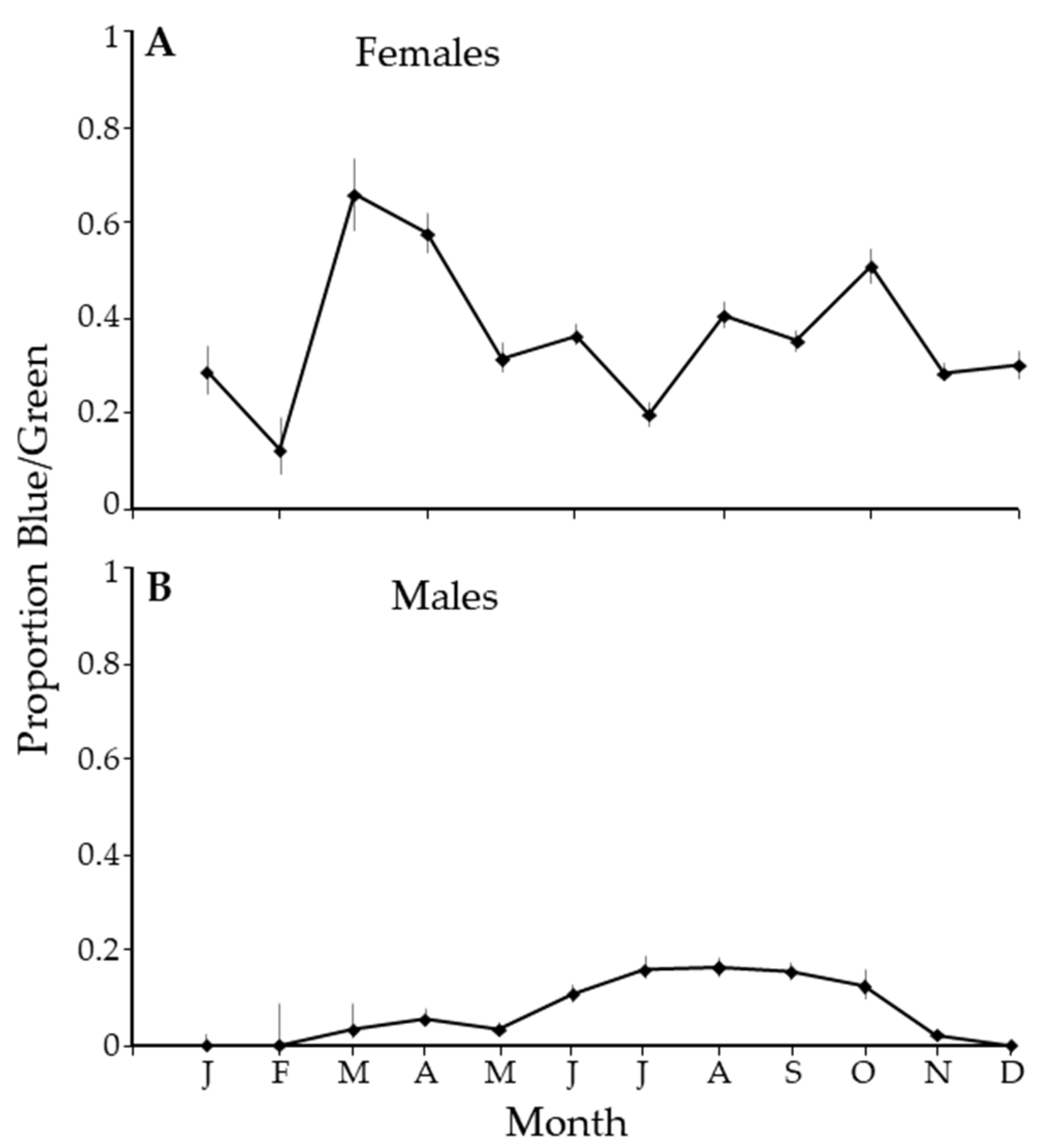
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivedi, P.; Sagaram, U.S.; Kim, J.-S.; Brlansky, R.H.; Rogers, M.E.; Stelinski, L.L.; Oswalt, C.; Wang, N. Quantification of viable Candidatus Liberibacter asiaticus in hosts using quantitative PCR with the aid of ethidium monoazide (EMA). Eur. J. Plant Pathol. 2009, 124, 553–563. [Google Scholar] [CrossRef]
- Gottwald, T.R.; da Graça, J.V.; Bassanezi, R.H.B. Citrus Huanglongbing: The Pathogen and its impact. Plant Heal. Prog. 2007, 8, 31. [Google Scholar] [CrossRef]
- Reynaud, B.; Turpin, P.; Molinari, F.M.; Grondin, M.; Roque, S.; Chiroleu, F.; Fereres, A.; Delatte, H. The African citrus psyllid Trioza erytreae: An efficient vector of Candidatus Liberibacter asiaticus. Front. Plant Sci. 2022, 13, 1089762. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Kikukawa, K.; Chikugi, H.; Itao, M.; Yokoyama, T. Seasonal variability of Candidatus Liberibactor asiaticus in Diaphorina citri Kuwayama. Res. Bull. Plant Prot. Serv. Jpn. 2010, 46, 79–84. [Google Scholar]
- Ukuda-Hosokawa, R.; Sadoyama, Y.; Kishaba, M.; Kuriwada, T.; Anbutsu, H.; Fukatsu, T. Infection density dynamics of the citrus greening bacterium “Candidatus Liberibacter asiaticus” in field populations of the psyllid Diaphorina citri and its relevance to the efficiency of pathogen transmission to citrus plants. Appl. Environ. Microbiol. 2015, 81, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Thind, S.K.; Nirmaljit, K.; Grewal, I.S. Population dynamics of citrus psylla, Diaphorina citri Kuwayama on Kinnow mandarin in relation to temperature and relative humidity. Indian J. Ecol. 1997, 24, 179–183. [Google Scholar]
- Ashari; Eveleens, K.G. The citrus psyllid (Diaphorina citri): Population ecology and distribution. In Research Reports 1968–1974; Section II. Technical Contributions; Agricultural Cooperation Indonesia-The Netherlands, Ed.; Ministry of Agriculture: Jakarta, Indonesia, 1974; pp. 370–373. [Google Scholar]
- Ashihara, W. Survival of Asian citrus psyllid, Diaphorina citri Kuwayama adults under winter temperature conditions in central and south Kyushu. Jpn. J. Appl. Èntomol. Zool. 2004, 48, 207–211. [Google Scholar] [CrossRef]
- Bhumannavar, B.S.; Singh, S.P. Studies on population-dynamics of citrus psylla, Diaphorina citri Kuwayama (Psyllidae, Hemiptera). Entomon 1986, 11, 63–66. [Google Scholar]
- Chavan, V.M.; Summanwar, A.S. Population dynamics and aspects of the biology of citrus psylla, Diaphorina citri Kuw., in Maharashtra. In Proceedings of the 12th Conference of the International Organization of Citrus Virologists; Department of Plant Pathology: Riverside, CA, USA, 1993; pp. 286–290. [Google Scholar]
- Chiaradia, L.A.; Milanez, J.M.; Smaniotto, M.A.; Davila, M.E.R.F. Population fluctuation and capture height of Diaphorina citri in citrus orchard. Rev. De Cienc. Agrovet. 2008, 7, 157–159. [Google Scholar]
- Hall, D.G.; Hentz, M.G.; Adair, R.C. Population ecology and phenology of Diaphorina citri (Hemiptera: Psyllidae) in two Florida citrus groves. Environ. Entomol. 2008, 37, 914–924. [Google Scholar] [CrossRef]
- Koli, S.Z.; Makar, P.V.; Choudhary, K.G. Seasonal abundance of citrus pests and their control. Indian J. Entomol. 1981, 43, 183–187. [Google Scholar]
- Tsai, J.H.; Wang, J.-J.; Liu, Y.-H. Seasonal abundance of the Asian citrus psyllid, Diaphorina citri (Homoptera: Psyllidae) in southern Florida. Fla. Èntomol. 2002, 85, 446–451. [Google Scholar] [CrossRef]
- Rakhshani, E.; Saeedifar, A. Seasonal fluctuations, spatial distribution and natural enemies of Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae) in Iran. Èntomol. Sci. 2013, 16, 17–25. [Google Scholar] [CrossRef]
- Arya, S. Extent of damage and seasonal abundance of citrus psylla (Diaphorina citri) on citrus plants. Acad. Soc. Res. 2016, 2, 131–136. [Google Scholar]
- Stucky, B.J.; Guralnick, R.; Deck, J.; Denny, E.G.; Bolmgren, K.; Walls, R. The plant phenology ontology: A new informatics resource for large-scale integration of plant phenology data. Front. Plant Sci. 2018, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Tsai, J.H. Effects of temperature on biology and life table parameters of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Ann. Appl. Biol. 2000, 137, 201–206. [Google Scholar] [CrossRef]
