Volatile Organic Compounds from Cassava Plants Confer Resistance to the Whitefly Aleurothrixus aepim (Goeldi, 1886)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Insects
2.3. Dynamic Headspace Collection
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.5. Olfactometer Assays
2.5.1. Y-Tube Olfactometer
2.5.2. Odour Treatments
2.6. Chemometric Analysis
2.7. Statistical Analysis
3. Results
3.1. Different Genotypes Constitutively Express Different VOC Blends
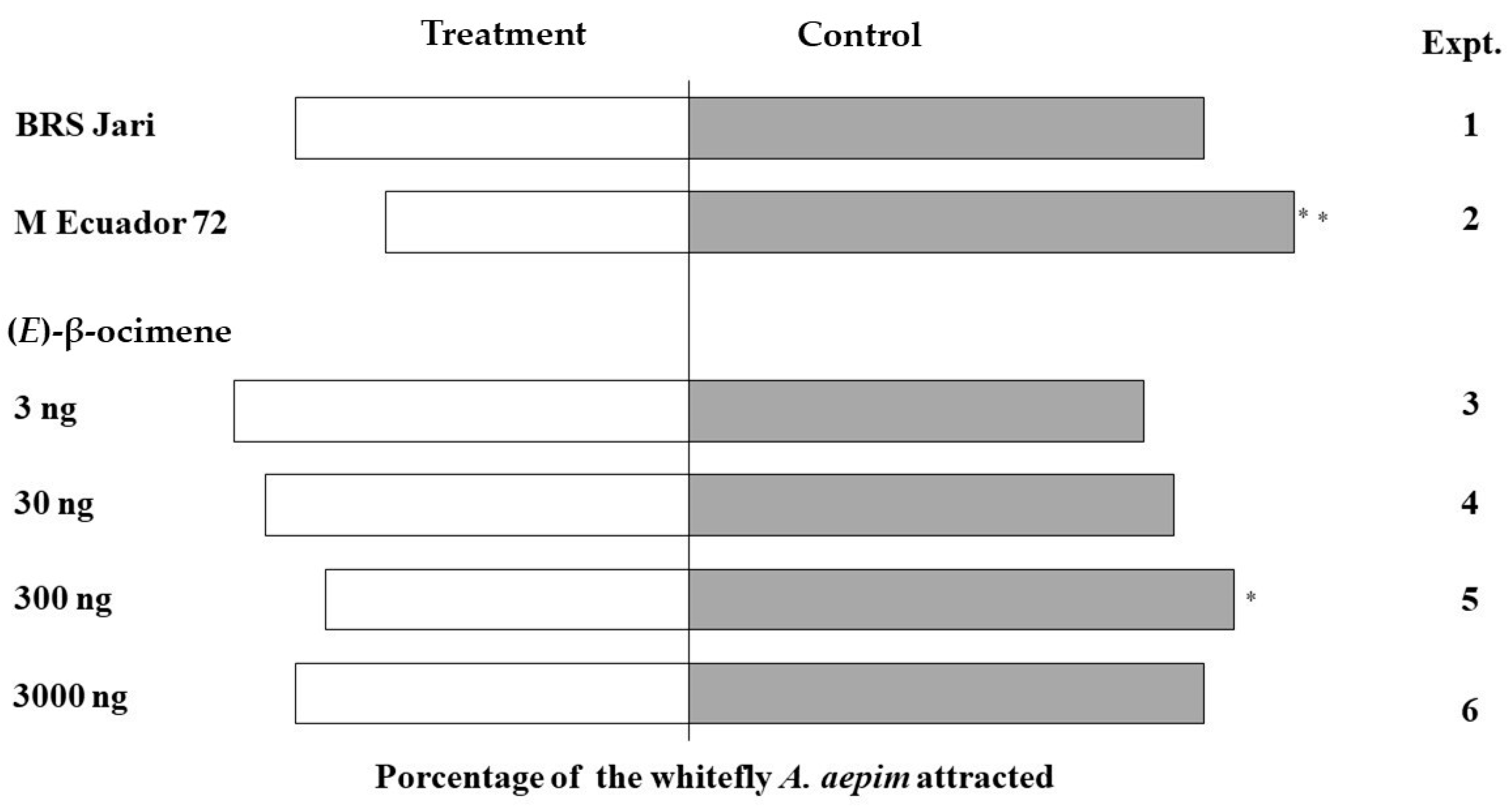
3.2. Behavioural Responses of Whiteflies to Volatiles Emitted by Two Cassava Genotypes
3.3. Comparative Analysis of the VOC Profiles of M Ecuador 72 and BRS Jari
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howeler, R.H.; Lutaladio, N.; Thomas, G. Food and Agriculture Organization of the United Nations. Save and Grow: Cassava: A Guide to Sustainable Production Intensification; Food and Agriculture Organization of the United Nations: Roma, Italy, 2013; ISBN 9251076413. [Google Scholar]
- Blagbrough, I.S.; Bayoumi, S.A.L.; Rowan, M.G.; Beeching, J.R. Cassava: An appraisal of its Phytochemistry and its Biotechnological Prospects. Phytochemistry 2010, 71, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Pastório, M.A.; Hoshino, A.T.; Kitzberger, C.S.G.; Bortolotto, O.C.; de Oliveira, L.M.; dos Santos, A.M.; Lima, W.F.; Menezes Junior, A.D.O.; Androcioli, H.G. The leaf color and trichome density influence the whitefly infestation in different cassava cultivars. Insects 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Amelework, A.B.; Bairu, M.W.; Marx, R.; Owoeye, L.; Laing, M.; Venter, S.L. On-Farm Multi-Environment Evaluation of Selected Cassava (Manihot esculenta Crantz) cultivars in South Africa. Plants 2022, 11, 3339. [Google Scholar] [CrossRef]
- Alves-Pereira, A.; Zucchi, M.I.; Clement, C.R.; Viana, J.P.G.; Pinheiro, J.B.; Veasey, E.A.; de Souza, A.P. Selective signatures and high genome-wide diversity in traditional brazilian manioc (Manihot esculenta Crantz) varieties. Sci. Rep. 2022, 12, 1268. [Google Scholar] [CrossRef]
- Kaur, K.; Ahluwalia, P. Cassava as potential crop for the food and fermentation industry: A review. Int. J. Food Ferment. Technol. 2017, 7, 1. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Bioeconomy 101. Available online: www.fao.org (accessed on 25 July 2013).
