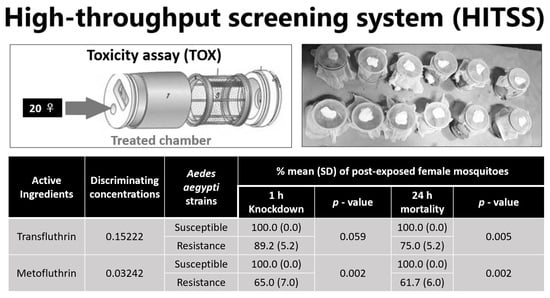
Transfluthrin and Metofluthrin as Effective Repellents against Pyrethroid-Susceptible and Pyrethroid-Resistant Aedes aegypti (L.) (Diptera: Culicidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Mosquito Rearing
2.3. Chemicals
2.4. Insecticide-Impregnated Filter Papers
2.5. High-Throughput Screening System
2.6. Data Analysis
3. Results
3.1. Toxicity Assay to Generate Lethal Concentrations
3.2. Toxicity Assay for Susceptibility Test Using Discriminating Concentrations
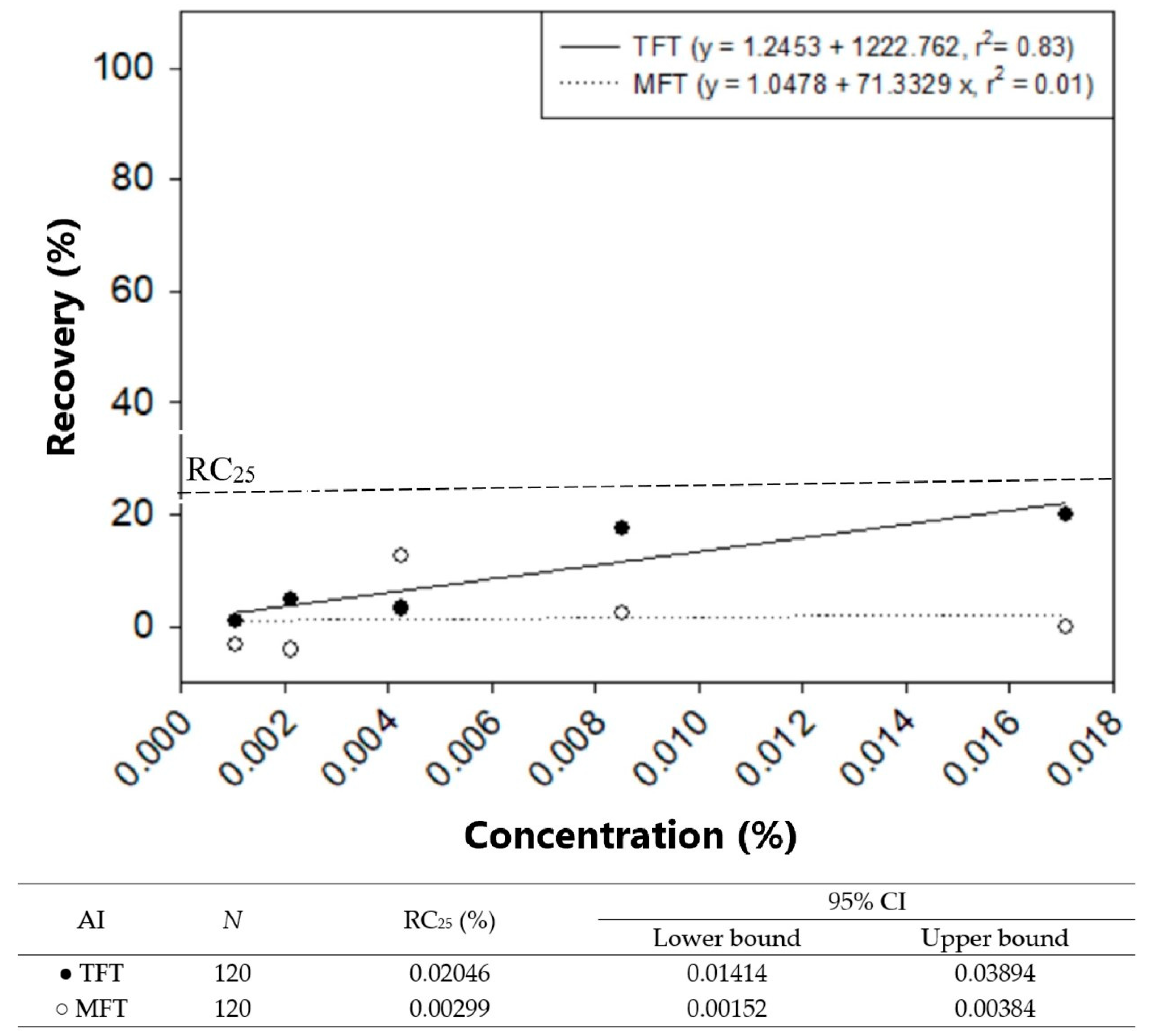
3.3. Comparison of Discriminating Concentrations of TFT and MFT Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, N.; Zheng, J.X.; Guo, Z.Y.; Li, L.H.; Xia, S.; Lv, S.; Zhou, X.N. Dengue incidence trends and its burden in major endemic regions from 1990 to 2019. Trop. Med. Infect. Dis. 2020, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Sasmono, R.T.; Santoso, M.S. Movement dynamics: Reduced dengue cases during the COVID-19 pandemic. Lancet Infect. Dis. 2022, 22, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Chareonviriyaphap, T.; Aum-Aung, B.; Ratanatham, S. Current insecticide resistance patterns in mosquito vectors in Thailand. South Asian J. Trop. Med. Public Health 1999, 1, 184–194. [Google Scholar]
- Chareonviriyaphap, T.; Bangs, M.J.; Suwonkerd, W.; Kongmee, M.; Corbel, V.; Ngoen-Klan, R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit. Vectors 2013, 6, 280. [Google Scholar] [CrossRef]
- Thanispong, K.; Sathantriphop, S.; Chareonviriyaphap, T. Insecticide resistance of Aedes aegypti and Culex quinquefasciatus in Thailand. J. Pestic. Sci. 2008, 33, 351–356. [Google Scholar] [CrossRef]
- Chuaycharoensuk, T.; Juntarajumnong, W.; Boonyuan, W.; Bangs, M.J.; Akratanakul, P.; Thammapalo, S.; Jirakanjanakit, N.; Tanasinchayakul, S.; Chareonviriyaphap, T. Frequency of pyrethroid resistance in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Thailand. J. Vector Ecol. 2011, 36, 204–212. [Google Scholar] [CrossRef]
- Thongwat, D.; Bunchu, N. Susceptibility to temephos, permethrin and deltamethrin of Aedes aegypti (Diptera: Culicidae) from Muang district, Phitsanulok Province, Thailand. Asian Pac. J. Trop. 2015, 8, 14–18. [Google Scholar] [CrossRef]
