A High Proportion of Malaria Vector Biting and Resting Indoors despite Extensive LLIN Coverage in Côte d’Ivoire
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
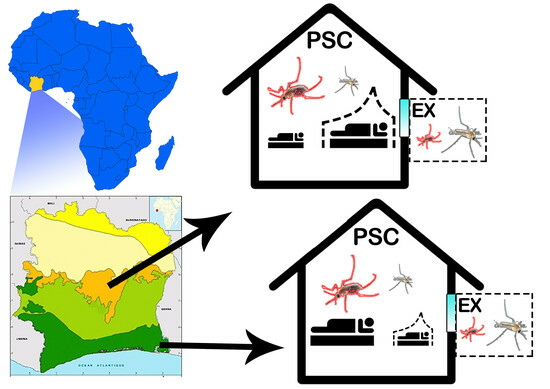
2.1. Study Sites
2.2. Mosquito Sampling and Field Processing
2.3. Molecular Analyses
2.4. Statistical Analysis
3. Results
3.1. Anopheles gambiae Relative Density and Plasmodium falciparum Infection Rate
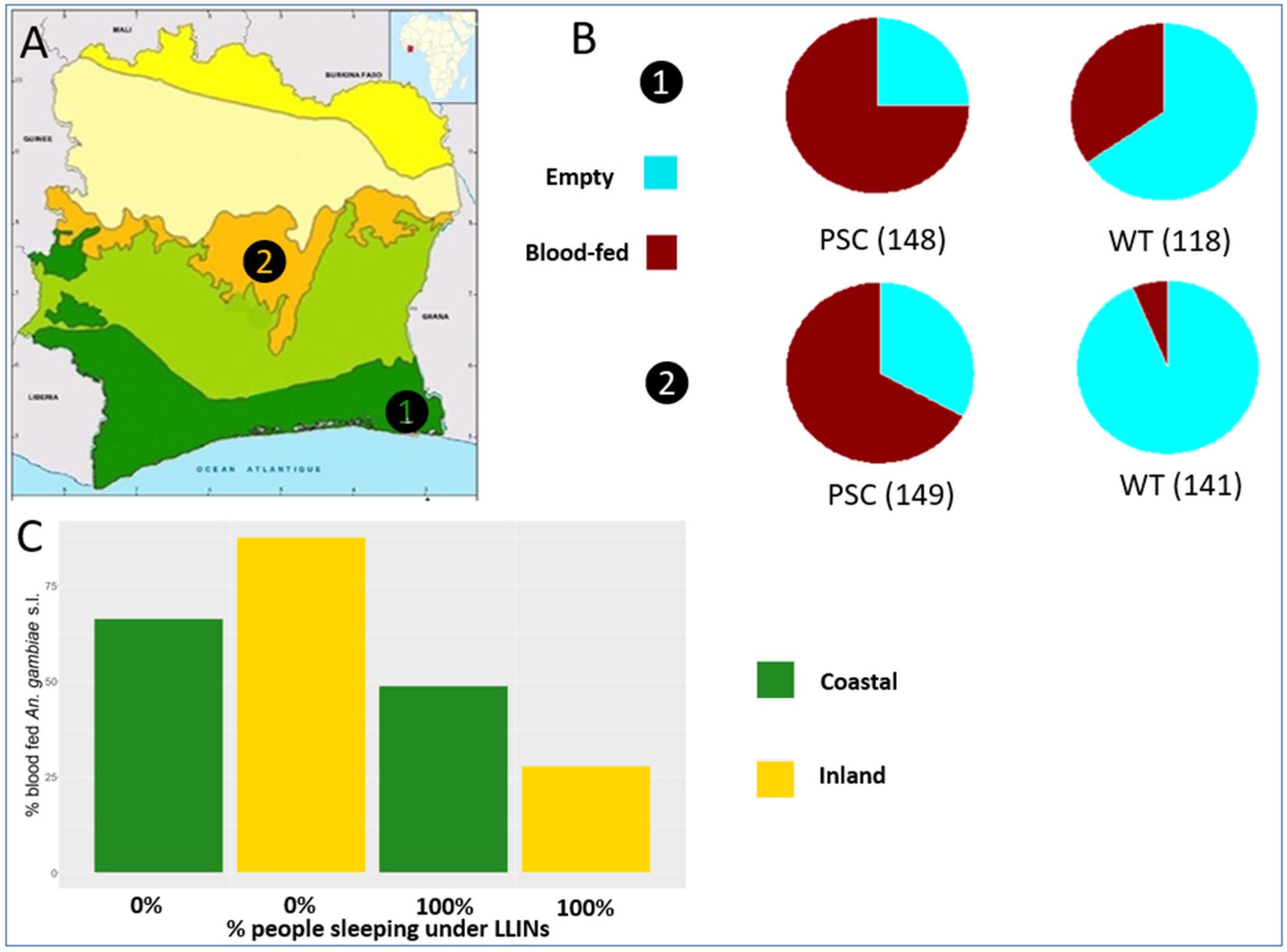
3.2. Anopheles gambiae Human Blood Index (HBI) and Physiological Status and Association with Bed-Net Usage
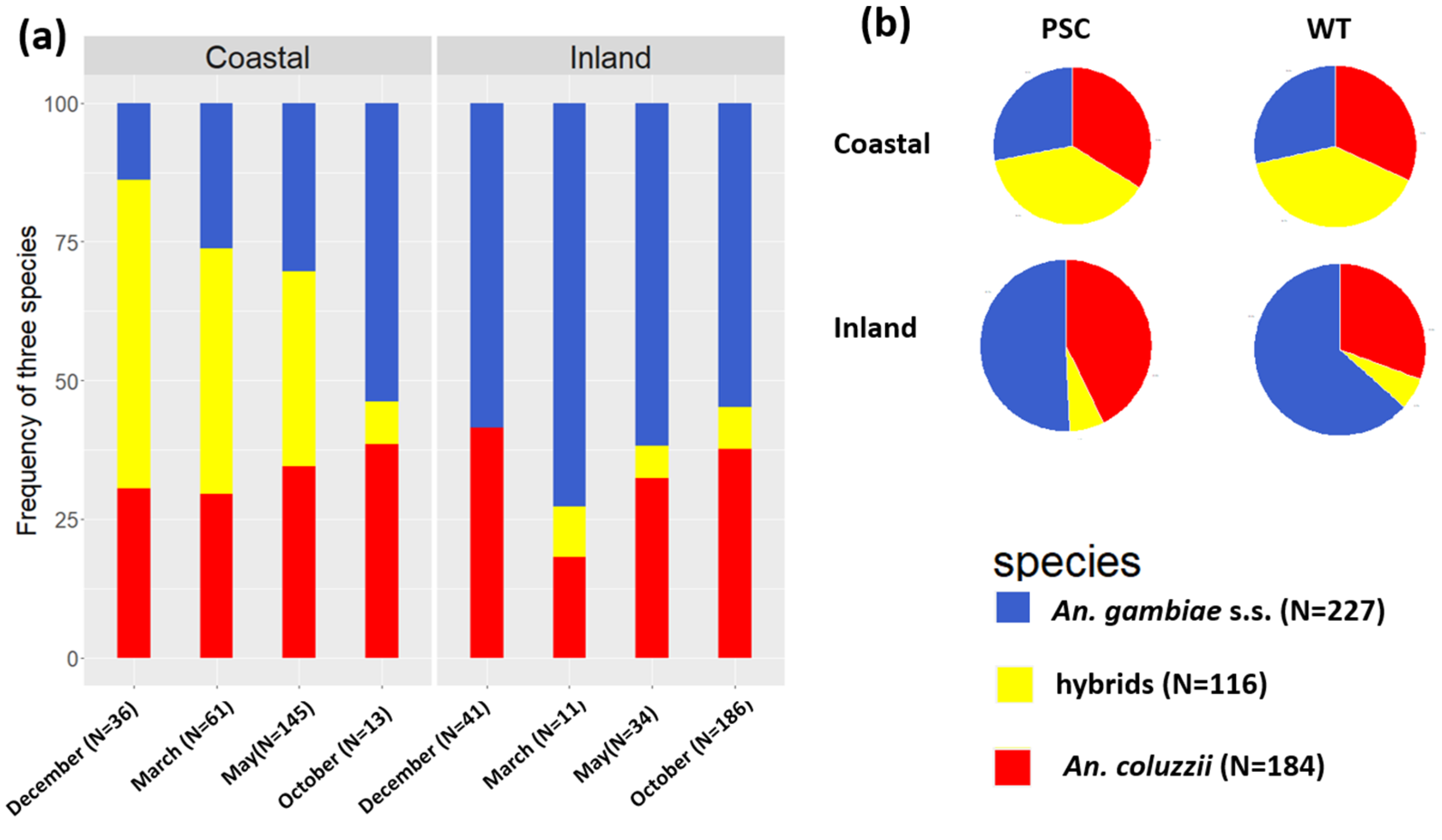
3.3. Anopheles gambiae s.l. Species Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Malaria Report. 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 5 June 2022).