- Conab-Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira Safra 2022–Segundo Levantamento. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 5 August 2022).
- Bellotti, A.C.; Arias, V.B.; Vargas, H.O.; Peña, J.E. Insects and mites causing yield losses in cassava. In Cassava in the Third Millennium: Modern production, Processing, Use, and Marketing Systems; Ospina Patiño, B., Ceballos, H., Eds.; Centro Internacional de Agricultura Tropical (CIAT); Latin American and Caribbean Consortium to support Cassava Research and Development (CLAYUCA); Technical Centre for Agricultural and Rural Cooperation (CTA): Cali, Colombia, 2012; pp. 251–264. [Google Scholar]
- Jacobson, A.L.; Duffy, S.; Sseruwagi, P. Whitefly-transmitted viruses threatening cassava production in Africa. Curr. Opin. Virol. 2018, 33, 167–176. [Google Scholar] [CrossRef]
- Mohammed, I.U.; Abarshi, M.M.; Muli, B.; Hillocks, R.J.; Maruthi, M.N. The symptom and genetic diversity of cassava brown streak viruses infecting cassava in East Africa. Adv. Virol. 2012, 2012, 795697. [Google Scholar] [CrossRef] [PubMed]
- Omongo, C.A.; Opio, S.M.; Bayiyana, I.; Otim, M.H.; Omara, T.; Wamani, S.; Ocitti, P.; Bua, A.; Macfadyen, S.; Colvin, J. African cassava whitefly and viral disease management through timed application of imidacloprid. Crop Prot. 2022, 158, 106015. [Google Scholar] [CrossRef]
- Patil, B.L.; Legg, J.P.; Kanju, E.; Fauquet, C.M. Cassava brown streak disease: A threat to food security in Africa. J. Gen. Virol. 2015, 96, 956–968. [Google Scholar] [CrossRef]
- Rey, C.; Vanderschuren, H. Cassava mosaic and brown streak diseases: Current perspectives and beyond. Annu. Rev. Virol. 2017, 4, 429–452. [Google Scholar] [CrossRef]
- Nyirakanani, C.; Bizimana, J.P.; Kwibuka, Y.; Nduwumuremyi, A.; Bigirimana, V.D.P.; Bucagu, C.; Lassois, L.; Malice, E.; Gengler, N.; Massart, S.; et al. Farmer and field survey in cassava-growing districts of rwanda reveals key factors associated with cassava brown streak disease incidence and cassava productivity. Front. Sustain. Food Syst. 2021, 5, 699655. [Google Scholar] [CrossRef]
- Legg, J.P.; Shirima, R.; Tajebe, L.S.; Guastella, D.; Boniface, S.; Jeremiah, S.; Nsami, E.; Chikoti, P.; Rapisarda, C. Biology and management of Bemisia whitefly vectors of cassava virus pandemics in Africa. Pest Manag. Sci. 2014, 70, 1446–1453. [Google Scholar] [CrossRef]