- Prapanthadara, L.A.; Promtet, N.; Koottathep, S.; Somboon, P.; Suwonkerd, W.; McCarroll, L.; Hemingway, J. Mechanisms of DDT and permethrin resistance in Aedes aegypti from Chiang Mai, Thailand. Dengue Bull. 2002, 26, 185–189. [Google Scholar]
- Sirisopa, P.; Thanispong, K.; Chareonviriyaphap, T.; Juntarajumnong, W. Resistance to synthetic pyrethroids in Aedes aegypti (Diptera: Culicidae) in Thailand. Agric. Nat. Resour. 2014, 48, 577–586. [Google Scholar]
- Plernsub, S.; Saingamsook, J.; Yanola, J.; Lumjuan, N.; Tippawangkosol, P.; Sukontason, K.; Walton, C.; Somboon, P. Additive effect of knockdown resistance mutations, S989P, V1016G and F1534C, in a heterozygous genotype conferring pyrethroid resistance in Aedes aegypti in Thailand. Parasit. Vectors 2016, 9, 417. [Google Scholar] [CrossRef]
- Achee, N.L.; Bangs, M.J.; Farlow, R.; Killeen, G.F.; Lindsay, S.; Logan, J.G.; Moore, S.J.; Rowland, M.; Sweeney, K.; Torr, S.J.; et al. Spatial repellents: From discovery and development to evidence-based validation. Malar. J. 2012, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Vijayalakshmi, A. Monitoring of allethrin, deltamethrin, esbiothrin, prallethrin and transfluthrin in air during the use of household mosquito repellents. J. Environ. Monit. 2001, 3, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.S.; Hien, D.F.; Bayili, K.; Yerbanga, R.S.; Cohuet, A.; Carrasco, D.; Guissou, E.; Gouagna, L.C.; Yaméogo, K.B.; Diabaté, A.; et al. Natural plant diet impacts phenotypic expression of pyrethroid resistance in Anopheles mosquitoes. Sci. Rep. 2022, 12, 21431. [Google Scholar] [CrossRef] [PubMed]
- Nazimek, T.; Wasak, M.; Zgrajka, W.; Turski, W.A. Content of transfluthrin in indoor air during the use of electro-vaporizers. Ann. Agric. Environ. Med. 2011, 18, 85–88. [Google Scholar] [PubMed]
- Argueta, T.B.O.; Kawada, H.; Takagi, M. Spatial repellency of metofluthrin-impregnated multilayer paper strip against Aedes albopictus under outdoor conditions, Nagasaki, Japan. Med. Entomol. Zool. 2004, 55, 211–216. [Google Scholar] [CrossRef][Green Version]
- Ogoma, S.B.; Ngonyani, H.; Simfukwe, E.T.; Mseka, A.; Moore, J.; Killeen, G.F. Spatial repellency of transfluthrin-treated hessian strips against laboratory-reared Anopheles arabiensis mosquitoes in a semi-field tunnel cage. Parasit. Vectors 2012, 5, 54–61. [Google Scholar] [CrossRef]