- Nakata, T. Temperature-dependent development of the citrus psyllid, Diaphorina citri (Homoptera: Psylloidea), and the predicted limit of its spread based on overwintering in the nymphal stage in temperate regions of Japan. Appl. Èntomol. Zool. 2006, 41, 383–387. [Google Scholar] [CrossRef]
- Nava, D.E.; Torres, M.L.G.; Rodrigues, M.D.L.; Bento, J.M.S.; Parra, J.R.P. Biology of Diaphorina citri (Hemiptera, Psyllidae) on different hosts and at different temperatures. J. Appl. Entomol. 2007, 131, 709–715. [Google Scholar] [CrossRef]
- Dunn, P.O.; Ahmed, I.; Armstrong, E.; Barlow, N.; Barnard, M.A.; Bélisle, M.; Benson, T.J.; Berzins, L.L.; Boynton, C.K.; Brown, T.A.; et al. Extensive regional variation in the phenology of insects and their response to temperature across North America. Ecology 2023, 104, e4036. [Google Scholar] [CrossRef] [PubMed]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the Huanglongbing pathogens. Annu. Rev. Èntomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Rosenblum, H.; Martini, X.; Tiwari, S.; Stelinski, L.L. Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. J. Econ. Èntomol. 2015, 108, 3–10. [Google Scholar] [CrossRef]
- Cifuentes-Arenas, J.C.; de Goes, A.; de Miranda, M.P.; Beattie, G.A.C.; Lopes, S.A. Citrus flush shoot ontogeny modulates biotic potential of Diaphorina citri. PLoS ONE 2018, 13, e0190563. [Google Scholar] [CrossRef] [PubMed]
- Arredondo de Ibarra, M.J. Abundance and Population Dynamics of the Asian Citrus Psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae) as Affected by Flush Shoots in Different Host Plants, in Plant and Soil Science; Texas A&M University: College Station, TX, USA, 2009; p. 80. [Google Scholar]
- Leong, S.C.T.; Abang, F.; Beattie, A.; Kueh, R.J.H.; Wong, S.K. Impacts of horticultural mineral oils and two insecticide practices on population fluctuation of Diaphorina citri and spread of Huanglongbing in a citrus orchard in Sarawak. Sci. World J. 2012, 2012, 651416. [Google Scholar] [CrossRef] [PubMed]
- Teck, L.C.; Abang, F.; Beattie, A.; Heng, K.; King, W. Seasonal population dynamics of the Asian citrus psyllid, Diaphorina citri Kuwayama in Sarawak. Am. J. Agric. Biol. Sci. 2011, 6, 527–535. [Google Scholar]
- Udell, B.; Monzo, C.; Paris, T.; Allan, S.; Stansly, P. Influence of limiting and regulating factors on populations of Asian citrus psyllid and the risk of insect and disease outbreaks. Ann. Appl. Biol. 2017, 171, 70–88. [Google Scholar] [CrossRef]
- Coletta-Filho, H.D.; Daugherty, M.P.; Ferreira, C.; Lopes, J.R.S. Temporal progression of ‘Candidatus Liberibacter asiaticus’ infection in citrus and acquisition efficiency by Diaphorina citri. Phytopathology 2014, 104, 416–421. [Google Scholar] [CrossRef]
- Canale, M.C.; Komada, K.M.A.; Lopes, J.R.S. Latency and incubation of ‘Candidatus Liberibacter asiaticus’ in citrus after vector inoculation. Trop. Plant Pathol. 2019, 45, 320–326. [Google Scholar] [CrossRef]
- Lee, J.A.; Halbert, S.E.; Dawson, W.O.; Robertson, C.J.; Keesling, J.E.; Singer, B.H. Asymptomatic spread of huanglongbing and implications for disease control. Proc. Natl. Acad. Sci. USA 2015, 112, 7605–7610. [Google Scholar] [CrossRef]
- Lopes, S.A.; Luiz, F.Q.B.F.; Oliveira, H.T.; Cifuentes-Arenas, J.C.; Raiol-Junior, L.L. Seasonal variation of ‘Candidatus Liberibacter asiaticus’ titers in new shoots of citrus in distinct environments. Plant Dis. 2017, 101, 583–590. [Google Scholar] [CrossRef]
- Bodaghi, S.; Meyering, B.; Bowman, K.D.; Albrecht, U. Different sweet orange–rootstock combinations infected by Candidatus Liberibacter asiaticus under greenhouse conditions: Effects on the Scion. HortScience 2022, 57, 144–153. [Google Scholar] [CrossRef]
- Tardivo, C.; Qureshi, J.; Bowman, K.D.; Albrecht, U. Relative influence of rootstock and scion on Asian citrus psyllid infestation and Candidatus Liberibacter asiaticus colonization. HortScience 2023, 58, 395–403. [Google Scholar] [CrossRef]
- Stover, E.; McCollum, G. Incidence and severity of Huanglongbing and Candidatus Liberibacter asiaticus titer among field-infected citrus cultivars. HortScience 2011, 46, 1344–1348. [Google Scholar] [CrossRef]