- Parry, H.; Kalyebi, A.; Bianchi, F.; Sseruwagi, P.; Colvin, J.; Schellhorn, N.; Macfadyen, S. Evaluation of cultural control and resistance breeding strategies for suppression of whitefly infestation of cassava at the landscape scale: A simulation modeling approach. Pest Manag. Sci. 2020, 76, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, A.; van Schoonhoven, A. Mite and insect pests of cassava. Annu. Rev. Entomol. 1978, 23, 39–67. [Google Scholar] [CrossRef] [PubMed]
- Pietrowski, V.; Rheinheimer, A.R.; Miranda, A.M.; Wengrat, A.P.G.D.S.; Barilli, D.R. Ocorrência de Aleurothrixus aepim (Goeldi, 1886) em mandioca na região oeste do Paraná. Arq. Inst. Biol. 2014, 81, 186–188. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Rani, K.; Arya, S.S.; Devi, S.; Kaur, V. Plant Volatiles and defense. In Volatiles and Food Security; Springer: Singapore, 2017; pp. 113–134. ISBN 9789811055539. [Google Scholar]
- Aljbory, Z.; Chen, M.S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Razo-Belman, R.; Ozuna, C. Volatile organic compounds: A review of their current applications as pest biocontrol and disease management. Horticulturae 2023, 9, 441. [Google Scholar] [CrossRef]
- Mishra, M.; Lomate, P.R.; Joshi, R.S.; Punekar, S.A.; Gupta, V.S.; Giri, A.P. Ecological turmoil in evolutionary dynamics of plant–insect interactions: Defense to offence. Planta 2015, 242, 761–771. [Google Scholar] [CrossRef]
- Heil, M. Indirect defence via tritrophic interactions. N. Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Gish, M.; De Moraes, C.M.; Mescher, M.C. Herbivore-induced plant volatiles in natural and agricultural ecosystems: Open questions and future prospects. Curr. Opin. Insect Sci. 2015, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Tumlinson, J.H. Plant Volatiles as a Defense against Insect Herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Ogburia, M.N. Occurrence, morphology, distribution and effect of extra-floral nectaries (EFNs) in cassava (Manihot esculenta Crantz) under greenhouse culture condition plantain/banana genetic conservation and breeding in Nigeria view project. Phytomorphology 2003, 53, 335–342. [Google Scholar]
- Hountondji, F.C.C.; Sabelis, M.W.; Hanna, R.; Janssen, A. Herbivore-induced plant volatiles trigger sporulation in entomopathogenic fungi: The case of neozygites tanajoae infecting the cassava green mite. J. Chem. Ecol. 2005, 31, 1003–1021. [Google Scholar] [CrossRef]