- Sukkanon, C.; Bangs, M.J.; Nararak, J.; Hii, J.; Chareonviriyaphap, T. Discriminating lethal concentrations for transfluthrin, a volatile pyrethroid compound for mosquito control in Thailand. J. Am. Mosq. Control Assoc. 2019, 35, 258–266. [Google Scholar] [CrossRef]
- World Health Organization. Determining Discriminating Concentrations of Insecticides for Monitoring Resistance in Mosquitoes: Report of a Multi-Centre Laboratory Study and WHO Expert Consultations; World Health Organization: Geneva, Switzerland, 2023; pp. 58–59.
- World Health Organization. Manual for Monitoring Insecticide Resistance in Mosquito Vectors and Selecting Appropriate Interventions; World Health Organization: Geneva, Switzerland, 2022.
- Khater, E.I.; Zhu, D.; Bibbs, C.S.; Xue, R.-D. Insecticide efficacy of spatial repellent compound-metofluthrin against susceptible and resistant strains of Aedes aegypti. J. Fla. Mosq. Control Assoc. 2021, 68, 86–91. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Leepasert, T.; Bangs, M.J.; Chareonviriyaphap, T. Dose–response assay for synthetic mosquito (Diptera: Culicidae) attractant using a high-throughput screening system. Insects 2021, 12, 355. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Leepasert, T.; Bangs, M.J.; Chareonviriyaphap, T. Evaluation of mosquito attractant candidates using a high-throughput screening system for Aedes aegypti (L.), Culex quinquefasciatus Say and Anopheles minimus Theobald (Diptera: Culicidae). Insects 2021, 12, 528. [Google Scholar] [CrossRef]
- Kim, D.Y.; Leepasert, T.; Bangs, M.J.; Chareonviriyaphap, T. Semi-field evaluation of novel chemical lures for Aedes aegypti, Culex quinquefasciatus, and Anopheles minimus (Diptera: Culicidae) in Thailand. Parasit. Vectors 2021, 14, 606. [Google Scholar] [CrossRef] [PubMed]
- Grieco, J.P.; Achee, N.L.; Chareonviriyaphap, T.; Suwonkerd, W.; Chauhan, K.; Sardelis, M.R.; Roberts, D.R. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS ONE 2007, 2, e716. [Google Scholar] [CrossRef] [PubMed]
- Wagman, J.M.; Achee, N.L.; Grieco, J.P. Insensitivity to the spatial repellent action of transfluthrin in Aedes aegypti: A heritable trait associated with decreased insecticide susceptibility. PLoS Negl. Trop. Dis. 2015, 9, e0003726. [Google Scholar] [CrossRef] [PubMed]