- Coetzee, M.; Hunt, R.H.; Wilkerson, R.; Torre, A.D.; Coulibaly, M.B.; Besansky, N.J. Anopheles coluzzii and Anopheles amharicus, New Members of the Anopheles gambiae Complex. Zootaxa 2013, 3619, 246–274. [Google Scholar] [CrossRef]
- PMI-U.S. President’s Malaria Initiative Côte d’Ivoire Malaria Operational Plan FY 2022; PMI: Arlington, VA, USA, 2022; pp. 1–79. Available online: https://www.pmi.go (accessed on 4 September 2023).
- Kouadio, J.C. Republique de Cote D’ivoire Union-Discipline-Travail; Ministère de la Santé et de l’Hygiène Publique: Abidjan, Côte d’Ivoire, 2021.
- PLNP. Evaluation de l’Efficacité Résiduelle des Moustiquaires Imprégnées à Longue Durée d’Action (MILDA) Dans les Sites Sentinelles de Côte d’Ivoire; Rapport Final; Ministère de la Santé et de l’Hygiène Publique: Abidjan, Côte d’Ivoire, 2019.
- Randriamaherijaona, S.; Briët, O.J.T.; Boyer, S.; Bouraima, A.; N’Guessan, R.; Rogier, C.; Corbel, V. Do Holes in Long-Lasting Insecticidal Nets Compromise Their Efficacy against Pyrethroid Resistant Anopheles gambiae and Culex Quinquefasciatus? Results from a Release-Recapture Study in Experimental Huts. Malar. J. 2015, 14, 332. [Google Scholar] [CrossRef]
- Wheldrake, A.; Guillemois, E.; Arouni, H.; Chetty, V.; Russell, S.J. The Causes of Holes and Loss of Physical Integrity in Long-Lasting Insecticidal Nets. Malar. J. 2021, 20, 45. [Google Scholar] [CrossRef]
- Obi, E.; Okoh, F.; Blaufuss, S.; Olapeju, B.; Akilah, J.; Okoko, O.O.; Okechukwu, A.; Maire, M.; Popoola, K.; Yahaya, M.A.; et al. Monitoring the Physical and Insecticidal Durability of the Long-Lasting Insecticidal Net DawaPlus® 2.0 in Three States in Nigeria. Malar. J. 2020, 19, 124. [Google Scholar] [CrossRef]
- Ochomo, E.O.; Bayoh, N.M.; Walker, E.D.; Abongo, B.O.; Ombok, M.O.; Ouma, C.; Githeko, A.K.; Vulule, J.; Yan, G.; Gimnig, J.E. The Efficacy of Long-Lasting Nets with Declining Physical Integrity May Be Compromised in Areas with High Levels of Pyrethroid Resistance. Malar. J. 2013, 12, 368. [Google Scholar] [CrossRef]
- Allan, R.; O’Reilly, L.; Gilbos, V.; Kilian, A. An Observational Study of Material Durability of Three World Health Organization–Recommended Long-Lasting Insecticidal Nets in Eastern Chad. Am. J. Trop. Med. Hyg. 2012, 87, 407. [Google Scholar] [CrossRef] [PubMed]
- Hiruy, H.N.; Zewde, A.; Irish, S.R.; Abdelmenan, S.; Woyessa, A.; Wuletaw, Y.; Solomon, H.; Haile, M.; Sisay, A.; Chibsa, S.; et al. The Effect of Long-Lasting Insecticidal Nets (LLINs) Physical Integrity on Utilization. Malar. J. 2021, 20, 468. [Google Scholar] [CrossRef]
- Bamou, R.; Rono, M.; Degefa, T.; Midega, J.; Mbogo, C.; Ingosi, P.; Kamau, A.; Ambelu, A.; Birhanu, Z.; Tushune, K.; et al. Entomological and Anthropological Factors Contributing to Persistent Malaria Transmission in Kenya, Ethiopia, and Cameroon. J. Infect. Dis. 2021, 223, S155. [Google Scholar] [CrossRef] [PubMed]
- Monroe, A.; Moore, S.; Koenker, H.; Lynch, M.; Ricotta, E. Measuring and Characterizing Night Time Human Behaviour as It Relates to Residual Malaria Transmission in Sub-Saharan Africa: A Review of the Published Literature. Malar. J. 2019, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Finda, M.F.; Moshi, I.R.; Monroe, A.; Limwagu, A.J.; Nyoni, A.P.; Swai, J.K.; Ngowo, H.S.; Minja, E.G.; Toe, L.P.; Kaindoa, E.W.; et al. Linking Human Behaviours and Malaria Vector Biting Risk in South-Eastern Tanzania. PLoS ONE 2019, 14, e0217414. [Google Scholar] [CrossRef]