- Husain, M.A.; Nath, D. The citrus psylla (Diaphorina citri, Kuw.) [Psyllidae: Homoptera]. Mem. Dept. Agric. Ind. Ent. 1927, 10, 1–27. [Google Scholar]
- Wenninger, E.J.; Hall, D.G. Daily and seasonal patterns in abdominal color in Diaphorina citri (Hemiptera: Psyllidae). Ann. Èntomol. Soc. Am. 2008, 101, 585–592. [Google Scholar] [CrossRef]
- Ebert, T.A.; Cartwright, B.O. Biology and ecology of Aphis gossypii Glover (Homoptera: Aphidiae). Southwest. Entomol. 1997, 22, 116–153. [Google Scholar]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Relationships between adult abdominal color and reproductive potential in Diaphorina citri (Hemiptera: Psyllidae). Ann. Èntomol. Soc. Am. 2009, 102, 476–483. [Google Scholar] [CrossRef]
- Ibanez, F.; Racine, K.; Hoyte, A.; Stelinski, L.L. Reproductive performance among color morphs of Diaphorina citri Kuwayama, vector of citrus greening pathogens. J. Insect Physiol. 2019, 117, 103904. [Google Scholar] [CrossRef]
- Martini, X.; Hoyte, A.; Stelinski, L.L. Abdominal color of the Asian citrus psyllid (Hemiptera: Liviidae) is associated with flight capabilities. Ann. Èntomol. Soc. Am. 2014, 107, 842–847. [Google Scholar] [CrossRef]
- Antolínez, C.A.; Szejbak, K.; Mauck, K.E.; Rivera, M.J. Assessment of variation in feeding behavior by color morph in the Asian citrus psyllid (Diaphorina citri). J. Insect Behav. 2021, 34, 312–318. [Google Scholar] [CrossRef]
- Ebert, T.A.; Rogers, M.E. Probing behavior of Diaphorina citri (Hemiptera: Liviidae) on Valencia orange influenced by sex, color, and size. J. Insect Sci. 2020, 20, 12. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Ramsey, J.; Mann, M.; Bennett, L.; Hunter, W.B.; Shams-Bakhsh, M.; Hall, D.G.; Heck, M. Color morphology of Diaphorina citri influences interactions with its bacterial endosymbionts and ‘Candidatus Liberibacter asiaticus’. PLoS ONE 2019, 14, e0216599. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.-X.; Shang, F.; Jiang, H.-B.; Dou, W.; Cernava, T.; Wang, J.-J. Candidatus Liberibacter asiaticus: An important factor affecting bacterial community composition and Wolbachia titers in Asian citrus psyllid. Front. Microbiol. 2023, 14, 1109803. [Google Scholar] [CrossRef]
- Jiang, R.-X.; Shang, F.; Jiang, H.-B.; Dou, W.; Cernava, T.; Wang, J.-J. Environmental factors affect the bacterial community in Diaphorina citri, an important vector of “Candidatus Liberibacter asiaticus”. Microbiol. Spectr. 2023, 11, e0529822. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Shams-Bakhsh, M.; Mann, M.; Fattah-Hosseini, S.; Bagheri, A.; Mehrabadi, M.; Heck, M. Distribution and variation of bacterial endosymbiont and “Candidatus Liberibacter asiaticus” titer in the Huanglongbing insect vector, Diaphorina citri Kuwayama. Microb. Ecol. 2019, 78, 206–222. [Google Scholar] [CrossRef]
- Tiwari, S.; Killiny, N.; Mann, R.S.; Wenninger, E.J.; Stelinski, L.L. Abdominal color of the Asian citrus psyllid, Diaphorina citri, is associated with susceptibility to various insecticides. Pest Manag. Sci. 2013, 69, 535–541. [Google Scholar] [CrossRef]
- Cooper, W.R.; Horton, D.R.; Wildung, M.E.R.; Jensen, A.S.; Thinakaran, J.; Rendon, D.; Nottingham, L.B.; Beers, E.H.; Wohleb, C.H.; Hall, D.G.; et al. Host and non-host ‘Whistle Stops’ for Psyllids: Molecular gut content analysis by high-throughput sequencing reveals landscape-level movements of Psylloidea (Hemiptera). Environ. Èntomol. 2019, 48, 554–566. [Google Scholar] [CrossRef]
- Stelinski, L.L. Ecological aspects of the vector-borne bacterial disease, citrus greening (Huanglongbing): Dispersal and host use by Asian citrus psyllid, Diaphorina Citri Kuwayama. Insects 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.B. Host plants of psyllids in south Texas. In Proceedings of the 2nd International Research Conference on Huanglongbing, Orlando, FL, USA, 10–14 January 2011. [Google Scholar]
- Horgan, F.G.; Garcia, C.P.F.; Haverkort, F.; de Jong, P.W.; Ferrater, J.B. Changes in insecticide resistance and host range performance of planthoppers artificially selected to feed on resistant rice. Crop. Prot. 2020, 127, 104963. [Google Scholar] [CrossRef]
- Graca, J.V. Citrus Greening Disease. Annu. Rev. Phytopathol. 1991, 29, 109–136. [Google Scholar] [CrossRef]