- Bibbs, C.S.; Tsikolia, M.; Bloomquist, J.R.; Bernier, U.R.; Xue, R.D.; Kaufman, P.E. Vapor toxicity of five volatile pyrethroids against Aedes aegypti, Aedes albopictus, Culex quinquefasciatus, and Anopheles quadrimaculatus (Diptera: Culicidae). Pest Manag. Sci. 2018, 74, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Sukkanon, C.; Nararak, J.; Bangs, M.J.; Hii, J.; Chareonviriyaphap, T. Behavioral responses to transfluthrin by Aedes aegypti, Anopheles minimus, Anopheles harrisoni, and Anopheles dirus (Diptera: Culicidae). PLoS ONE 2020, 15, e0237353. [Google Scholar] [CrossRef]
- Dang, K.; Singham, G.V.; Doggett, S.L.; Lilly, D.G.; Lee, C.Y. Effects of different surfaces and insecticide carriers on residual insecticide bioassays against bed bugs, Cimex spp. (Hemiptera: Cimicidae). J. Econ. Entomol. 2017, 110, 558–566. [Google Scholar]
- World Health Organization. Standard Operating Procedure for Impregnation of Filter Papers for Testing Insecticide Susceptibility of Adult Mosquitoes in WHO Tube Tests; World Health Organization: Geneva, Switzerland, 2022; pp. 4–5.
- Grieco, J.P.; Achee, N.L.; Sardelis, M.R.; Chauhan, K.R.; Roberts, D.R. A novel high-throughput screening system to evaluate the behavioral response of adult mosquitoes to chemicals. J. Am. Mosq. Control Assoc. 2005, 21, 404–411. [Google Scholar] [CrossRef]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016; pp. 19–20.
- Phonjatturas, K.; Grieco, J.P.; Corbel, V.; Chareonviriyaphap, T.; Juntarajumnong, W. Laboratory evaluation of novel long-lasting insecticidal nets on Aedes aegypti L., using a high-throughput screening system. Agric. Nat. Resour. 2021, 55, 213–218. [Google Scholar]
- Owusu, H.F.; Jančáryová, D.; Malone, D.; Müller, P. Comparability between insecticide resistance bioassays for mosquito vectors: Time to review current methodology? Parasit. Vectors 2015, 8, 357. [Google Scholar] [CrossRef]
- Achee, N.; Masuoka, P.; Smith, P.; Martin, P.; Chareonviriyaphap, T.; Polsomboon, T.; Hendarto, J.; Grieco, J.P. Identifying the effective concentration for spatial repellency of the dengue vector Aedes aegypti. Parasit. Vectors 2012, 5, 300. [Google Scholar] [CrossRef]
- Lucas, J.R.; Shono, Y.; Iwasaki, T.; Ishiwatari, T.; Spero, N.; Benzon, G. US laboratory and field trials of metofluthrin (Sumione®) emanators for reducing mosquito biting outdoor. J. Am. Mosq. Control Assoc. 2007, 23, 47–54. [Google Scholar] [CrossRef]
