Plasticity of Life-History Traits and Adult Fitness of Fall Webworm in Relation to Climate Change
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Experimental Design
2.3. Measurement Parameters
2.4. Pupal Energy Reserves
2.5. Statistical Analysis
3. Results
3.1. Larval Development Duration and Body Mass
3.2. Body Mass and Size of Pupae
3.3. Body Composition of Pupae
3.4. Adult Fitness
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fordyce, J.A. The Evolutionary Consequences of Ecological Interactions Mediated through Phenotypic Plasticity. J. Exp. Biol. 2006, 209, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F. Development and Evolution of Adaptive Polyphenisms. Evol. Dev. 2003, 5, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Blanckenhorn, W.U.; Fairbairn, D.J. Life History Adaptation along a Latitudinal Cline in the Water Strider Aquarius Remigis (Heteroptera: Gerridae). J. Evol. Biol. 1995, 8, 21–41. [Google Scholar] [CrossRef]
- HaiMin, H.; Liang, X.; QianWu, C.; FangSen, X. A life-history trait of the Asian corn borer, Ostrinia furnacalis (Lepidoptera: Pyralidae)-positive correlation between body weight and temperature. Acta Entomol. Sin. 2018, 61, 340–347. [Google Scholar]
- Dillon, M.E.; Wang, G.; Garrity, P.A.; Huey, R.B. Thermal Preference in Drosophila. J. Therm. Biol. 2009, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D. Temperature and Organism Size—A Biological Law for Ectotherms? In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1994; Volume 25, pp. 1–58. ISBN 978-0-12-013925-5. [Google Scholar]
- Angilletta, M.J.; Dunham, A.E. The Temperature-Size Rule in Ectotherms: Simple Evolutionary Explanations May Not Be General. Am. Nat. 2003, 162, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D. Ectotherm Life-History Responses to Developmental Temperature. In Animals and Temperature: Phenotypic and Evolutionary Adaptation; Cambridge University Press: Cambridge, UK, 1996; pp. 183–204. [Google Scholar]
- Molet, M.; Péronnet, R.; Couette, S.; Canovas, C.; Doums, C. Effect of Temperature and Social Environment on Worker Size in the Ant Temnothorax Nylanderi. J. Therm. Biol. 2017, 67, 22–29. [Google Scholar] [CrossRef]
- Kammenga, J.E.; Doroszuk, A.; Riksen, J.A.G.; Hazendonk, E.; Spiridon, L.; Petrescu, A.-J.; Tijsterman, M.; Plasterk, R.H.A.; Bakker, J. A Caenorhabditis Elegans Wild Type Defies the Temperature-Size Rule Owing to a Single Nucleotide Polymorphism in Tra-3. PLoS Genet. 2007, 3, e34. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Massie, K.R.; Ragland, G.J.; Smith, M.H. Rapid Population Divergence in Thermal Reaction Norms for an Invading Species: Breaking the Temperature?Size Rule. J. Evol. Biol. 2007, 20, 892–900. [Google Scholar] [CrossRef]
- Xiao, L.; He, H.-M.; Huang, L.-L.; Geng, T.; Fu, S.; Xue, F.-S. Variation of Life-History Traits of the Asian Corn Borer, Ostrinia Furnacalis in Relation to Temperature and Geographical Latitude. Ecol. Evol. 2016, 6, 5129–5143. [Google Scholar] [CrossRef]
- Fu, D.-M.; He, H.-M.; Zou, C.; Xiao, H.-J.; Xue, F.-S. Life-History Responses of the Rice Stem Borer Chilo Suppressalis to Temperature Change: Breaking the Temperature–Size Rule. J. Therm. Biol. 2016, 61, 115–118. [Google Scholar] [CrossRef]
- Chown, S.L.; Gaston, K.J. Body Size Variation in Insects: A Macroecological Perspective. Biol. Rev. 2010, 85, 139–169. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A.; Simmons, L.W. Evolution of Phenotypic Optima and Copula Duration in Dungflies. Nature 1994, 370, 53–56. [Google Scholar] [CrossRef]
- Rivero, A.; West, S.A. The Physiological Costs of Being Small in a Parasitic Wasp. Evol. Ecol. Res. 2002, 4, 407–420. [Google Scholar]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee Foraging Ranges and Their Relationship to Body Size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.Z.; Crone, E.E.; Williams, N.M. Integrating Vital Rates Explains Optimal Worker Size for Resource Return by Bumblebee Workers. Funct. Ecol. 2019, 33, 467–478. [Google Scholar] [CrossRef]