- Pulici, J.D.V.S.; Murata, M.M.; Johnson, E.G. Early physiological plant response and systemic effects of Huanglongbing infection in split-root plants. Phytopathology 2022, 112, 1833–1843. [Google Scholar] [CrossRef]
- Coates, L.C.; Mahoney, J.; Ramsey, J.S.; Warwick, E.; Johnson, R.; MacCoss, M.J.; Krasnoff, S.B.; Howe, K.J.; Moulton, K.; Saha, S.; et al. Development on Citrus medica infected with ‘Candidatus Liberibacter asiaticus’ has sex-specific and -nonspecific impacts on adult Diaphorina citri and its endosymbionts. PLoS ONE 2020, 15, e0239771. [Google Scholar] [CrossRef]
- Chu, C.-C.; Gill, T.A.; Hoffmann, M.; Pelz-Stelinski, K.S. Inter-population variability of endosymbiont densities in the Asian citrus psyllid (Diaphorina citri Kuwayama). Microb. Ecol. 2016, 71, 999–1007. [Google Scholar] [CrossRef]
- Gill, T.A.; Chu, C.; Pelz-Stelinski, K.S. Comparative proteomic analysis of hemolymph from uninfected and Candidatus Liberibacter asiaticus-infected Diaphorina citri. Amino Acids 2017, 49, 389–406. [Google Scholar] [CrossRef]
- Fagan, J.R.; Giongo, A.; Brown, C.T.; Davis-Richardson, A.G. Characterization of the relative abundance of the citrus pathogen Ca. Liberibacter asiaticus in the microbiome of its insect vector, Diaphorina citri, using high throughput 16S rRNA sequencing. Open Microbiol. J. 2012, 6, 29–33. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of Huanglongbing or greening disease on orange juice quality, a Review. Front. Plant Sci. 2018, 9, 1976. [Google Scholar] [CrossRef]
- Ebert, T.A.; Brlansky, R.; Rogers, M. Reexamining the pooled sampling approach for estimating prevalence of infected insect vectors. Ann. Èntomol. Soc. Am. 2010, 103, 827–837. [Google Scholar] [CrossRef]
- Manjunath, K.L.; Webb, S.; Chen, Y.; Bendix, C.; Lewis, J.D.; Cifuentes-Arenas, J.C.; Beattie, G.A.C.; Peña, L.; Lopes, S.A.; Shimwela, M.M.; et al. Detection of ‘Candidatus Liberibacter asiaticus’ in Diaphorina citri and Its Importance in the Management of Citrus Huanglongbing in Florida. Phytopathology 2008, 98, 387–396. [Google Scholar] [CrossRef]
- Miranda, M.P.; dos Santos, F.L.; Bassanezi, R.H.B.; Montesino, L.H.; Barbosa, J.C.; Sétamou, M. Monitoring methods for Diaphorina citri Kuwayama (Hemiptera: Liviidae) on citrus groves with different insecticide application programmes. J. Appl. Èntomol. 2018, 142, 89–96. [Google Scholar] [CrossRef]
- Monzo, C.; Arevalo, H.A.; Jones, M.M.; Vanaclocha, P.; Croxton, S.D.; Qureshi, J.A.; Stansly, P.A. Sampling methods for detection and monitoring of the Asian citrus psyllid (Hemiptera: Psyllidae). Environ. Èntomol. 2015, 44, 780–788. [Google Scholar] [CrossRef]
- Setamou, M.; Flores, D.; French, J.V.; Hall, D.G. Dispersion patterns and sampling plans for Diaphorina citri (Hemiptera: Psyllidae) in citrus. J. Econ. Entomol. 2008, 101, 1478–1487. [Google Scholar] [CrossRef]
- Sétamou, M.; Alabi, O.J.; Tofangsazi, N.; Grafton-Cardwell, E. COPF: Citrus orchard perimeter fencing as a strategy for reducing Asian citrus psyllid (Hemiptera: Liviidae) infestation. J. Appl. Èntomol. 2018, 142, 959–966. [Google Scholar] [CrossRef]
- Meyer, J.M.; Hoy, M.A.; Singh, R. Low incidence of Candidatus Liberibacter asiaticus in Diaphorina citri (Hemiptera: Psyllidae) populations between Nov 2005 and Jan 2006: Relevance to management of citrus greening disease in Florida. Fla. Èntomol. 2007, 90, 394–397. [Google Scholar] [CrossRef]
- Mann, R.S.; Pelz-Stelinski, K.; Hermann, S.L.; Tiwari, S.; Stelinski, L.L. Sexual transmission of a plant pathogenic bacterium, Candidatus Liberibacter asiaticus, between conspecific insect vectors during mating. PLoS ONE 2011, 6, e29197. [Google Scholar] [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef]
- Anonymous. Florida Automated Weather Network. 2023. Available online: https://fawn.ifas.ufl.edu/ (accessed on 9 September 2023).
- Ebert, T.; Downer, R. Macrolepidoptera biodiversity in Wooster, Ohio from 2001 through 2009. ZooKeys 2014, 452, 79–105. [Google Scholar] [CrossRef] [PubMed]
- Biggerstaff, B.J. PooledInfRate, Version 3.0: A Microsoft® Excel® Add-In to Compute Prevalence Estimates from Pooled Samples. 2006. Available online: http://www.cdc.gov/westnile/resourcepages/mosqsurvsoft.html (accessed on 9 September 2023).