- Darbro, J.M.; Muzari, M.O.; Giblin, A.; Adamczyk, R.M.; Ritchie, S.A.; Devine, G.J. Reducing biting rates of Aedes aegypti with metofluthrin: Investigations in time and space. Parasit. Vectors 2017, 10, 300. [Google Scholar] [CrossRef]
- Rapley, L.P.; Russell, R.C.; Montgomery, B.L.; Ritchie, S.A. The effects of sustained release metofluthrin on the biting, movement, and mortality of Aedes aegypti in a domestic setting. Am. J. Trop. Med. Hyg. 2009, 81, 94–99. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Devine, G.J. Confusion, knock-down and kill of Aedes aegypti using metofluthrin in domestic settings: A powerful tool to prevent dengue transmission? Parasit. Vectors 2013, 6, 262. [Google Scholar] [CrossRef]
- Haynes, K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef]
- Tambwe, M.M.; Swai, J.K.; Moore, S.J. Chapter 10. Semi-field system and experimental huts bioassays for the evaluation of spatial (and topical) repellents for indoor and outdoor use. In Advances in Arthropod Repellents; Coats, J., Corona, C., Debboun, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 163–192. [Google Scholar]
- Kawada, H.; Maekawa, Y.; Takagi, M. Field trial on the spatial repellency of metofluthrin-impregnated plastic strips for mosquitoes in shelters without walls (beruga) in Lombok, Indonesia. J. Vector Ecol. 2005, 30, 181. [Google Scholar]
- Kawada, H.; Temu, E.A.; Minjas, J.N.; Matsumoto, O.; Iwasaki, T.; Takagi, M. Field evaluation of spatial repellency of metofluthrin-impregnated plastic strips against Anopheles gambiae complex in Bagamoyo, coastal Tanzania. J. Am. Mosq. Control Assoc. 2008, 24, 404–409. [Google Scholar] [CrossRef][Green Version]
- Argueta, T.B.O.; Kawada, H.; Sugano, M.; Kubota, S.I.; Shono, Y.; Tsushima, K.; Takagi, M. Comparative insecticidal efficacy of a new pyrethroid, metofluthrin, against colonies of Asian Culex quinquefasciatus and Culex pipiens pallens. Med. Entomol. Zool. 2004, 55, 289–294. [Google Scholar] [CrossRef]
- Ponlawat, A.; Kankaew, P.; Chanaimongkol, S.; Pongsiri, A.; Richardson, J.H.; Evans, B.P. Semi-field evaluation of metofluthrin-impregnated nets on host-seeking Aedes aegypti and Anopheles dirus. J. Am. Mosq. Control Assoc. 2016, 32, 130–138. [Google Scholar] [CrossRef]