- Sokhna, C.; Ndiath, M.O.; Rogier, C. The Changes in Mosquito Vector Behaviour and the Emerging Resistance to Insecticides Will Challenge the Decline of Malaria. Clin. Microbiol. Infect. 2013, 19, 902–907. [Google Scholar] [CrossRef]
- Kawada, H.; Ohashi, K.; Dida, G.O.; Sonye, G.; Njenga, S.M.; Mwandawiro, C.; Minakawa, N. Preventive Effect of Permethrin-Impregnated Long-Lasting Insecticidal Nets on the Blood Feeding of Three Major Pyrethroid-Resistant Malaria Vectors in Western Kenya. Parasites Vectors 2014, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Strode, C.; Donegan, S.; Garner, P.; Enayati, A.A.; Hemingway, J. The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis. PLoS Med. 2014, 11, e1001619. [Google Scholar] [CrossRef]
- Ranson, H.; Edi, C.V.A.; Koudou, B.G.; Jones, C.M.; Weetman, D. Multiple-Insecticide Resistance in Anopheles gambiae Mosquitoes, Southern Côte d’Ivoire. Emerg. Infect. Dis. 2012, 18, 1508–1511. [Google Scholar] [CrossRef]
- Koffi, A.A.; Ahoua Alou, L.P.; Adja, M.A.; Chandre, F.; Pennetier, C. Insecticide Resistance Status of Anopheles gambiae s.s Population from M’Bé: A WHOPES-Labelled Experimental Hut Station, 10 Years after the Political Crisis in Côte d’Ivoire. Malar. J. 2013, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Koffi, A.A.; Alou, L.P.A.; Adja, M.A.; Koné, M.; Chandre, F.; N’Guessan, R. Update on Resistance Status of Anopheles gambiae s.s. to Conventional Insecticides at a Previous WHOPES Field Site, “Yaokoffikro”, 6 Years after the Political Crisis in Côte d’Ivoire. Parasites Vectors 2012, 5, 68. [Google Scholar] [CrossRef]
- Sangbakembi-Ngounou, C.; Costantini, C.; Longo-Pendy, N.M.; Ngoagouni, C.; Akone-Ella, O.; Rahola, N.; Cornelie, S.; Kengne, P.; Nakouné, E.R.; Komas, N.P.; et al. Diurnal Biting of Malaria Mosquitoes in the Central African Republic Indicates Residual Transmission May Be “out of Control”. Proc. Natl. Acad. Sci. USA 2022, 119, e2104282119. [Google Scholar] [CrossRef] [PubMed]
- Caputo, B.; Tondossoma, N.; Virgillito, C.; Pichler, V.; Serini, P.; Calzetta, M.; Manica, M.; Coulibaly, Z.I.; Dia, I.; Akré, M.; et al. Is Côte D’Ivoire a New High Hybridization Zone for the Two Major Malaria Vectors, Anopheles coluzzii and An. Gambiae (Diptera, Culicidae)? Infect. Genet. Evol. 2022, 98, 105215. [Google Scholar] [CrossRef]
- Wolie, R.Z.; Koffi, A.A.; Ayuk-Taylor, L.; Alou, L.P.A.; Sternberg, E.D.; N’Nan-Alla, O.; N’Guessan, Y.; Dahounto, A.; Oumbouke, W.A.; Tia, I.Z.; et al. Entomological Indicators of Malaria Transmission Prior to a Cluster-Randomized Controlled Trial of a ‘Lethal House Lure’ Intervention in Central Côte d’Ivoire. Malar. J. 2022, 21, 188. [Google Scholar] [CrossRef]
- Zogo, B.; Soma, D.D.; Tchiekoi, B.N.C.; Somé, A.; Alou, L.P.A.; Kof, A.A.; Fournet, F.; Dahounto, A.; Coulibaly, B.; Kandé, S.; et al. Anopheles Bionomics, Insecticide Resistance Mechanisms, and Malaria Transmission in the Korhogo Area, Northern Côte d’Ivoire: A Pre-Intervention Study. Parasite 2019, 26, 40. [Google Scholar] [CrossRef]
- WHO. Morbidity, Managing Disability, Preventing Nationa. In 2013 World Health Organization Global Programme to Eliminate Lymphatic Filariasis; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- WHO. Manual on Practical Entomology in Malaria/Prepared by the WHO Division of Malaria and Other Parasitic Diseases. 1995; Available online: https://apps.who.int/iris/handle/10665/42481 (accessed on 25 October 2022).
