Composition and Diversity of the Endobacteria and Ectobacteria of the Invasive Bark Beetle Hylurgus ligniperda (Fabricius) (Curculionidae: Scolytinae) in Newly Colonized Areas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
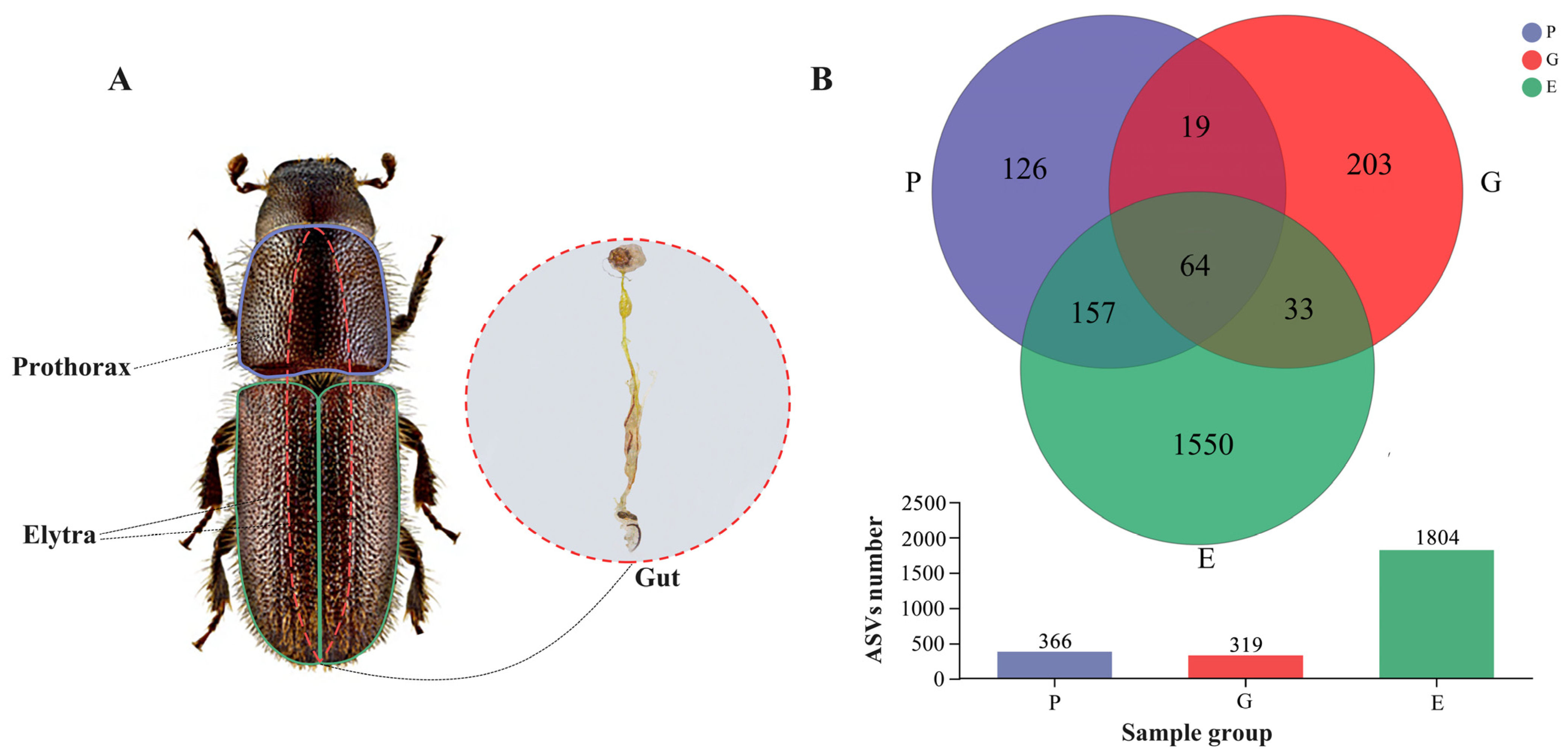
2.1. Sample Collection and Dissections
2.2. DNA Extraction, PCR Amplification, and 16S rRNA Gene Illumina Sequencing
2.3. Sequence Data Processing
2.4. Statistical Analysis
3. Results
3.1. ASV Sequencing Results of the Prothorax, Elytra, and Gut
3.2. Bacterial Diversity Associated with the Prothorax, Elytra, and Gut of H. ligniperda
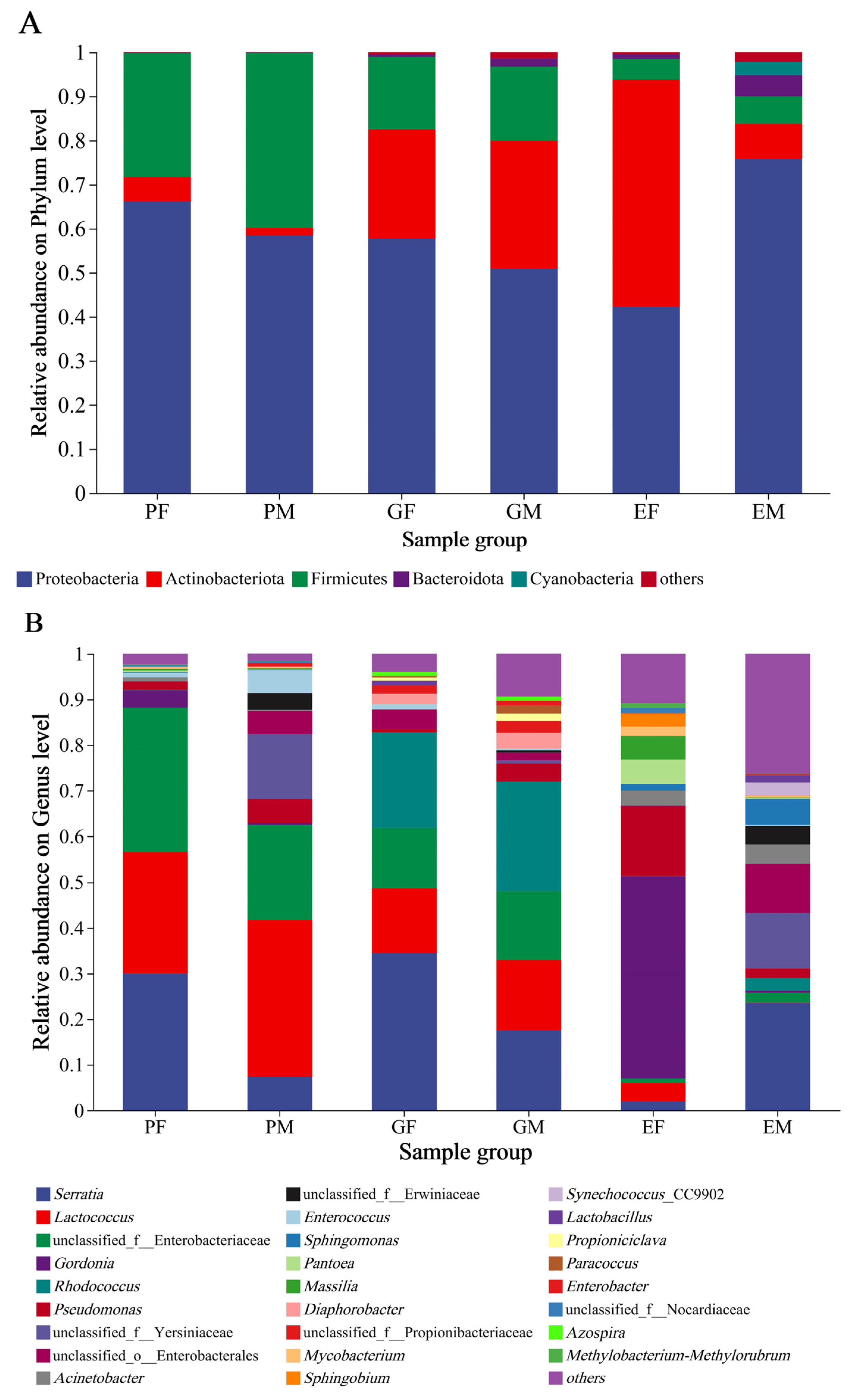
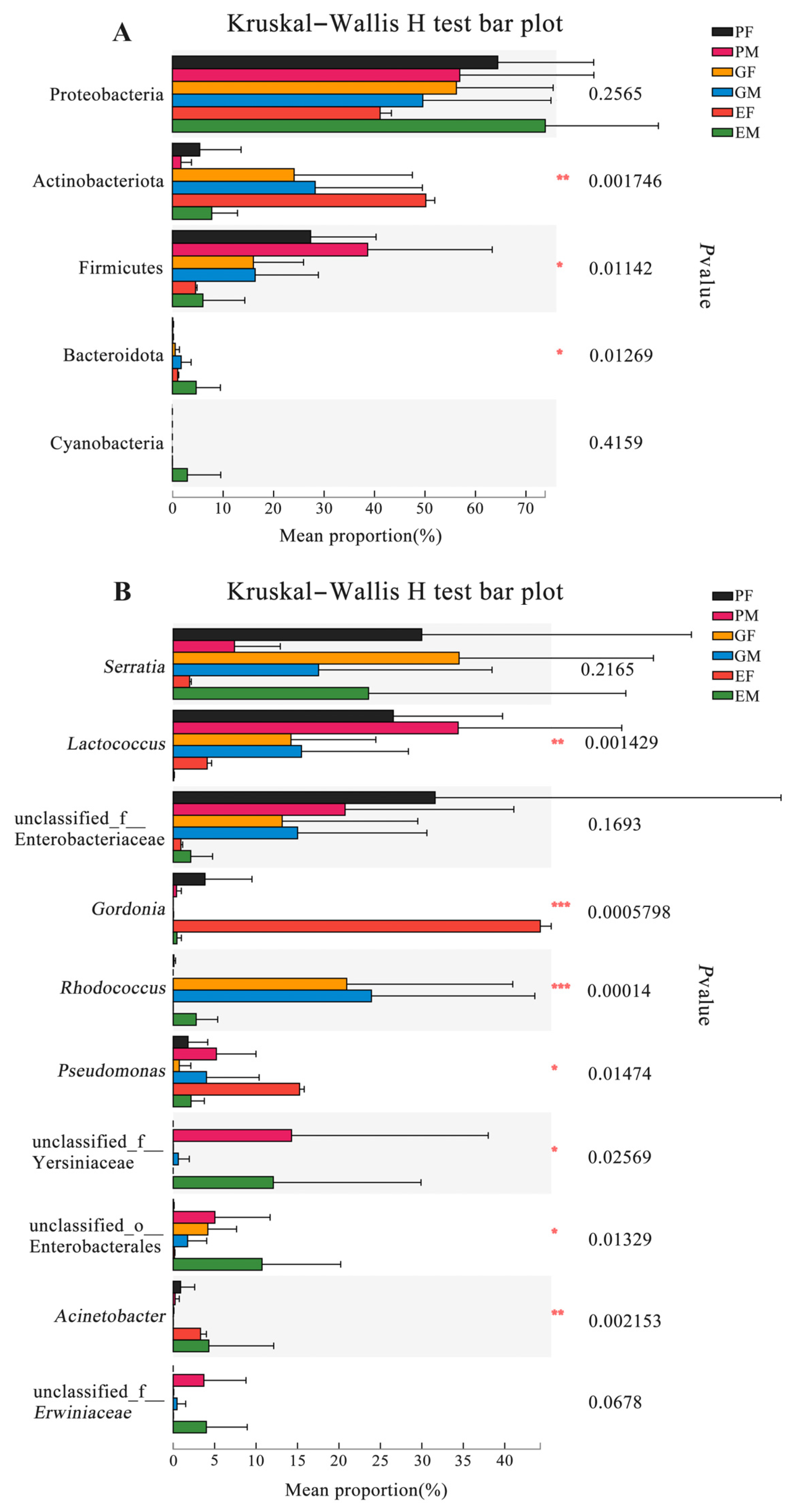
3.3. Bacterial Community Composition Associated with the Prothorax, Elytra, and Gut of H. ligniperda
3.4. Functional Predictions of the Endobacteria and Ectobacteria of H. ligniperda