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W.; et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705. [Google Scholar] [CrossRef]
- Pinto-Zevallos, D.M.; Pareja, M.; Ambrogi, B.G. Current knowledge and future research perspectives on cassava (Manihot Esculenta Crantz) chemical defenses: An agroecological view. Phytochemistry 2016, 130, 10–21. [Google Scholar] [PubMed]
- Carabalí, A.; Bellotti, A.C.; Montoya-Lerma, J.; Fregene, M. Resistance to the whitefly, Aleurotrachelus Socialis, in wild populations of cassava, Manihot Tristis. J. Insect Sci. 2010, 10, 1–10. [Google Scholar] [CrossRef][Green Version]
- Ricardo Barilli, D.; Paula Gonçalves da Silva Wengrat, A.; Tereza Bittencourt Guimarães, A.; Pietrowski, V.; Ringenberg, R.; Silveira Garcia, M. Resistance of cassava genotypes to Bemisia Tuberculata. Arthropod. Plant. Interact. 2019, 13, 663–669. [Google Scholar] [CrossRef]
- Bellotti, A.; Arias, B. Host Plant Resistance to whiteflies with emphasis on cassava as a case study. Crop Prot. 2001, 20, 813–823. [Google Scholar] [CrossRef]
- Reddy, G.V. Plant volatiles mediate orientation and plant preference by the predator Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). Biol. Control 2002, 25, 49–55. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, S.; Qin, Y.; Zhang, S.; Gao, Z.; Dang, Z.; Pan, W. Identification of plant chemicals attracting and repelling whiteflies. Arthropod. Plant. Interact. 2014, 8, 183–190. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, 68. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D. Genes—A software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 2013, 35, 271–276. [Google Scholar] [CrossRef]
- Kang, Z.-W.; Liu, F.-H.; Zhang, Z.-F.; Tian, H.-G.; Liu, T.-X. Volatile β-ocimene can regulate developmental performance of peach aphid myzus persicae through activation of defense responses in chinese cabbage Brassica pekinensis. Front. Plant Sci. 2018, 9, 708. [Google Scholar] [CrossRef]
- Tu, H.; Qin, Y. Repellent effects of different celery varieties in Bemisia tabaci (Hemiptera: Aleyrodidae) Biotype Q. J. Econ. Entomol. 2017, 110, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Jian, G.; Jia, Y.; Li, J.; Zhou, X.; Liao, Y.; Dai, G.; Zhou, Y.; Tang, J.; Zeng, L. Elucidation of the regular emission mechanism of volatile β-ocimene with anti-insect function from tea plants (Camellia Sinensis) exposed to herbivore attack. J. Agric. Food Chem. 2021, 69, 11204–11215. [Google Scholar] [CrossRef]