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region); South African Institute for Medical Research: Cape Town, South Africa, 1968. [Google Scholar]
- Detinova, T. Age-Grouping Methods in Diptera of Medical Importance, with Special Reference to Some Vectors of Malaria; World Health Organization Monograph Series; WHO: Geneva, Switzerland, 1962; Volume 47, pp. 13–191. Available online: https://apps.who.int/iris/handle/10665/41724 (accessed on 25 October 2022).
- Rider, M.A.; Byrd, B.D.; Keating, J.; Wesson, D.M.; Caillouet, K.A. PCR Detection of Malaria Parasites in Desiccated Anopheles Mosquitoes Is Uninhibited by Storage Time and Temperature. Malar. J. 2012, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.E.; Howell, P.I.; Benedict, M.Q. IMP PCR Primers Detect Single Nucleotide Polymorphisms for Anopheles gambiae Species Identification, Mopti and Savanna RDNA Types, and Resistance to Dieldrin in Anopheles arabiensis. Malar. J. 2006, 5, 125. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Blagborough, A.M.; Vontas, J.; Sinden, R.E.; Williamson, M.S.; Field, L.M. PCR-Based Detection of Plasmodium in Anopheles Mosquitoes: A Comparison of a New High-Throughput Assay with Existing Methods. Malar. J. 2008, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.J.; Norris, D.E. Identification of Mammalian Blood Meals in Mosquitoes by a Multiplexed Polymerase Chain Reaction Targeting Cytochrome B. Am. J. Trop. Med. Hyg. 2005, 73, 336. [Google Scholar] [CrossRef] [PubMed]
- Entomologie du Paludisme et Contrôle des Vecteurs Guide du Stagiaire Organisation Mondiale de la Santé Mobilisation Sociale et Formation Département du Contrôle, de la Prévention et de l’Eradication Groupe des Maladies Transmissibles Edition Provisoire; World Health Organization, Social Mobilization and Training Team. 2003. Available online: https://apps.who.int/iris/bitstream/handle/10665/68376/WHO_CDS_CPE_SMT_2002.18_Rev.1_PartieI.pdf (accessed on 22 May 2023).
- R Project. R: The R Project for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 22 May 2023).