4. Discussion
4.1. Diversity Differences of H. ligniperda-Associated Bacteria and Its Potential Causes
4.2. Possible Ecological Role of High-Abundance Bacteria in the Invasion and Colonization of H. ligniperda
4.3. The Limitations and Prospects of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haack, R.A. Exotic bark- and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Tribe, G.D. Colonisation sites on Pinus radiata logs of the bark beetles, Orthotomicus erosus, Hylastes angustatus and Hylurgus ligniperda (Coleoptera: Scolytidae). J. Entomol. Soc. South. Afr. 1992, 55, 77–84. [Google Scholar]
- Liang, Z.; Zhang, J.H.; Yang, D.; Chen, N.Z. Overview of alien insects intercepted at China ports from 2005 to 2015. Plant Quar. 2017, 31, 64–68. [Google Scholar]
- Lin, W.; Park, S.; Jiang, Z.R.; Ji, Y.C.; Ernstsons, A.S.; Li, J.J.; Li, Y.; Hulcr, J. Native or Invasive? The Red-Haired Pine Bark Beetle Hylurgus ligniperda (Fabricius) (Curculionidae: Scolytinae) in East Asia. Forests 2021, 12, 7. [Google Scholar] [CrossRef]
- Ren, L.L.; Tao, J.; Wu, H.W.; Zong, S.X.; Zhen, W.C.; Hua, D.; Shi, J.; Liu, Y.Z.; Luo, Y.Q. The First Discovery and Infective Characteristics of A Major Invasive Pest Hylurgus ligniperda(Coleoptera: Scolytidae) in China. Sci. Silvae Sin. 2021, 57, 140–150. [Google Scholar] [CrossRef]
- Lee, J.C.; Haack, R.A.; Negrón, J.F.; Witcosky, J.J.; Seybold, S.J. Invasive bark beetles; Forest Insect and Disease Leaflet 176; US Department of Agriculture, US Forest Service: Washington, DC, USA, 2007; 12p. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=912a320e5aa7aeb88483eed2eae3d372db7c126d (accessed on 26 December 2023).