- Jing, T.; Qian, X.; Du, W.; Gao, T.; Li, D.; Guo, D.; He, F.; Yu, G.; Li, S.; Schwab, W.; et al. Herbivore-induced volatiles influence moth preference by increasing the β-ocimene emission of neighbouring tea plants. Plant. Cell Environ. 2021, 44, 3667–3680. [Google Scholar] [CrossRef] [PubMed]
- Celedon, J.M.; Bohlmann, J. Oleoresin defenses in conifers: Chemical diversity, terpene synthases and limitations of oleoresin defense under climate change. New Phytol. 2019, 224, 1444–1463. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The Role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Karban, R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 2010, 25, 137–144. [Google Scholar] [CrossRef]
- Murali-Baskaran, R.K.; Mooventhan, P.; Das, D.; Dixit, A.; Sharma, K.C.; Senthil-Nathan, S.; Kaushal, P.; Ghosh, P.K. The future of plant volatile organic compounds (PVOCs) research: Advances and applications for sustainable agriculture. Environ. Exp. Bot. 2022, 200, 104912. [Google Scholar] [CrossRef]
- Bellotti, A.; Peña, J.; Arias, B.; Guerrero, J.M.; Trujillo, H.; Holguín, C.; Ortega, A. Biological control of whiteflies by indigenous natural enemies for major food crops in the neotropics. In Whitefly and Whitefly-Borne Viruses in the Tropics: Building a Knowledge Base for Global Action; Anderson, P.K., Morales, F.J., Eds.; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 2005; pp. 313–323. [Google Scholar]
- Omongo, C.A.; Kawuki, R.; Bellotti, A.C.; Alicai, T.; Baguma, Y.; Maruthi, M.; Bua, A.; Colvin, J. African cassava whitefly, Bemisia tabaci, resistance in African and south American cassava genotypes. J. Integr. Agric. 2012, 11, 327–336. [Google Scholar] [CrossRef]
- Lima, W.H.; Ringenberg, R.; Fancelli, M.; Alberto, C.; Ledo, S. Resistance of Manihot esculenta and its intraspecific hybrids to the whitefly Aleurothrixus aepim (Hemiptera: Aleyrodidae). Pesq. Agropec. Bras 2018, 53, 885–891. [Google Scholar] [CrossRef]
- Parsa, S.; Medina, C.; Rodríguez, V. Sources of pest resistance in cassava. Crop Prot. 2015, 68, 79–84. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Lengwiler, U.B.; Bernasconi, M.L.; Wechsler, D. Timing of induced volatile emissions in maize seedlings. Planta 1998, 207, 146–152. [Google Scholar] [CrossRef]