- Biggerstaff, B.J. PooledInfRate. 2023. Available online: https://github.com/CDCgov/PooledInfRate (accessed on 9 September 2023).
- Anonymous. Mosquito surveillance software. 2023. Available online: https://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html (accessed on 9 September 2023).
- Dorfman, R. The Detection of defective members of large populations. Ann. Math. Stat. 1943, 14, 436–440. [Google Scholar] [CrossRef]
- Hepworth, G. Exact Confidence intervals for proportions estimated by group testing. Biometrics 1996, 52, 1134. [Google Scholar] [CrossRef]
- Hepworth, G. Estimation of Proportions by Group Testing, in Mathematics and Statistics; University of Melbourne: Melbourne, Australia, 1999; p. 232. [Google Scholar]
- Hepworth, G. Mid-P confidence intervals based on the likelihood ratio for proportions estimated by group testing. Aust. N. Z. J. Stat. 2004, 46, 391–405. [Google Scholar] [CrossRef]
- Hepworth, G. Confidence intervals for proportions estimated by group testing with groups of unequal size. J. Agric. Biol. Environ. Stat. 2005, 10, 478–497. [Google Scholar] [CrossRef]
- Hepworth, G.; Watson, R. Debiased estimation of proportions in group testing. J. R. Stat. Soc. Ser. C-Appl. Stat. 2009, 58, 105–121. [Google Scholar] [CrossRef]
- Balding, D.J.; Torney, D.C. Optimal pooling designs with error detection. J. Comb. Theory Ser. A 1996, 74, 131–140. [Google Scholar] [CrossRef]
- Bar-Lev, S.K.; Stadje, W.; Schouten, F.A.v.d.D. Optimal group testing with processing times and incomplete identification. Methodol. Comput. Appl. Probab. 2004, 6, 55–72. [Google Scholar] [CrossRef]
- Bar-Lev, S.K.; Stadje, W.; Schouten, F.A.V.d.D. Group testing procedures with incomplete identification and unreliable testing results. Appl. Stoch. Model. Bus. Ind. 2006, 22, 281–296. [Google Scholar] [CrossRef]
- Bargmann, R.E.; Carter, F.L. Group testing in binomial and multinomial situations. Va. J.Sci. 1960, 11, 230. [Google Scholar]
- Burrows, P.M. Improved estimation of pathogen transmission rates by group testing. Phytopathology 1987, 77, 363–365. [Google Scholar] [CrossRef]
- Chen, C.L.; Swallow, W.H. Sensitivity analysis of variable-size group testing and its related continuous models. Biom. J. 1995, 37, 173–181. [Google Scholar] [CrossRef]
- de Bonis, A.; Gargano, L.; Vaccaro, U. Group testing with unreliable tests. Inf. Sci. 1997, 96, 1–14. [Google Scholar] [CrossRef]
- Federer, W.T. Pooling and other designs for analyzing laboratory samples more efficiently. Statistician 1994, 43, 413–422. [Google Scholar] [CrossRef]
- Juan, J.S.-T.; Chang, G.J. Adaptive group testing for consecutive positives. Discret. Math. 2008, 308, 1124–1129. [Google Scholar] [CrossRef]
- Katholi, C.R.; Unnasch, T.R. Important experimental parameters for determining infection rates in arthropod vectors using pool screening approaches. Am. J. Trop. Med. Hyg. 2006, 74, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.L. Multistage group testing procedure (group screening). Commun. Stat. Simul. Comput. 2004, 33, 621–637. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Hudgens, M.G.; Dreyfuss, J.M.; Westreich, D.J.; Pilcher, C.D. Comparison of group testing algorithms for case identification in the presence of test error. Biometrics 2007, 63, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Macula, A.J.; Popyack, L.J. A group testing method for finding patterns in data. Discret. Appl. Math. 2004, 144, 149–157. [Google Scholar] [CrossRef]
- Mantel, N. Group testing with goal of estimation. Biometrics 1975, 31, 994–995. [Google Scholar] [PubMed]
- Montesinos-López, O.A.; Montesinos-López, A.; Crossa, J.; Eskridge, K. Sample size under inverse negative binomial group testing for accuracy in parameter estimation. PLoS ONE 2012, 7, e32250. [Google Scholar] [CrossRef]
- Sobel, M.; Elashoff, R.M. Group testing with a new goal, estimation. Biometrika 1975, 62, 181–193. [Google Scholar] [CrossRef]
- Westreich, D.J.; Hudgens, M.G.; Fiscus, S.A.; Pilcher, C.D. Optimizing screening for acute Human Immunodeficiency Virus infection with pooled nucleic acid amplification tests. J. Clin. Microbiol. 2008, 46, 1785–1792. [Google Scholar] [CrossRef]
- Xie, M.G. Regression analysis of group testing samples. Stat. Med. 2001, 20, 1957–1969. [Google Scholar] [CrossRef]
- Yamamura, K.; Hino, A. Estimation of the proportion of defective units by using group testing under the existence of a threshold of detection. Commun. Stat. Simul. Comput. 2007, 36, 949–957. [Google Scholar] [CrossRef]
- Zhang, B.; Bilder, C.R.; Biggerstaff, B.; Schaarschmidt, F.; Hitt, B. binGroup: Evaluation and experimental design for binomial group testing. R Package Version 2.2-1. 2018. Available online: https://CRAN.R-project.org/package=binGroup (accessed on 9 September 2023).