- Hoebeke, E.R. Hylurgus ligniperda: A new exotic pine bark beetle in the United States. Newsl. Themichigan Entomol. Soc. 2001, 46, 1–2. [Google Scholar]
- Wood, S.L. Bark and Ambrosia Beetles of South America; Brigham Young University Press: Provo, UT, USA, 2007. [Google Scholar]
- Kirkendall, L.R. Invasive Bark Beetles (Coleoptera, Curculionidae, Scolytinae) in Chile and Argentina, Including Two Species New for South America, and the Correct Identity of the Orthotomicus Species in Chile and Argentina. Diversity 2018, 10, 40. [Google Scholar] [CrossRef]
- Ciesla, W.M. Pine bark beetles: A new pest management challenge for Chilean foresters. J. For. 1988, 86, 27–31. [Google Scholar]
- Fabre, J.; Carle, P. Contribution to the biological study of Hylurgus ligniperda F.(Toleoptera scoly cidae) biology in the South eastern part of France. Ann. Des. Sci. For. 1974, 32, 55–71. [Google Scholar] [CrossRef]
- Clare, G.K.; George, E.M. Life cycle and mass-rearing of Hylurgus ligniperda using a novel egg-collection method. New Zealand Plant Prot. 2016, 69, 143–152. [Google Scholar] [CrossRef]
- Kim, S.; Harrington, T.C.; Lee, J.C.; Seybold, S.J. Leptographium tereforme sp. nov. and other Ophiostomatales isolated from the root-feeding bark beetle Hylurgus ligniperda in California. Mycologia 2011, 103, 152–163. [Google Scholar] [CrossRef]
- Zhou, X.D.; De Beer, Z.; Ahumada, R.; Wingfield, B.; Wingfield, M. Ophiostoma and Ceratocystiopsis spp. associated with two pine-infesting bark beetles in Chile. Fungal Divers. 2004, 15, 261–274. [Google Scholar] [CrossRef]
- Ray, S.; Thwaites, J.; Farrell, R. Survey of Ophiostomataceae associated with Hylurgus ligniperda (Curculionidae: Scolytinae) in New Zealand. New Zealand Entomol. 2006, 29, 21–26. [Google Scholar] [CrossRef]
- Jankowiak, R.; Bilański, P. Ophiostomatoid fungi associated with root-feeding bark beetles on Scotspine in Poland. For. Pathol. 2013, 43, 422–428. [Google Scholar] [CrossRef]
- Davydenko, K.; Vasaitis, R.; Meshkova, V.; Menkis, A. Fungi associated with the red-haired bark beetle, Hylurgus ligniperda (Coleoptera: Curculionidae) in the forest-steppe zone in eastern Ukraine. Eur. J. Entomol. 2014, 111, 561–565. [Google Scholar] [CrossRef]
- Zhou, X.D.; Beer, Z.; Wingfield, B.D.; Wingfield, M.J. Ophiostomatoid fungi associated with three pine-infesting bark beetles in South Africa. Sydowia-Horn- 2001, 53, 290–300. [Google Scholar] [CrossRef]
- Lu, M.; Wingfield, M.J.; Gillette, N.E.; Mori, S.R.; Sun, J.H. Complex interactions among host pines and fungi vectored by an invasive bark beetle. New Phytol. 2010, 187, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Torrini, G.; Paoli, F.; Mazza, G.; Simoncini, S.; Strangi, A.; Guidotti, A.; Mori, E.; Roversi, P.F.; Marianelli, L. First detection of Bursaphelenchus abietinus and B. áandrassyi in Italy. For. Pathol. 2020, 50, e12627. [Google Scholar] [CrossRef]
- Penas, A.C.; Bravo, M.A.; Naves, P.; Bonifácio, L.; Sousa, E.; Mota, M. Species of Bursaphelenchus Fuchs, 1937 (Nematoda: Parasitaphelenchidae) and other nematode genera associated with insects from Pinus pinaster in Portugal. Ann. Appl. Biol. 2006, 148, 121–131. [Google Scholar] [CrossRef]
- Mejri, M.; Fonseca, L.; Cardoso, J.; Ben JamÔa, M.; Abrantes, I. Bursaphelenchus tusciae in Tunisia associated with Hylurgus ligniperda. For. Pathol. 2016, 46, 663–665. [Google Scholar] [CrossRef]
- Keeling, C.I.; Bohlmann, J. Diterpene resin acids in conifers. Phytochemistry 2006, 67, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Huber, D.; Carroll, A. The legacy of attack: Implications of high phloem resin monoterpene levels in lodgepole pines following mass attack by mountain pine beetle, Dendroctonus ponderosae Hopkins. Environ. Entomol. 2012, 41, 392–398. [Google Scholar] [CrossRef]