- Zollner, G.; Orshan, L. Evaluation of a metofluthrin fan vaporizer device against phlebotomine sand flies (Diptera: Psychodidae) in a cutaneous leishmaniasis focus in the Judean Desert, Israel. J. Vector Ecol. 2011, 36, 157–165. [Google Scholar] [CrossRef]
- Blouquy, L.; Mottet, C.; Olivares, J.; Plantamp, C.; Siegwart, M.; Barrès, B. How varying parameters impact insecticide resistance bioassay: An example on the worldwide invasive pest Drosophila suzukii. PLoS ONE 2021, 16, e0247756. [Google Scholar] [CrossRef]
- Praulins, G.; McDermott, D.T.; Spiers, A.; Lees, R.S. Reviewing the WHO tube bioassay methodology: Accurate method reporting and numbers of mosquitoes are key to producing robust results. Insects 2022, 13, 544. [Google Scholar] [CrossRef]
- Fisher, R.A. Chapter 6. The correlation coefficient. In Statistical Methods for Research Workers, 6th ed.; Crew, F.A.E., Cutler, D.W., Eds.; Oliver and Boyd: Edinburgh, UK, 1936; pp. 176–213. [Google Scholar]
- Finney, D.J. Chapter 3. The estimation of the median effective dose. In Probit Analysis a Statistical Treatment of the Sigmoid Response Curve, 2nd ed.; Finney, D.J., Tattersfield, F., Eds.; The Syndics of the Cambridge University Press: London, UK, 1952; pp. 36–47. [Google Scholar]
- Oliver, S.V.; Brooke, B.D. The effect of larval nutritional deprivation on the life history and DDT resistance phenotype in laboratory strains of the malaria vector Anopheles arabiensis. Malar. J. 2013, 12, 44. [Google Scholar] [CrossRef]
- Grossman, M.K.; Uc-Puc, V.; Flores, A.E.; Manrique-Saide, P.C.; Vazquez-Prokopec, G.M. Larval density mediates knockdown resistance to pyrethroid insecticides in adult Aedes aegypti. Parasit. Vectors 2018, 11, 282. [Google Scholar] [CrossRef]
- Balmert, N.J.; Rund, S.S.; Ghazi, J.P.; Zhou, P.; Duffield, G.E. Time-of-day specific changes in metabolic detoxification and insecticide resistance in the malaria mosquito Anopheles gambiae. J. Insect Physiol. 2014, 64, 30–39. [Google Scholar] [CrossRef]
- Glunt, K.D.; Oliver, S.V.; Hunt, R.H.; Paaijmans, K.P. The impact of temperature on insecticide toxicity against the malaria vectors Anopheles arabiensis and Anopheles funestus. Malar. J. 2018, 17, 131. [Google Scholar] [CrossRef]
- Choi, D.B.; Grieco, J.P.; Apperson, C.S.; Schal, C.; Ponnusamy, L.; Wesson, D.W.; Achee, N.L. Effect of spatial repellent exposure on dengue vector attraction to oviposition sites. PLoS Negl. Trop. Dis. 2016, 10, e0004850. [Google Scholar] [CrossRef]