- Verdun, C.M.; Fuchs, T.; Harar, P.; Elbrächter, D.; Fischer, D.S.; Berner, J.; Grohs, P.; Theis, F.J.; Krahmer, F. Group testing for SARS-CoV-2 allows for up to 10-fold efficiency increase across realistic scenarios and testing strategies. Front. Public Health 2021, 9, 583377. [Google Scholar] [CrossRef]
- Tan, J.; Omar, A.; Lee, W.; Wong, M. Considerations for group testing: A practical approach for the clinical laboratory. Clin. Biochem. Rev. 2020, 41, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Keremane, M.L.; Ramadugu, C.; Rodriguez, E.; Kubota, R.; Shibata, S.; Hall, D.G.; Roose, M.L.; Jenkins, D.; Lee, R.F. A rapid field detection system for citrus huanglongbing associated ‘Candidatus Liberibacter asiaticus’ from the psyllid vector, Diaphorina citri Kuwayama and its implications in disease management. Crop. Prot. 2015, 68, 41–48. [Google Scholar] [CrossRef]
- SAS Institute. SAS/Stat User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2023; Available online: http://documentation.sas.com/doc/en/pgmsascdc/9.4_3.4/statug/titlepage.htm (accessed on 9 September 2023).
- Ebert, T.A.; Hernandez, J.C.; Russell, M.P. Ocean conditions and bottom-up modifications of gonad development in the sea urchin Strongelocentrotus purpuratus over space and time. Mar. Ecol. Prog. Ser. 2012, 467, 147–166. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodal Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Hall, D.G.; Albrigo, L. Estimating the relative abundance of flush shoots in citrus with implications on monitoring insects associated with flush. HortScience 2007, 42, 364–368. [Google Scholar] [CrossRef]
- Hall, D.G.; Hentz, M.G. Seasonal flight activity by the Asian citrus psyllid in east central Florida. Èntomol. Exp. Appl. 2011, 139, 75–85. [Google Scholar] [CrossRef]
- Monzo, C.; Stansly, P.A. Economic injury levels for Asian citrus psyllid control in process oranges from mature trees with high incidence of huanglongbing. PLoS ONE 2017, 12, e0175333. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Stockton, D.; Gossett, H.; Qureshi, J.A.; Ibanez, F.; Pelz-Stelinski, K.S.; Stelinski, L.L. Comparisons of economic thresholds for Asian citrus psyllid management suggest a revised approach to reduce management costs and improve yield. Front. Sustain. Food Syst. 2022, 6, 948278. [Google Scholar] [CrossRef]
- Li, W.; Levy, L.; Hartung, J.S.; Junior, L.L.R.; Cifuentes-Arenas, J.C.; Cunniffe, N.J.; Turgeon, R.; Lopes, S.A.; Raiol-Junior, L.L.; de Carvalho, E.V.; et al. Quantitative distribution of ‘Candidatus Liberibacter asiaticus’ in citrus plants with citrus Huanglongbing. Phytopathology 2009, 99, 139–144. [Google Scholar] [CrossRef]
- Gottwald, T.; Parnell, S.; Taylor, E.; Poole, K.; Hodge, J.; Ford, A.; Therrien, L.; Mayo, S.; Irey, M. Within-tree spatial distribution of Candidatus Liberibacter asiaticus. In Proceedings of the International Research Conference on Huanglongbing, Orlando, FL, USA, 1–5 December 2008; pp. 310–313. Available online: https://swfrec.ifas.ufl.edu/hlb/database/pdf/22_IRCHLB_08.pdf (accessed on 9 September 2023).
- Kunta, M.; da Graça, J.V.; Malik, N.S.; Louzada, E.S.; Sétamou, M. Quantitative distribution of Candidatus Liberibacter asiaticus in the aerial parts of the Huanglongbing-infected citrus trees in Texas. HortScience 2014, 49, 65–68. [Google Scholar] [CrossRef]
- Tatineni, S.; Sagaram, U.S.; Gowda, S.; Robertson, C.J.; Dawson, W.O.; Iwanami, T.; Wang, N.; Li, J.; Pang, Z.; Vincent, C.; et al. In planta distribution of ‘Candidatus Liberibacter asiaticus’ as revealed by polymerase chain reaction (PCR) and real-time PCR. Phytopathology 2008, 98, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, C.; Turner, D.; Wang, C.; Welker, S.; Achor, D.; Artiga, Y.A.; Turgeon, R.; Levy, A. Candidatus Liberibacter asiaticus accumulation in the phloem inhibits callose and reactive oxygen species. Plant Physiol. 2022, 190, 1090–1094. [Google Scholar] [CrossRef]
- Achor, D.; Welker, S.; Ben-Mahmoud, S.; Wang, C.; Folimonova, S.Y.; Dutt, M.; Gowda, S.; Levy, A. Dynamics of Candidatus Liberibacter asiaticus movement and sieve-pore plugging in citrus sink cells. Plant Physiol. 2020, 182, 882–891. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Z.; Liu, S.; Chi, Y.; Jia, D.; Wei, T. Infection and distribution of Candidatus Liberibacter asiaticus in citrus plants and psyllid vectors at the cellular level. Microb. Biotechnol. 2022, 15, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.A.; Backus, E.A.; Shugart, H.J.; Rogers, M.E. Behavioral plasticity in probing by Diaphorina citri (Hemiptera, Liviidae): Ingestion from phloem vs. xylem is influenced by leaf age and surface. J. Insect Behav. 2018, 31, 119–137. [Google Scholar] [CrossRef]
- George, J.; Ammar, E.D.; Hall, D.G.; Shatters, R.G., Jr.; Lapointe, S.L. Prolonged phloem ingestion by Diaphorina citri nymphs compared to adults is correlated with increased acquisition of citrus greening pathogen. Sci. Rep. 2018, 8, 10352. [Google Scholar] [CrossRef]