- Therrien, J.; Mason, C.J.; Cale, J.A.; Adams, A.; Aukema, B.H.; Currie, C.R.; Raffa, K.F.; Erbilgin, N. Bacteria influence mountain pine beetle brood development through interactions with symbiotic and antagonistic fungi: Implications for climate-driven host range expansion. Oecologia 2015, 179, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Six, D.L. The bark beetle holobiont: Why microbes matter. J. Chem. Ecol. 2013, 39, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- García-Fraile, P. Roles of bacteria in the bark beetle holobiont–How do they shape this forest pest? Ann. Appl. Biol. 2018, 172, 111–125. [Google Scholar] [CrossRef]
- Adams, A.; Currie, C.; Cardoza, Y.; Klepzig, K.; Raffa, K. Effects of symbiotic bacteria and tree chemistry on the growth and reproduction of bark beetle fungal symbionts. Can. J. For. Res. 2009, 39, 1133–1147. [Google Scholar] [CrossRef]
- Bridges, J.R. Nitrogen-fixing bacteria associated with bark beetles. Microb. Ecol. 1981, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Morales-Jiménez, J.; Zúñiga, G.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Bacterial community and nitrogen fixation in the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Curculionidae: Scolytinae). Microb. Ecol. 2009, 58, 879–891. [Google Scholar] [CrossRef]
- Morales-Jiménez, J.; Vera-Ponce de León, A.; García-Domínguez, A.; Martínez-Romero, E.; Zúñiga, G.; Hernández-Rodríguez, C. Nitrogen-fixing and uricolytic bacteria associated with the gut of Dendroctonus rhizophagus and Dendroctonus valens (Curculionidae: Scolytinae). Microb. Ecol. 2013, 66, 200–210. [Google Scholar] [CrossRef]
- Fabryová, A.; Kostovčík, M.; Díez-Méndez, A.; Jiménez-Gómez, A.; Celador-Lera, L.; Saati-Santamaría, Z.; Sechovcová, H.; Menéndez, E.; Kolařik, M.; García-Fraile, P. On the bright side of a forest pest-the metabolic potential of bark beetles’ bacterial associates. Sci. Total Environ. 2018, 619, 9–17. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Wang, C.; Chen, H. Cellulolytic bacteria associated with the gut of Dendroctonus armandi larvae (Coleoptera: Curculionidae: Scolytinae). Forests 2014, 5, 455–465. [Google Scholar] [CrossRef]
- Menendez, E.; Ramírez-Bahena, M.H.; Fabryova, A.; Igual, J.M.; Benada, O.; Mateos, P.F.; Peix, A.; Kolařík, M.; García-Fraile, P. Pseudomonas coleopterorum sp. nov., a cellulase-producing bacterium isolated from the bark beetle Hylesinus fraxini. Int. J. Syst. Evol. Microbiol. 2015, 65, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.K.; Keefover-Ring, K.; Mapes, A.C.; Adams, A.S.; Bohlmann, J.; Raffa, K.F. Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J. Chem. Ecol. 2013, 39, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.T.; Lu, M.; Sun, J.H. Invasive bark beetle-associated microbes degrade a host defensive monoterpene. Insect Sci. 2016, 23, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Skrodenytė-Arbačiauskienė, V.; Radžiutė, S.; Stunžėnas, V.; Būda, V. Erwiniatypographi sp. nov., isolated from bark beetle (Ips typographus) gut. Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 4, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.S.; Aylward, F.O.; Adams, S.M.; Erbilgin, N.; Aukema, B.H.; Currie, C.R.; Suen, G.; Raffa, K.F. Mountain pine beetles colonizing historical and naive host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl. Environ. Microbiol. 2013, 79, 3468–3475. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Kaltenpoth, M. Beetle-Bacterial Symbioses: Endless Forms Most Functional. Annu. Rev. Entomol. 2022, 67, 201–219. [Google Scholar] [CrossRef]