- Sougoufara, S.; Yorkston-Dives, H.; Aklee, N.M.; Rus, A.C.; Zairi, J.; Tripet, F. Standardised bioassays reveal that mosquitoes learn to avoid compounds used in chemical vector control after a single sub-lethal exposure. Sci. Rep. 2022, 12, 2206. [Google Scholar] [CrossRef]
- Lees, R.S.; Fornadel, C.; Snetselaar, J.; Wagman, J.; Spiers, A. Insecticides for mosquito control: Improving and validating methods to strengthen the evidence base. Insects 2023, 14, 116. [Google Scholar] [CrossRef]
| Concentration (%) | KD60 (%) | MT (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TFT | MFT | p-Value ** | TFT | MFT | p-Value ** | |||||
| Mean (SE) | Mean Rank * (Min.–Max.) | Mean (SE) | Mean Rank * (Min.–Max.) | Mean (SE) | Mean Rank * (Min.–Max.) | Mean (SE) | Mean Rank * (Min.–Max.) | |||
| 0.00107 | 1.7 | 5.0 a, A | 6.7 | 4.9 a, A | 0.120 | 0.8 | 6.2 a, A | 10.0 | 5.7 a, A | 0.152 |
| (1.1) | (0.0–5.0) | (2.5) | (0.0–15.0) | (0.8) | (0.0–5.0) | (6.3) | (0.0–40.0) | |||
| 0.00213 | 6.7 | 9.0 a, A | 24.2 | 8.8 a, A | 0.097 | 1.7 | 7.3 a, A | 28.3 | 8.9 a, A | 0.087 |
| (2.5) | (0.0–15.0) | (8.5) | (0.0–55.0) | (1.1) | (0.0–5.0) | (11.9) | (0.0–75.0) | |||
| 0.00427 | 17.5 | 14.8 ab, A | 73.3 | 17.2 ab, B | 0.006 ** | 14.2 | 15.6 ab, A | 60.8 | 14.5 ab, B | 0.020 ** |
| (3.6) | (10.0–30.0) | (12.6) | (20.0–100.0) | (3.5) | (5.0–25.0) | (14.2) | (15.0–95.0) | |||
| 0.00853 | 63.3 | 22.1 bc, A | 99.2 | 23.3 b, B | 0.003 ** | 45.8 | 22.6 bc, A | 96.7 | 23.4 bc, B | 0.005 ** |
| (8.4) | (25.0–85.0) | (0.8) | (95.0–100.0) | (12.7) | (15.0–85.0) | (2.5) | (85.0–100.0) | |||
| 0.01706 | 89.2 | 26.7 c, A | 99.2 | 23.3 b, A | 0.072 | 69.2 | 25.8 c, A | 99.2 | 25.0 c, B | 0.003 ** |
| (6.2) | (60.0–100.0) | (0.8) | (95.0–100.0) | (5.1) | (60.0–85.0) | (0.8) | (95.0–100.0) | |||
| Concentration (%) | MRE (%) * | p-Value *** | |||
|---|---|---|---|---|---|
| TFT | MFT | ||||
| Mean (SE) | Mean Rank ** (Min.–Max.) | Mean (SE) | Mean Rank ** (Min.–Max.) | ||
| 0.00107 | 0.8 | 10.17 a | −3.3 | 13.50 a, A | 0.598 |
| (0.8) | (0.0–5.0) | (4.4) | (−25.0–5.0) | ||
| 0.00213 | 5.0 | 14.58 a | −4.2 | 12.00 a, A | 0.091 |
| (2.9) | (−5.0–15.0) | (4.0) | (−20.0–5.0) | ||
| 0.00427 | 3.3 | 13.33 a | 12.5 | 20.17 a, B | 0.317 |
| (4.8) | (−10.0–20.0) | (7.3) | (−5.0–40.0) | ||