- Yokoly, F.N.; Zahouli, J.B.Z.; Small, G.; Ouattara, A.F.; Opoku, M.; de Souza, D.K.; Koudou, B.G. Assessing Anopheles Vector Species Diversity and Transmission of Malaria in Four Health Districts along the Borders of Côte d’Ivoire. Malar. J. 2021, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, A.L.; Esayas, E.; Sutcliffe, A.C.; Irish, S.R.; Gadisa, E.; Tadesse, F.G.; Lobo, N.F. A Comparison of PCR and ELISA Methods to Detect Different Stages of Plasmodium vivax in Anopheles arabiensis. Parasites Vectors 2021, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Hawley, W.A.; Phillips-Howard, P.A.; Ter Kuile, F.O.; Terlouw, D.J.; Vulule, J.M.; Ombok, M.; Nahlen, B.L.; Gimnig, J.E.; Kariuki, S.K.; Kolczak, M.S.; et al. Community-Wide Effects of Permethrin-Treated Bed Nets on Child Mortality and Malaria Morbidity in Western Kenya. Am. J. Trop. Med. Hyg. 2003, 68, 121–127. [Google Scholar] [CrossRef]
- Russell, T.L.; Lwetoijera, D.W.; Maliti, D.; Chipwaza, B.; Kihonda, J.; Charlwood, J.D.; Smith, T.A.; Lengeler, C.; Mwanyangala, M.A.; Nathan, R.; et al. Impact of Promoting Longer-Lasting Insecticide Treatment of Bed Nets upon Malaria Transmission in a Rural Tanzanian Setting with Pre-Existing High Coverage of Untreated Nets. Malar. J. 2010, 9, 187. [Google Scholar] [CrossRef]
- Killeen, G.F.; Smith, T.A.; Ferguson, H.M.; Mshinda, H.; Abdulla, S.; Lengeler, C.; Kachur, S.P. Preventing Childhood Malaria in Africa by Protecting Adults from Mosquitoes with Insecticide-Treated Nets. PLoS Med. 2007, 4, e229. [Google Scholar] [CrossRef] [PubMed]
- Govella, N.J.; Okumu, F.O.; Killeen, G.F. Insecticide-Treated Nets Can Reduce Malaria Transmission by Mosquitoes Which Feed Outdoors. Am. J. Trop. Med. Hyg. 2010, 82, 415. [Google Scholar] [CrossRef]
- Bayoh, M.N.; Walker, E.D.; Kosgei, J.; Ombok, M.; Olang, G.B.; Githeko, A.K.; Killeen, G.F.; Otieno, P.; Desai, M.; Lobo, N.F.; et al. Persistently High Estimates of Late Night, Indoor Exposure to Malaria Vectors despite High Coverage of Insecticide Treated Nets. Parasites Vectors 2014, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Oumbouke, W.A.; Koffi, A.A.; Alou, L.P.A.; Rowland, M.; N’Guessan, R. Evaluation of Standard Pyrethroid Based LNs (MiraNet and MagNet) in Experimental Huts against Pyrethroid Resistant Anopheles gambiae s.l. M’bé, Côte d’Ivoire: Potential for Impact on Vectorial Capacity. PLoS ONE 2019, 14, e0215074. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.L.; Govella, N.J.; Azizi, S.; Drakeley, C.J.; Kachur, S.P.; Killeen, G.F. Increased Proportions of Outdoor Feeding among Residual Malaria Vector Populations Following Increased Use of Insecticide-Treated Nets in Rural Tanzania. Malar. J. 2011, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Moshi, I.R.; Manderson, L.; Ngowo, H.S.; Mlacha, Y.P.; Okumu, F.O.; Mnyone, L.L. Outdoor Malaria Transmission Risks and Social Life: A Qualitative Study in South-Eastern Tanzania. Malar. J. 2018, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Tondossama, N.; Coulibaly, Z.I.; Traoré, I.; Ako, B.A.; Zoh, D.D.; Virgillito, C.; Guindo-Coulibaly, N.; Serini, P.; Assouho, F.K.; Dia, I.; et al. High Levels of Admixture in Anopheles gambiae Populations from Côte d’Ivoire Revealed by Multilocus Genotyping. Insects 2022, 13, 1090. [Google Scholar] [CrossRef] [PubMed]
- Asidi, A.; N’Guessan, R.; Akogbeto, M.; Curtis, C.; Rowland, M. Loss of Household Protection from Use of Insecticide-Treated Nets against Pyrethroid-Resistant Mosquitoes, Benin. Emerg. Infect. Dis. 2012, 18, 1101. [Google Scholar] [CrossRef] [PubMed]