- Berasategui, A.; Salem, H.; Paetz, C.; Santoro, M.; Gershenzon, J.; Kaltenpoth, M.; Schmidt, A. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 2017, 26, 4099–4110. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef]
- Reis, F.; Kirsch, R.; Pauchet, Y.; Bauer, E.; Bilz, L.; Fukumori, K.; Fukatsu, T.; Kölsch, G.; Kaltenpoth, M. Bacterial symbionts support larval sap feeding and adult folivory in (semi-) aquatic reed beetles. Nat. Commun. 2020, 11, 2964. [Google Scholar] [CrossRef]
- Salem, H.; Bauer, E.; Kirsch, R.; Berasategui, A.; Cripps, M.; Weiss, B.; Koga, R.; Fukumori, K.; Vogel, H.; Fukatsu, T. Drastic genome reduction in an herbivore’s pectinolytic symbiont. Cell 2017, 171, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Kirsch, R.; Pauchet, Y.; Berasategui, A.; Fukumori, K.; Moriyama, M.; Cripps, M.; Windsor, D.; Fukatsu, T.; Gerardo, N.M. Symbiont digestive range reflects host plant breadth in herbivorous beetles. Curr. Biol. 2020, 30, 2875–2886. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.P.; Vogel, H.; Heckel, D.G.; Vilcinskas, A.; Kaltenpoth, M. Burying beetles regulate the microbiome of carcasses and use it to transmit a core microbiota to their offspring. Mol. Ecol. 2018, 27, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rozen, D.E. Gut microbiota in the burying beetle, Nicrophorus vespilloides, provide colonization resistance against larval bacterial pathogens. Ecol. Evol. 2018, 8, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Anbutsu, H.; Moriyama, M.; Nikoh, N.; Hosokawa, T.; Futahashi, R.; Tanahashi, M.; Meng, X.-Y.; Kuriwada, T.; Mori, N.; Oshima, K. Small genome symbiont underlies cuticle hardness in beetles. Proc. Natl. Acad. Sci. USA 2017, 114, E8382–E8391. [Google Scholar] [CrossRef] [PubMed]
- Hirota, B.; Okude, G.; Anbutsu, H.; Futahashi, R.; Moriyama, M.; Meng, X.-Y.; Nikoh, N.; Koga, R.; Fukatsu, T. A novel, extremely elongated, and endocellular bacterial symbiont supports cuticle formation of a grain pest beetle. Microbiology 2017, 8, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Maire, J.; Parisot, N.; Galvao Ferrarini, M.; Vallier, A.; Gillet, B.; Hughes, S.; Balmand, S.; Vincent-Monégat, C.; Zaidman-Rémy, A.; Heddi, A. Spatial and morphological reorganization of endosymbiosis during metamorphosis accommodates adult metabolic requirements in a weevil. Proc. Natl. Acad. Sci. USA 2020, 117, 19347–19358. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Kaltenpoth, M. Bacteriome-localized intracellular symbionts in pollen-feeding beetles of the genus Dasytes (Coleoptera, Dasytidae). Front. Microbiol. 2016, 7, 1486. [Google Scholar] [CrossRef]
- Dirren, S.; Salcher, M.M.; Blom, J.F.; Schweikert, M.; Posch, T. Ménage-à-trois: The Amoeba Nuclearia sp. from Lake Zurich with its Ecto- and Endosymbiotic Bacteria. Protist 2014, 165, 745–758. [Google Scholar] [CrossRef]
- Bellec, L.; Bonavita, M.-A.C.; Hourdez, S.; Jebbar, M.; Tasiemski, A.; Durand, L.; Gayet, N.; Zeppilli, D. Chemosynthetic ectosymbionts associated with a shallow-water marine nematode. Sci. Rep. 2019, 9, 7019. [Google Scholar] [CrossRef]
- Durand, A.A.; Bergeron, A.; Constant, P.; Buffet, J.P.; Deziel, E.; Guertin, C. Surveying the endomicrobiome and ectomicrobiome of bark beetles: The case of Dendroctonus simplex. Sci. Rep. 2015, 5, 17190. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Ophiostomatales Isolated from Two European Bark Beetles, Hylurgus ligniperda and Orthotomicus erosus, in California. Master’s Thesis, Iowa State University, Ames, IA, USA, 2010. [Google Scholar] [CrossRef]