- Ramalho, M.; Imperatriz-Fonseca, V.L.; Giannini, T.C. Within-Colony Size Variation of Foragers and Pollen Load Capacity in the Stingless Bee Melipona Quadrifasciata Anthidioides Lepeletier (Apidae, Hymenoptera). Apidologie 1998, 29, 221–228. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, K.; Tang, F.; Xu, M. Activation of the Host Immune Response in Hyphantria Cunea (Drury) (Lepidoptera: Noctuidae) Induced by Serratia Marcescens Bizio. Insects 2021, 12, 983. [Google Scholar] [CrossRef]
- Xin, B.; Liu, P.; Zhang, S.; Yang, Z.; Daane, K.M.; Zheng, Y. Research and Application of Chouioia Cunea Yang (Hymenoptera: Eulophidae) in China. Biocontrol Sci. Technol. 2017, 27, 301–310. [Google Scholar] [CrossRef]
- Gomi, T.; Nagasaka, M.; Fukuda, T.; Hagihara, H. Shifting of the Life Cycle and Life-History Traits of the Fall Webworm in Relation to Climate Change. Entomol. Exp. Appl. 2007, 125, 179–184. [Google Scholar] [CrossRef]
- Chen, C.; Wei, X.; Xiao, H.; He, H.; Xia, Q.; Xue, F. Diapause Induction and Termination in Hyphantria Cunea (Drury) (Lepidoptera: Arctiinae). PLoS ONE 2014, 9, e98145. [Google Scholar] [CrossRef]
- Rehnberg, B.G. Temperature Profiles inside Webs of the Fall Webworm, Hyphantria Cunea (Lepidoptera: Arctiidae): Influence of Weather, Compass Orientation, and Time of Day. J. Therm. Biol. 2006, 31, 274–279. [Google Scholar] [CrossRef]
- Loewy, K.J.; Flansburg, A.L.; Grenis, K.; Kjeldgaard, M.K.; Mccarty, J.; Montesano, L.; Vernick, J.; Murphy, S.M. Life History Traits and Rearing Techniques for Fall Webworms (Hyphantria Cunea Drury) in Colorado. J. Lepidopterists Soc. 2013, 67, 196–205. [Google Scholar] [CrossRef]
- Williams, C.M.; Chick, W.D.; Sinclair, B.J. A Cross-Seasonal Perspective on Local Adaptation: Metabolic Plasticity Mediates Responses to Winter in a Thermal-Generalist Moth. Funct. Ecol. 2015, 29, 549–561. [Google Scholar] [CrossRef]
- Lorenz, M.W. Adipokinetic Hormone Inhibits the Formation of Energy Stores and Egg Production in the Cricket Gryllus Bimaculatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, E.B.; Gusev, I.A. A Novel Form of Phenotypic Plasticity of the Thermal Reaction Norms for Development in the Bug Graphosoma Lineatum (L.) (Heteroptera, Pentatomidae). Entomol. Rev. 2019, 99, 417–436. [Google Scholar] [CrossRef]
- Morgan Fleming, J.; Carter, A.W.; Sheldon, K.S. Dung Beetles Show Metabolic Plasticity as Pupae and Smaller Adult Body Size in Response to Increased Temperature Mean and Variance. J. Insect Physiol. 2021, 131, 104215. [Google Scholar] [CrossRef]
- Schlesener, D.C.H.; Wollmann, J.; Krüger, A.P.; Martins, L.N.; Teixeira, C.M.; Bernardi, D.; Garcia, F.R.M. Effect of Temperature on Reproduction, Development, and Phenotypic Plasticity of Drosophila Suzukii in Brazil. Entomol. Exp. Appl. 2020, 168, 817–826. [Google Scholar] [CrossRef]
- Filina, O.; Demirbas, B.; Haagmans, R.; van Zon, J.S. Temporal Scaling in C. Elegans Larval Development. Proc. Natl. Acad. Sci. USA 2022, 119, e2123110119. [Google Scholar] [CrossRef]
- Howe, R.W. Temperature Effects on Embryonic Development in Insects. Annu. Rev. Entomol. 1967, 12, 15–42. [Google Scholar] [CrossRef]
- Aguilon, D.J.; Velasco, L.R. Effects of Larval Rearing Temperature and Host Plant Condition on the Development, Survival, and Coloration of African Armyworm, Spodoptera Exempta Walker (Lepidoptera: Noctuidae). J. Environ. Sci. Manag. 2015, 18, 54–60. [Google Scholar] [CrossRef]
- Jaworski, T.; Hilszczański, J. The Effect of Temperature and Humidity Changes on Insects Development Their Impact on Forest Ecosystems in the Context of Expected Climate Change. For. Res. Pap. 2013, 74, 345–355. [Google Scholar] [CrossRef]
- Xia, Q.-W.; Chen, C.; Tang, J.-J.; He, H.-M.; Xue, F.-S. A Reverse Temperature-Size Rule Associated with a Negative Relationship between Larval Development Time and Pupal Weight in a Tropical Population of Ostrinia Furnacalis. Physiol. Entomol. 2019, 44, 209–214. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Huey, R.B. Size, Temperature, and Fitness: Three Rules. Evol. Ecol. Res. 2008, 10, 251–268. [Google Scholar] [CrossRef]