- Ammar, E.-D.; George, J.; Sturgeon, K.; Stelinski, L.L.; Shatters, R.G. Asian citrus psyllid adults inoculate huanglongbing bacterium more efficiently than nymphs when this bacterium is acquired by early instar nymphs. Sci. Rep. 2020, 10, 18244. [Google Scholar] [CrossRef] [PubMed]
- Ammar, E.-D.; Hall, D.G.; Shatters, R.G., Jr. Stylet morphometrics and citrus leaf vein structure in relation to feeding behavior of the Asian citrus psyllid Diaphorina citri, vector of citrus huanglongbing bacterium. PLoS ONE 2013, 8, e59914. [Google Scholar] [CrossRef]
- Hartung, J.S.; Paul, C.; Achor, D.; Brlansky, R.H. Colonization of dodder, Cuscuta indecora, by ‘Candidatus Liberibacter asiaticus’ and ‘Ca. L. americanus’. Phytopathology 2010, 100, 756–762. [Google Scholar] [CrossRef]
- Shokrollah, H.; Abdullah, T.L.; Sijam, K.; Abdullah, S.N.A. Ultrastructures of Candidatus Liberibacter asiaticus and its damage in huanglongbing (HLB) infected citrus. Afr. J. Biotechnol. 2010, 9, 5897–5901. [Google Scholar]
- Hilf, M.E.; Sims, K.R.; Folimonova, S.Y.; Achor, D.S.; Junior, L.L.R.; Cifuentes-Arenas, J.C.; Cunniffe, N.J.; Turgeon, R.; Lopes, S.A.; Raiol-Junior, L.L.; et al. Visualization of ‘Candidatus Liberibacter asiaticus’ cells in the vascular bundle of citrus seed coats with fluorescence in situ hybridization and transmission electron microscopy. Phytopathology 2013, 103, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.A. The probing behavior component of disease transmission in insect-transmitted bacterial plant pathogens. Insects 2019, 10, 212. [Google Scholar] [CrossRef]
- Alves, M.N.; Cifuentes-Arenas, J.C.; Raiol-Junior, L.L.; Ferro, J.A.; Peña, L. Early population dynamics of “Candidatus Liberibacter asiaticus” in susceptible and resistant genotypes after inoculation with infected Diaphorina citri feeding on young shoots. Front. Microbiol. 2021, 12, 683923. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of trifoliate citrus rootstock hybrids to Candidatus Liberibacter asiaticus. Sci. Hortic. 2012, 147, 71–80. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Reciprocal influences of rootstock and scion citrus cultivars challenged with Ca. Liberibacter asiaticus. Sci. Hortic. 2019, 254, 133–142. [Google Scholar] [CrossRef]
- Kunwar, S.; Meyering, B.; Grosser, J.; Gmitter, F.G.; Castle, W.S.; Albrecht, U. Field performance of ‘Valencia’ orange trees on diploid and tetraploid rootstocks in different huanglongbing-endemic growing environments. Sci. Hortic. 2023, 309, 111635. [Google Scholar] [CrossRef]
- Villegas, C. Development of a Technique for Reliable Recovery of Testable Asian Citrus Psyllid from Field Deployed Sticky Cards, in Plant and Soil Sciences; Texas A&M University: Kingsville, TX, USA, 2020; p. 90. [Google Scholar]
- Russell, D.N.; Qureshi, J.A.; Halbert, S.E.; Stansly, P.A. Host suitability of Citrus and Zanthoxylum spp. for Leutonota fagarae Burckhardt and Diaphorina citri Kuwayama (Hemiptera: Psylloidea). Fla. Entomol. 2014, 97, 1481–1492. [Google Scholar] [CrossRef]
- Ferguson, L.V.; Adamo, S.A. From perplexing to predictive: Are we ready to forecast insect disease susceptibility in a warming world? J. Exp. Biol. 2023, 226, 244911. [Google Scholar] [CrossRef]
- Bisht, K.; Velthuis, A.J.W.T. Decoding the role of temperature in RNA virus infections. mBio 2023, 13, e0202122. [Google Scholar] [CrossRef]
- I El-Saadi, M.; A MacMillan, H.; Ferguson, L.V. Cold-induced immune activation in chill-susceptible insects. Curr. Opin. Insect Sci. 2023, 58, 101054. [Google Scholar] [CrossRef]
- Killiny, N.; Hijaz, F.; Ebert, T.A.; Rogers, M.E. A plant bacterial pathogen manipulates its insect vector’s energy metabolism. Appl. Environ. Microbiol. 2017, 83, e03005-16. [Google Scholar] [CrossRef]
- Sétamou, M.; Simpson, C.R.; Alabi, O.J.; Nelson, S.D.; Telagamsetty, S.; Jifon, J.L. Quality matters: Influences of citrus flush physicochemical characteristics on population dynamics of the Asian citrus psyllid (Hemiptera: Liviidae). PLoS ONE 2016, 11, e0168997. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Higgins, S.A.; Ramsey, J.; Howe, K.; Griggs, M.; Castrillo, L.; Heck, M. Proteomic polyphenism in color morphotypes of Diaphorina citri, insect vector of citrus greening disease. J. Proteome Res. 2021, 20, 2851–2866. [Google Scholar] [CrossRef]
- Mann, M.; Fattah-Hosseini, S.; Ammar, E.-D.; Stange, R.; Warrick, E.; Sturgeon, K.; Shatters, R.; Heck, M. Diaphorina citri nymphs are resistant to morphological changes induced by “Candidatus Liberibacter asiaticus” in midgut epithelial cells. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Goolsby, J.A.; Adamczyk, J.J.; Crosslin, J.M.; Troxclair, N.N.; Anciso, J.R.; Bester, G.G.; Bradshaw, J.D.; Bynum, E.D.; Carpio, L.A.; Henne, D.C.; et al. Seasonal population dynamics of the potato psyllid (Hemiptera: Triozidae) and its associated pathogen “Candidatus Liberibacter solanacearum” in potatoes in the Southern Great Plains of North America. J. Econ. Èntomol. 2012, 105, 1268–1276. [Google Scholar] [CrossRef]