| 0.00853 | 17.5 | 18.92 a | 2.5 | 17.83 a, B | 0.212 |
| (8.3) | (−10.0–40.0) | (1.7) | (0.0–10.0) | ||
| 0.01706 | 20.0 | 20.50 a | 0.0 | 14.00 a, A | 0.021 * |
| (7.1) | (0.0–35.0) | (0.0) | (0.0–0.0) | ||
| AIs | % LC50 (95% FL) | % LC75 (95% FL) | % LC99 (95% FL) | %DCs | χ2(df) * | p-Value |
|---|---|---|---|---|---|---|
| TFT | 0.01040 (0.00925–0.01189) a | 0.01852 (0.01576–0.02280) a | 0.07611 (0.05455–0.11971) a | 0.15222 | 3.217 (3) | 0.359 |
| MFT | 0.00307 (0.00279–0.00338) b | 0.00497 (0.00447–0.00562) b | 0.01621 (0.01312–0.02123) b | 0.03242 | 7.692 (3) | 0.053 |
| Conc. (%) | Mean (SE) | |||||
|---|---|---|---|---|---|---|
| KD60 (%) * | MT (%) * | |||||
| USDA | Pu-Teuy | p-Value ** | USDA | Pu-Teuy | p-Value ** | |
| 0.01040 (LC50) | 98.3 (1.1) a, A | 20.8 (4.9) a, B | 0.003 ** | 45.0 (3.7) a, A | 12.5 (4.4) a, B | 0.000 ** |
| 0.01852 (LC75) | 100.0 (0.0) b, A | 15.0 (2.6) a, B | 0.000 ** | 65.8 (7.0) b, A | 9.2 (3.5) a, B | 0.001 ** |
| 0.07611 (LC99) | 100.0 (0.0) b, A | 80.0 (7.3) b, A | 0.041 | 100.0 (0.0) c, A | 55.0 (9.9) b, B | 0.006 ** |
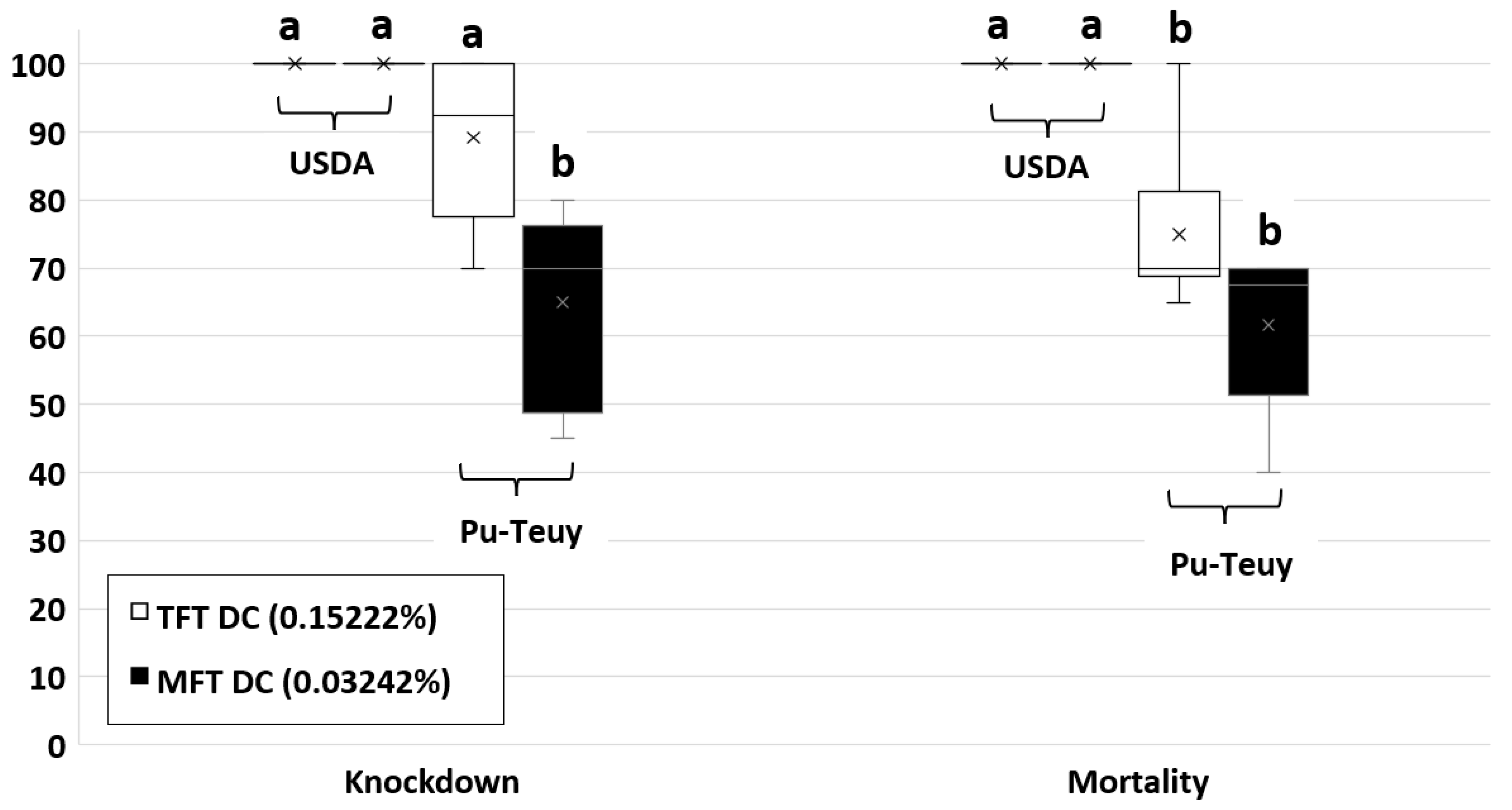
| 0.15222 (DC) | 100.0 (0.0) b, A | 89.2 (5.2) b, A | 0.093 | 100.0 (0.0) c, A | 75.0 (5.2) b, B | 0.007 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Hii, J.; Chareonviriyaphap, T. Transfluthrin and Metofluthrin as Effective Repellents against Pyrethroid-Susceptible and Pyrethroid-Resistant Aedes aegypti (L.) (Diptera: Culicidae). Insects 2023, 14, 767. https://doi.org/10.3390/insects14090767
Kim D-Y, Hii J, Chareonviriyaphap T. Transfluthrin and Metofluthrin as Effective Repellents against Pyrethroid-Susceptible and Pyrethroid-Resistant Aedes aegypti (L.) (Diptera: Culicidae). Insects. 2023; 14(9):767. https://doi.org/10.3390/insects14090767
Chicago/Turabian StyleKim, Dae-Yun, Jeffrey Hii, and Theeraphap Chareonviriyaphap. 2023. "Transfluthrin and Metofluthrin as Effective Repellents against Pyrethroid-Susceptible and Pyrethroid-Resistant Aedes aegypti (L.) (Diptera: Culicidae)" Insects 14, no. 9: 767. https://doi.org/10.3390/insects14090767
APA StyleKim, D.-Y., Hii, J., & Chareonviriyaphap, T. (2023). Transfluthrin and Metofluthrin as Effective Repellents against Pyrethroid-Susceptible and Pyrethroid-Resistant Aedes aegypti (L.) (Diptera: Culicidae). Insects, 14(9), 767. https://doi.org/10.3390/insects14090767