- Takabayashi, J.; Dicke, M.; Posthumus, M.A. Variation in Composition of Predator-Attracting Allelochemicals Emitted by Herbivore-Infested Plants: Relative Influence of Plant and Herbivore. Chemoecology 1991, 2, 1–6. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Manukian, A.; Heath, R.R.; Tumlinson, J.H. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J. Chem. Ecol. 1995, 21, 1217–1227. [Google Scholar] [PubMed]
- Halitschke, R.; Keßler, A.; Kahl, J.; Lorenz, A.; Baldwin, I.T. Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia 2000, 124, 408–417. [Google Scholar] [CrossRef]
- Aartsma, Y.; Leroy, B.; Van Der Werf, W.; Dicke, M.; Poelman, E.H.; Bianchi, F.J.J.A.; Aartsma, Y.; Leroy, B.; Bianchi, F.J.J.A. Intraspecific variation in herbivore-induced plant volatiles influences the spatial range of plant-parasitoid interactions. Nord. Soc. Oikos 2019, 128, 77-68. [Google Scholar]
- Yang, F.; Dong, W.; Zhang, X.; Li, Y.; Zhou, S.; Zhu, G.; Xiao, C. Volatile-organic compound changes in rose twigs consequent to infection with rose powdery mildew. Chil. J. Agric. Res. 2019, 79, 596–608. [Google Scholar] [CrossRef]
- Adhikary, P.; Mukherjee, A.; Barik, A. Role of surface wax alkanes from Lathyrus sativus L. Seeds for Attraction of Callosobruchus Maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2014, 59, 113–119. [Google Scholar] [CrossRef]
- Zhu, G.; Pan, L.; Zhao, Y.; Zhang, X.; Wang, F.; Yu, Y.; Fan, W.; Liu, Q.; Zhang, S.; Li, M. Chemical investigations of volatile kairomones produced by Hyphantria cunea (Drury), a host of the parasitoid Chouioia cunea Yang. Bull. Entomol. Res. 2017, 107, 234–240. [Google Scholar] [CrossRef]
- Fürstenau, B.; Adler, C.; Schulz, H.; Hilker, M. Host habitat volatiles enhance the olfactory response of the larval parasitoid Holepyris sylvanidis to specifically host-associated cues. Chem. Senses 2016, 41, 611–621. [Google Scholar] [PubMed]
- Zhang, Y.-R.; Wang, R.; Yu, L.-F.; Lu, P.-F.; Luo, Y.-Q. Identification of caragana plant volatiles, overlapping profiles, and olfactory attraction to Chlorophorus caragana in the Laboratory. J. Plant Interact. 2015, 10, 41–50. [Google Scholar]