- Belasque, J., Jr.; Bassanezi, R.H.B.; Yamamoto, P.T.; Ayres, A.J.; Tachibana, A.; Violante, A.R.; Tank Jr, A.; Di Giorgi, F.; Tersi, F.E.; Menezes, G.M.; et al. Lessons from huanglongbing management in Sao Paulo State, Brazil. J. Plant Pathol. 2010, 92, 285–302. [Google Scholar]
- Zhang, J.; Liu, Y.; Gao, J.; Yuan, C.; Zhan, X.; Cui, X.; Zheng, Z.; Deng, X.; Xu, M. Current epidemic situation and control status of citrus Huanglongbing in Guangdong China: The space–time pattern analysis of specific orchards. Life 2023, 13, 749. [Google Scholar] [CrossRef] [PubMed]
- Uechi, N.; Katoh, H.; Iwanami, T.; Fujikawa, T.; Miyata, S.-I.; Yasuda, K.; Ooishi, T.; Kawano, S.; Uesato, T. Management of Huanglongbing (HLB) by an intensive vector and disease control in the surroundings of the orchard, in addition to planting HLB-free trees in Okinawa, Japan. Jpn. Agric. Res. Quarterly JARQ 2019, 53, 103–108. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, C.; Bassanezi, R.H.B.; Wu, F.; Feng, Z.; Shi, D.; Li, J.; Du, Y.; Zhong, L.; Zhong, B.; et al. Region-wide comprehensive implementation of roguing infected trees, tree replacement, and insecticide applications successfully controls citrus Huanglongbing. Phytopathology 2021, 111, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Wulff, N.A.; Daniel, B.; Sassi, R.S.; Moreira, A.S.; Bassanezi, R.H.B.; Sala, I.; Coletti, D.A.B.; Rodrigues, J.C. Incidence of Diaphorina citri carrying Candidatus Liberibacter asiaticus in Brazil’s citrus belt. Insects 2020, 11, 372. [Google Scholar] [CrossRef]
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H.W. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef]


| Study Site Name | Station Name | Station ID | Latitude (deg) | Longitude (deg) | Elevation (ft) |
|---|---|---|---|---|---|
| Arcadia | Arcadia | 490 | N 27.22621 | W 81.83838 | 64 |
| Conserv II | Avalon | 304 | N 28.47485 | W 81.65300 | 196 |
| Homestead | Homestead | 440 | N 25.5126 | W 80.5031 | 8 |
| Lake Alfred | Lake Alfred | 330 | N 28.10185 | W 81.71128 | 154 |
| Ft. Meade | Ona | 380 | N 27.39750 | W 81.93973 | 75 |
| Lake Wales | Frostproof * | 390 | N 27.76551 | W 81.53735 | 164 |
| Lake Placid | Sebring | 470 | N 27.42108 | W 81.40095 | 117 |
| Site | N | Cycles/Year | k | R2 |
|---|---|---|---|---|
| All | 967 | 2 | 5 | 0.25 |
| Conserv II | 141 | 1 | 6 | 0.50 |
| Lake Alfred | 214 | 1 | 5 | 0.65 |
| Arcadia | 116 | 1 | 6 | 0.13 |
| Homestead | 143 | 2 | 6 | 0.40 |
| Lake Wales | 184 | 2 | 5 | 0.17 |
| Lake Placid | 61 | 1 | 5 | 0.63 |
| Fort Meade | 108 | 2 | 6 | 0.56 |
| Categorical | DF | SS | F | P > F | Source | DF | SS | F | P > F |
|---|---|---|---|---|---|---|---|---|---|
| Model | 42 | 104.82 | 34.63 | <0.01 | location | 6 | 72.10 | 166.73 | <0.01 |
| Error | 658 | 47.42 | color | 1 | 1.46 | 20.22 | <0.01 | ||
| Total | 700 | 152.24 | R2= | 0.69 | year | 3 | 5.01 | 23.17 | <0.01 |
| month | 11 | 4.84 | 6.11 | <0.01 | |||||
| year × month | 21 | 3.83 | 2.53 | <0.01 | |||||
| Continuous | |||||||||
| Model | 9 | 100.27 | 137.98 | <0.01 | location | 6 | 73.60 | 151.92 | <0.01 |
| Error | 715 | 57.73 | JulianFrac | 1 | 6.30 | 78.04 | <0.01 | ||
| Total | 724 | 158.00 | R2= | 0.63 | NM2Solar | 1 | 2.07 | 25.61 | <0.01 |
| PerMG | 1 | 0.80 | 9.97 | <0.01 |
| Df | SS III | F | P > F | Source | df | SS III | F | P > F | |
|---|---|---|---|---|---|---|---|---|---|
| Model | 23 | 101.10 | 58.19 | <0.0001 | Location | 6 | 72.04 | 166.46 | <0.0001 |
| Error | 677 | 51.14 | SandC | 3 | 1.57 | 7.23 | <0.0001 | ||
| Corrected Total | 700 | 152.24 | R2= | 0.66 | Year | 3 | 5.02 | 23.20 | <0.0001 |
| month | 11 | 4.81 | 6.06 | <0.0001 | |||||
| year × month | 20 | 3.82 | 2.53 | 0.0002 | |||||
| Raw Mean % | |||||||||
| Brown female | 35.4 | A | |||||||
| Brown male | 33.2 | AB | |||||||
| Green female | 28.2 | BC | |||||||
| Green male | 25.4 | C | |||||||
| Female | Male | ||||
|---|---|---|---|---|---|
| Brown | Green | Brown | Green | ||
| N | 193 | 187 | 191 | 130 | |
| Female | Brown | 0.848 | 0.942 | 0.808 | |
| Green | 0.861 | 0.786 | |||
| Male | Brown | 0.816 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebert, T.A.; Shawer, D.; Brlansky, R.H.; Rogers, M.E. Seasonal Patterns in the Frequency of Candidatus Liberibacter Asiaticus in Populations of Diaphorina citri (Hemiptera: Psyllidae) in Florida. Insects 2023, 14, 756. https://doi.org/10.3390/insects14090756
Ebert TA, Shawer D, Brlansky RH, Rogers ME. Seasonal Patterns in the Frequency of Candidatus Liberibacter Asiaticus in Populations of Diaphorina citri (Hemiptera: Psyllidae) in Florida. Insects. 2023; 14(9):756. https://doi.org/10.3390/insects14090756
Chicago/Turabian StyleEbert, Timothy A., Dalia Shawer, Ron H. Brlansky, and Michael E. Rogers. 2023. "Seasonal Patterns in the Frequency of Candidatus Liberibacter Asiaticus in Populations of Diaphorina citri (Hemiptera: Psyllidae) in Florida" Insects 14, no. 9: 756. https://doi.org/10.3390/insects14090756