- Zhang, J.H.; Yu, N.; Xu, X.X.; Liu, Z.W. Community structure, dispersal ability and functional profiling of microbiome existing in fat body and ovary of the brown planthopper, Nilaparvata lugens. Insect Sci. 2019, 26, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community ecology package 1.18-2. Time Int. 2010, 1997, 15–17. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.; Maffei, V.; Zaneveld, J.; Yurgel, S.; Brown, J.; Taylor, C.; Huttenhower, C.; Langille, M. PICRUSt2: An improved and extensible approach for metagenome inference. bioRxiv 2019, 672295. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Chen, H.; Ma, J. Differences in the structure of the gut bacteria communities in development stages of the Chinese white pine beetle (Dendroctonus armandi). Int. J. Mol. Sci. 2013, 14, 21006–21020. [Google Scholar] [CrossRef]
- Xu, L.; Lu, M.; Xu, D.; Chen, L.; Sun, J. Sexual variation of bacterial microbiota of Dendroctonus valens guts and frass in relation to verbenone production. J. Insect Physiol. 2016, 95, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Nones, S.; Simões, F.; Trindade, C.S.; Matos, J.; Sousa, E. Microbiome Associated with the Mycangia of Female and Male Adults of the Ambrosia Beetle Platypus cylindrus Fab. (Coleoptera: Curculionidae). Insects 2021, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, S.L.; Zhou, W.C. Identification of Hylurgus ligniperda. Plant Quar. 2006, 1, 30–31. [Google Scholar] [CrossRef]
- Chi, G.L. The Research of Pathogenicity and Ophiostomatoid Fungi Associated with Endemic Bark Beetles in Occurence Area of Dendroctonus valens. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2010. [Google Scholar] [CrossRef]
- Houck, M.; OConnor, B.M. Ecological and evolutionary significance of phoresy in the Astigmata. Annu. Rev. Entomol. 1991, 36, 611–636. [Google Scholar] [CrossRef]
- Briones-Roblero, C.I.; Hernández-García, J.A.; Gonzalez-Escobedo, R.; Soto-Robles, L.V.; Rivera-Orduña, F.N.; Zúñiga, G. Structure and dynamics of the gut bacterial microbiota of the bark beetle, Dendroctonus rhizophagus (Curculionidae: Scolytinae) across their life stages. PloS ONE 2017, 12, e0175470. [Google Scholar] [CrossRef]
- Li, J.L.; Li, C.C.; Wang, M.; Wang, L.X.; Liu, X.B.; Gao, C.L.; Ren, L.L.; Luo, Y.Q. Gut Structure and microbial communities in Sirex noctilio (Hymenoptera: Siricidae) and their predicted contribution to larval nutrition. Front. Microbiol. 2021, 12, 641141. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.K.; Wang, Y.; Li, Z.K.; Xue, H.J.; Zhou, X.D.; Huang, J.H. Two Apriona species sharing a host niche have different gut microbiome diversity. Microb. Ecol. 2022, 83, 1059–1072. [Google Scholar] [CrossRef]
- Vigneron, A.; Masson, F.; Vallier, A.; Balmand, S.; Rey, M.; Vincent-Monégat, C.; Aksoy, E.; Aubailly-Giraud, E.; Zaidman-Rémy, A.; Heddi, A. Insects recycle endosymbionts when the benefit is over. Curr. Biol. 2014, 24, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Xiang, X.J.; Wan, X. Divergence in gut bacterial community among life stages of the rainbow stag beetle Phalacrognathus muelleri (Coleptera: Lucanidae). Insects 2020, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Human, Z.R.; Slippers, B.; de Beer, Z.W.; Wingfield, M.J.; Venter, S.N. Antifungal actinomycetes associated with the pine bark beetle, Orthotomicus erosus, in South Africa. South. Afr. J. Sci. 2017, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nones, S.; Fernandes, C.; Duarte, L.; Cruz, L.; Sousa, E. Bacterial community associated with the ambrosia beetle Platypus cylindrus on declining Quercus suber trees in the Alentejo region of Portugal. Plant Pathol. 2022, 71, 966–979. [Google Scholar] [CrossRef]
- Wang, Y.J. The Diversity Research of Periplaneta Americana Intestinal Endogenous Actinomyces. Master’s Thesis, Guang dong Pharmaceutical University, Guangzhou, China, 2016. [Google Scholar]