- Xiu, C.; Zhang, W.; Xu, B.; Wyckhuys, K.A.G.; Cai, X.; Su, H.; Lu, Y. Volatiles from aphid-infested plants attract adults of the multicolored asian lady beetle Harmonia axyridis. Biol. Control 2019, 129, 1–11. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Zhang, Y.; Xie, W.; Wu, Q.; Wang, S. Virus-infected plants altered the host selection of Encarsia formosa, a parasitoid of whiteflies. Front. Physiol. 2017, 8, 937. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, C.; Fereres, A.; Reina, M.; Cabrera, R.; González-Coloma, A. Behavioral and sublethal effects of structurally related lower terpenes on Myzus persicae. J. Chem. Ecol. 1997, 23, 1641–1650. [Google Scholar] [CrossRef]
- Halbert, S.E.; Corsini, D.; Wiebe, M.; Vaughn, S.F. Plant-derived compounds and extracts with potential as aphid repellents. Ann. Appl. Biol. 2009, 154, 303–307. [Google Scholar] [CrossRef]
- Cantó-Tejero, M.; Guirao, P.; Pascual-Villalobos, M.J. Aphicidal activity of farnesol against the green peach aphid—Myzus persicae. Pest Manag. Sci. 2022, 78, 2714–2721. [Google Scholar] [CrossRef]
- Schlaeger, S.; Pickett, J.A.; Birkett, M.A. Prospects for management of whitefly using plant semiochemicals, compared with related pests. Pest Manag. Sci. 2018, 74, 2405–2411. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef]
- Godard, K.A.; White, R.; Bohlmann, J. Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry 2008, 69, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Marcu, J. Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar]
- Elzinga, S.; Fischedick, J.; Podkolinski, R.; Raber, J.C. Cannabinoids and terpenes as chemotaxonomic markers in cannabis. Nat. Prod. Chem. Res 2015, 3, 10–4172. [Google Scholar]
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Tamiru, A.; Khan, Z.R.; Bruce, T.J. New directions for improving crop resistance to insects by breeding for egg induced defence. Curr. Opin. Insect Sci. 2015, 9, 51–55. [Google Scholar] [CrossRef] [PubMed]
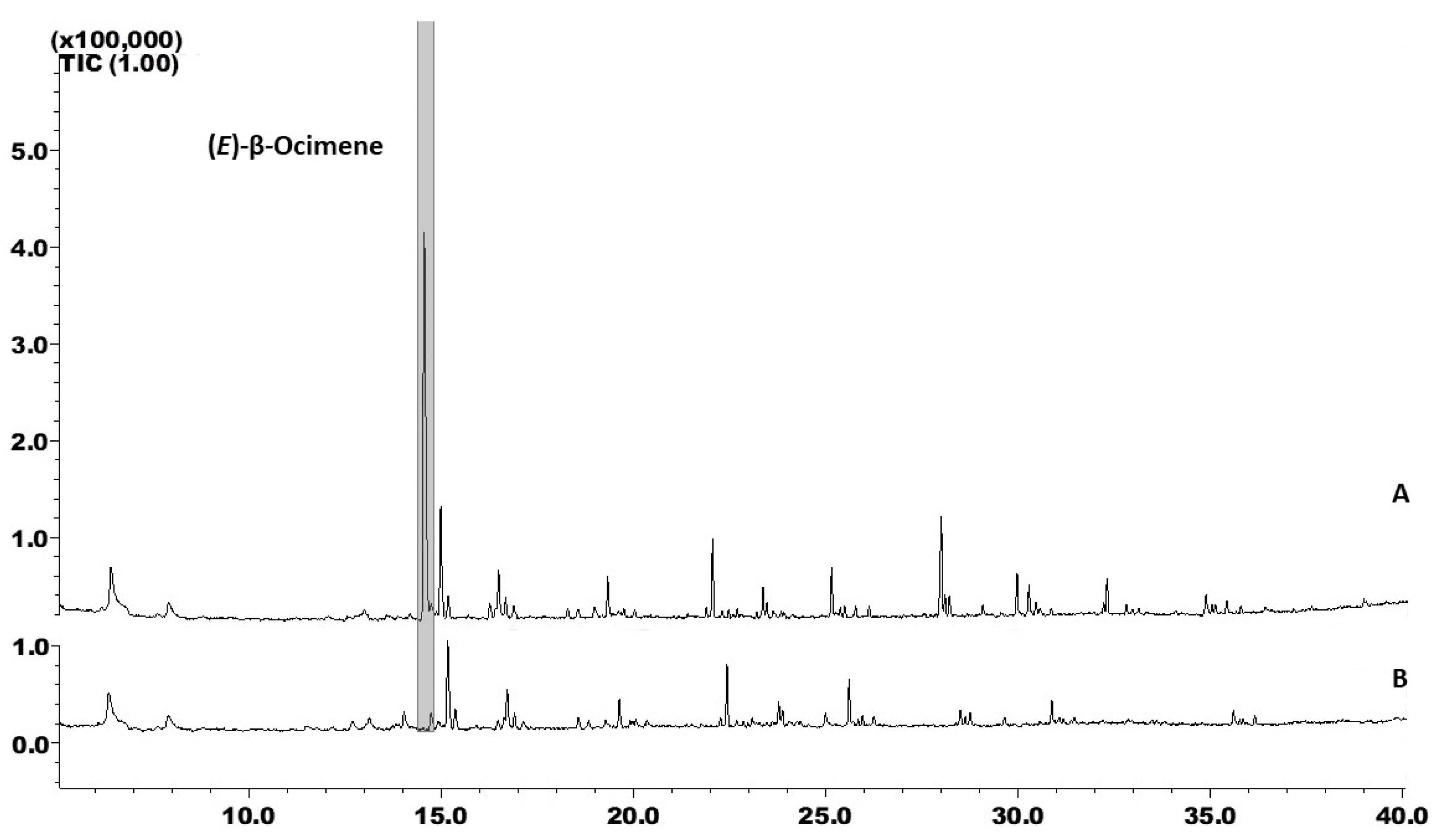


| No | Compounds | RI | M Ecuador 72 (ng/µL) | BRS Jari (ng/µL) |
|---|---|---|---|---|
| 1 | 4-Methyl-octane | 857 | 26.02 ± 11.64 | - |
| 2 | 4-Octen-3-one | 958 | 19.47 ± 8.73 | - |
| 3 | (Z)-β-ocimene | 1044 | 18.33 ± 8.20 | - |
| 4 | (E)-β-ocimene | 1046 | 250.20 ± 32.29 a | 105.43 ± 16.98 b |
| 5 | 5-Ethyl-2-methyl-octane | 1052 | 27.43 ± 12.78 a | 58.80 ± 11.76 a |
| 6 | Linalool | 1094 | 21.04 ± 9.41 a | 27.26 ± 8.71 a |
| 7 | 2,6 Dimethyl-decane | 1129 | - | 26.83 ± 12.89 |
| 8 | 5-Butyl-nonane | 1152 | 19.28 ± 8.62 | - |
| 9 | 5-Methyl-undecane | 1153 | 18.61 ± 8.32 a | 22.48 ± 10.55 a |
| 10 | Methyl salicylate | 1188 | 39.35 ± 8.32 a | 21.35 ± 9.56 a |
| 11 | Dodec-1-ene | 1193 | 19.29 ± 8.62 | - |