- Zahouli, J.Z.B.; Edi, C.A.V.; Yao, L.A.; Lisro, E.G.; Adou, M.; Koné, I.; Small, G.; Sternberg, E.D.; Koudou, B.G. Small-Scale Field Evaluation of PermaNet® Dual (a Long-Lasting Net Coated with a Mixture of Chlorfenapyr and Deltamethrin) against Pyrethroid-Resistant Anopheles gambiae Mosquitoes from Tiassalé, Côte d’Ivoire. Malar. J. 2023, 22, 36. [Google Scholar] [CrossRef]
- Barreaux, P.; Koella, J.C.; N’Guessan, R.; Thomas, M.B. Use of Novel Lab Assays to Examine the Effect of Pyrethroid-Treated Bed Nets on Blood-Feeding Success and Longevity of Highly Insecticide-Resistant Anopheles gambiae s.l. Mosquitoes. Parasites Vectors 2022, 15, 111. [Google Scholar] [CrossRef]
- Glunt, K.D.; Coetzee, M.; Huijben, S.; Koffi, A.A.; Lynch, P.A.; N’Guessan, R.; Oumbouke, W.A.; Sternberg, E.D.; Thomas, M.B. Empirical and Theoretical Investigation into the Potential Impacts of Insecticide Resistance on the Effectiveness of Insecticide-Treated Bed Nets. Evol. Appl. 2018, 11, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Hauser, G.; Thiévent, K.; Koella, J.C. The Ability of Anopheles gambiae Mosquitoes to Bite through a Permethrin-Treated Net and the Consequences for Their Fitness. Sci. Rep. 2019, 9, 8141. [Google Scholar] [CrossRef] [PubMed]
- Mwangangi, J.M.; Mbogo, C.M.; Orindi, B.O.; Muturi, E.J.; Midega, J.T.; Nzovu, J.; Gatakaa, H.; Githure, J.; Borgemeister, C.; Keating, J.; et al. Shifts in Malaria Vector Species Composition and Transmission Dynamics along the Kenyan Coast over the Past 20 Years. Malar. J. 2013, 12, 13. [Google Scholar] [CrossRef]
| Village | Sampling Methods | N | m/p/n (95%CI) | M | PfR (95%CI) |
|---|---|---|---|---|---|
| Coastal | PSC | 48 | 1.24 (0.63/1.85) | 89 (18) | 20% (13–29%) |
| WT | 80 | 0.76 (0.29/1.24) | 118 (6) | 5% (2–10%) | |
| Inland | PSC | 78 | 1.08 (0.60/1.56) | 97 (34) | 35% (26–45%) |
| WT | 80 | 0.50 (0.03/0.97) | 141 (7) | 4% (2–10%) |
| Physiological Status | ||||||
|---|---|---|---|---|---|---|
| Village | Samplig Method | Unfed | Half Gravid | Freshly Fed | Gravid | Tot |
| Coastal | PSC | 20% | 45% | 30% | 5% | 148 |
| WT | 51% | 18% | 17% | 14% | 118 | |
| Inland | PSC | 16% | 39% | 28% | 17% | 149 |
| WT | 74% | 4% | 2% | 20% | 141 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tondossama, N.; Virgillito, C.; Coulibaly, Z.I.; Pichler, V.; Dia, I.; della Torre, A.; Touré, A.O.; Adja, A.M.; Caputo, B. A High Proportion of Malaria Vector Biting and Resting Indoors despite Extensive LLIN Coverage in Côte d’Ivoire. Insects 2023, 14, 758. https://doi.org/10.3390/insects14090758
Tondossama N, Virgillito C, Coulibaly ZI, Pichler V, Dia I, della Torre A, Touré AO, Adja AM, Caputo B. A High Proportion of Malaria Vector Biting and Resting Indoors despite Extensive LLIN Coverage in Côte d’Ivoire. Insects. 2023; 14(9):758. https://doi.org/10.3390/insects14090758
Chicago/Turabian StyleTondossama, Naminata, Chiara Virgillito, Zanakoungo Ibrahima Coulibaly, Verena Pichler, Ibrahima Dia, Alessandra della Torre, Andre Offianan Touré, Akré Maurice Adja, and Beniamino Caputo. 2023. "A High Proportion of Malaria Vector Biting and Resting Indoors despite Extensive LLIN Coverage in Côte d’Ivoire" Insects 14, no. 9: 758. https://doi.org/10.3390/insects14090758
APA StyleTondossama, N., Virgillito, C., Coulibaly, Z. I., Pichler, V., Dia, I., della Torre, A., Touré, A. O., Adja, A. M., & Caputo, B. (2023). A High Proportion of Malaria Vector Biting and Resting Indoors despite Extensive LLIN Coverage in Côte d’Ivoire. Insects, 14(9), 758. https://doi.org/10.3390/insects14090758