- Wu, X.L.; Liang, R.X.; Dai, Q.Y.; Jin, D.C.; Wang, Y.Y.; Chao, W.L. Complete degradation of di-n-octyl phthalate by biochemical cooperation between Gordonia sp. strain JDC-2 and Arthrobacter sp. strain JDC-32 isolated from activated sludge. J. Hazard. Mater. 2010, 176, 262–268. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Lin, Z.; Liu, B.; Wang, G.; Weng, L.Y.; Zhou, J.L.; Hu, H.Q.; He, H.; Huang, Y.X.; Chen, J.J. Bioremediation of di-(2-ethylhexyl) phthalate contaminated red soil by Gordonia terrae RL-JC02: Characterization, metabolic pathway and kinetics. Sci. Total Environ. 2020, 733, 139138. [Google Scholar] [CrossRef]
- Táncsics, A.; Banerjee, S.; Soares, A.; Bedics, A.; Kriszt, B. Combined Omics Approach Reveals Key Differences between Aerobic and Microaerobic Xylene-Degrading Enrichment Bacterial Communities: Rhodoferax—A Hitherto Unknown Player Emerges from the Microbial Dark Matter. Environ. Sci. Technol. 2023, 57, 2846–2855. [Google Scholar] [CrossRef]
- Kundu, A.; Harrisson, O.; Ghoshal, S. Impacts of Arctic diesel contamination on microbial community composition and degradative gene abundance during hydrocarbon biodegradation with and without nutrients: A case study of seven sub-Arctic soils. Sci. Total Environ. 2023, 871, 161777. [Google Scholar] [CrossRef]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef]
- Briones-Roblero, C.I.; Rodríguez-Díaz, R.; Santiago-Cruz, J.A.; Zúñiga, G.; Rivera-Orduña, F.N. Degradation capacities of bacteria and yeasts isolated from the gut of Dendroctonus rhizophagus (Curculionidae: Scolytinae). Folia Microbiol. 2017, 62, 1–9. [Google Scholar] [CrossRef]
- Xu, L.; Lou, Q.; Cheng, C.; Lu, M.; Sun, J. Gut-associated bacteria of Dendroctonus valens and their involvement in verbenone production. Microb. Ecol. 2015, 70, 1012–1023. [Google Scholar] [CrossRef]
- Chakravarthy, S.S.; Pande, S.; Kapoor, A.; Nerurkar, A.S. Comparison of denitrification between Paracoccus sp. and Diaphorobacter sp. Appl. Biochem. Biotechnol. 2011, 165, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Oba, K.; Suenaga, T.; Kuroiwa, M.; Riya, S.; Terada, A. Exploring the Functions of Efficient Canonical Denitrifying Bacteria as N2O Sinks: Implications from 15N Tracer and Transcriptome Analyses. Environ. Sci. Technol. 2022, 56, 11694–11706. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.H.; Ye, F.Y.; Cheng, C.H.; Kang, Z.W.; Kou, H.R.; Sun, J.H. Symbiotic microbes aid host adaptation by metabolizing a deterrent host pine carbohydrate d-pinitol in a beetle-fungus invasive complex. Sci. Adv. 2022, 8, eadd5051. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J. Complex relationships at the intersection of insect gut microbiomes and plant defenses. J. Chem. Ecol. 2020, 46, 793–807. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Ge, S.; Li, J.; Ren, L.; Wang, C.; Luo, Y. Composition and Diversity of the Endobacteria and Ectobacteria of the Invasive Bark Beetle Hylurgus ligniperda (Fabricius) (Curculionidae: Scolytinae) in Newly Colonized Areas. Insects 2024, 15, 12. https://doi.org/10.3390/insects15010012
Gu Y, Ge S, Li J, Ren L, Wang C, Luo Y. Composition and Diversity of the Endobacteria and Ectobacteria of the Invasive Bark Beetle Hylurgus ligniperda (Fabricius) (Curculionidae: Scolytinae) in Newly Colonized Areas. Insects. 2024; 15(1):12. https://doi.org/10.3390/insects15010012
Chicago/Turabian StyleGu, Ying, Sixun Ge, Jiale Li, Lili Ren, Chuanzhen Wang, and Youqing Luo. 2024. "Composition and Diversity of the Endobacteria and Ectobacteria of the Invasive Bark Beetle Hylurgus ligniperda (Fabricius) (Curculionidae: Scolytinae) in Newly Colonized Areas" Insects 15, no. 1: 12. https://doi.org/10.3390/insects15010012