| 12 | 3-Ethylacetophenone | 1281 | 22.42 ± 10.03 | - |
| 13 | Tridecane | 1300 | 18.59 ± 8.31 | - |
| 14 | 4,6-Dimethyl-dodecane | 1321 | 26.84 ± 12.00 | - |
| 15 | Cyclododecane | 1328 | 18.60 ± 8.31 | - |
| 16 | Tetradec-1-ene | 1393 | 24.75 ± 7.82 | - |
| 17 | (E)-β-caryophyllene | 1417 | 38.16 ± 0.58 a | 28.94 ± 9.71 a |
| 18 | Pentadecane | 1500 | 33.28 ± 10.96 a | 33.79 ± 13.01 a |
| 19 | Hexadec-1-ene | 1586 | 18.76 ± 8.39 a | 19.86 ± 8.90 a |
| 20 | Hexadecane | 1600 | 20.20 ± 9.04 a | 29.97 ± 15.92 a |
| 21 | Cyclotetradecane | 1671 | 18.88 ± 8.44 | - |
| 22 | Heptadec-3-ene | 1676 | - | 42.25 ± 9.54 |
| 23 | Heptadecane | 1700 | - | 25.21 ± 12.44 |
| 24 | Farnesol | 1719 | 18.96 ± 8.47 | - |
| 24 | Octadecane | 1800 | 26.45 ± 8.39 a | 20.25 ± 9.07 a |
| 25 | Phytane | 1811 | - | 28.12 ± 9.31 |
| 26 | Heneicosane | 2100 | - | 25.68 ± 8.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.F.L.; Oliveira, D.J.d.A.; da Costa, J.G.; Gutierrez, M.A.M.; de Oliveira, E.J.; Ribeiro Junior, K.A.L.; Goulart, H.F.; Riffel, A.; Santana, A.E.G. Volatile Organic Compounds from Cassava Plants Confer Resistance to the Whitefly Aleurothrixus aepim (Goeldi, 1886). Insects 2023, 14, 762. https://doi.org/10.3390/insects14090762
Ribeiro TFL, Oliveira DJdA, da Costa JG, Gutierrez MAM, de Oliveira EJ, Ribeiro Junior KAL, Goulart HF, Riffel A, Santana AEG. Volatile Organic Compounds from Cassava Plants Confer Resistance to the Whitefly Aleurothrixus aepim (Goeldi, 1886). Insects. 2023; 14(9):762. https://doi.org/10.3390/insects14090762
Chicago/Turabian StyleRibeiro, Thyago Fernando Lisboa, Demetrios José de Albuquerque Oliveira, João Gomes da Costa, Miguel Angel Martinez Gutierrez, Eder Jorge de Oliveira, Karlos Antonio Lisboa Ribeiro Junior, Henrique Fonseca Goulart, Alessandro Riffel, and Antonio Euzebio Goulart Santana. 2023. "Volatile Organic Compounds from Cassava Plants Confer Resistance to the Whitefly Aleurothrixus aepim (Goeldi, 1886)" Insects 14, no. 9: 762. https://doi.org/10.3390/insects14090762
APA StyleRibeiro, T. F. L., Oliveira, D. J. d. A., da Costa, J. G., Gutierrez, M. A. M., de Oliveira, E. J., Ribeiro Junior, K. A. L., Goulart, H. F., Riffel, A., & Santana, A. E. G. (2023). Volatile Organic Compounds from Cassava Plants Confer Resistance to the Whitefly Aleurothrixus aepim (Goeldi, 1886). Insects, 14(9), 762. https://doi.org/10.3390/insects14090762