- Zuo, W.; Moses, M.E.; West, G.B.; Hou, C.; Brown, J.H. A General Model for Effects of Temperature on Ectotherm Ontogenetic Growth and Development. Proc. R. Soc. B Biol. Sci. 2011, 279, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Fukuchi, A.; Nanbara, S.; Takagi, M. Effect of Body Size and Sugar Meals on Oviposition of the Yellow Fever Mosquito, Aedes Aegypti (Diptera: Culicidae). J. Vector Ecol. 2010, 35, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.A.; Denlinger, D.L. Energetics of Insect Diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Aguila, J.R.; Suszko, J.; Gibbs, A.G.; Hoshizaki, D.K. The Role of Larval Fat Cells in Adult Drosophila Melanogaster. J. Exp. Biol. 2007, 210, 956–963. [Google Scholar] [CrossRef]
- Crompton, M.; Birt, L.M. Changes in the Amounts of Carbohydrates, Phosphagen, and Related Compounds during the Metamorphosis of the Blowfly, Lucilia Cuprina. J. Insect Physiol. 1967, 13, 1575–1592. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W. Effects of Autumn Warming on Energy Consumption of Diapausing Fall Webworm (Lepidoptera: Arctiidae) Pupae. J. Insect Sci. 2021, 21, 8. [Google Scholar] [CrossRef]
- Gomi, T. Effects of Timing of Diapause Induction on Winter Survival and Reproductive Success in Hyphantria Cunea in a Transition Area of Voltinism. Entomol. Sci. 2000, 3, 433–438. [Google Scholar]
- Verberk, W.C.; Buchwalter, D.B.; Kefford, B.J. Energetics as a Lens to Understanding Aquatic Insect’s Responses to Changing Temperature, Dissolved Oxygen and Salinity Regimes. Curr. Opin. Insect Sci. 2020, 41, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.E.; Tuchinskaya, I.; Buck, N.A.; Tabashnik, B.E. Hexameric Storage Proteins during Metamorphosis and Egg Production in the Diamondback Moth, Plutella Xylostella (Lepidoptera). J. Insect Physiol. 2000, 46, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Jahant-Miller, C.; Miller, R.; Parry, D. Size-Dependent Flight Capacity and Propensity in a Range-Expanding Invasive Insect. Insect Sci. 2022, 29, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.D. Sexual Selection, Phenotypic Variation, and Allometry in Genitalic and Non-Genitalic Traits in the Sexually Size-Dimorphic Stick Insect Micrarchus Hystriculeus. Biol. J. Linn. Soc. 2014, 113, 471–484. [Google Scholar] [CrossRef]
- Sota, T.; Mogi, M. Interspecific Variation in Desiccation Survival Time of Aedes (Stegomyia) Mosquito Eggs Is Correlated with Habitat and Egg Size. Oecologia 1992, 90, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Flinn, P.W.; Hagstrum, D.W. Movement of Rhyzopertha Dominica in Response to Temperature Gradients in Stored Wheat. J. Stored Prod. Res. 2011, 47, 407–410. [Google Scholar] [CrossRef]
- Bonsignore, C.P.; Bellamy, C. Daily Activity and Flight Behaviour of Adults of Capnodis Tenebrionis (Coleoptera: Buprestidae). Eur. J. Entomol. 2007, 104, 425–431. [Google Scholar] [CrossRef]
- Soule, A.J.; Decker, L.E.; Hunter, M.D. Effects of Diet and Temperature on Monarch Butterfly Wing Morphology and Flight Ability. J. Insect Conserv. 2020, 24, 961–975. [Google Scholar] [CrossRef]
- Jyothi, P.; Aralimarad, P.; Wali, V.; Dave, S.; Bheemanna, M.; Ashoka, J.; Shivayogiyappa, P.; Lim, K.S.; Chapman, J.W.; Sane, S.P. Evidence for Facultative Migratory Flight Behavior in Helicoverpa Armigera (Noctuidae: Lepidoptera) in India. PLoS ONE 2021, 16, e0245665. [Google Scholar] [CrossRef]
- Matzek, V. Trait Values, Not Trait Plasticity, Best Explain Invasive Species’ Performance in a Changing Environment. PLoS ONE 2012, 7, e48821. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do Invasive Species Show Higher Phenotypic Plasticity than Native Species and, If so, Is It Adaptive? A Meta-Analysis: Invasive Species Have Higher Phenotypic Plasticity. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
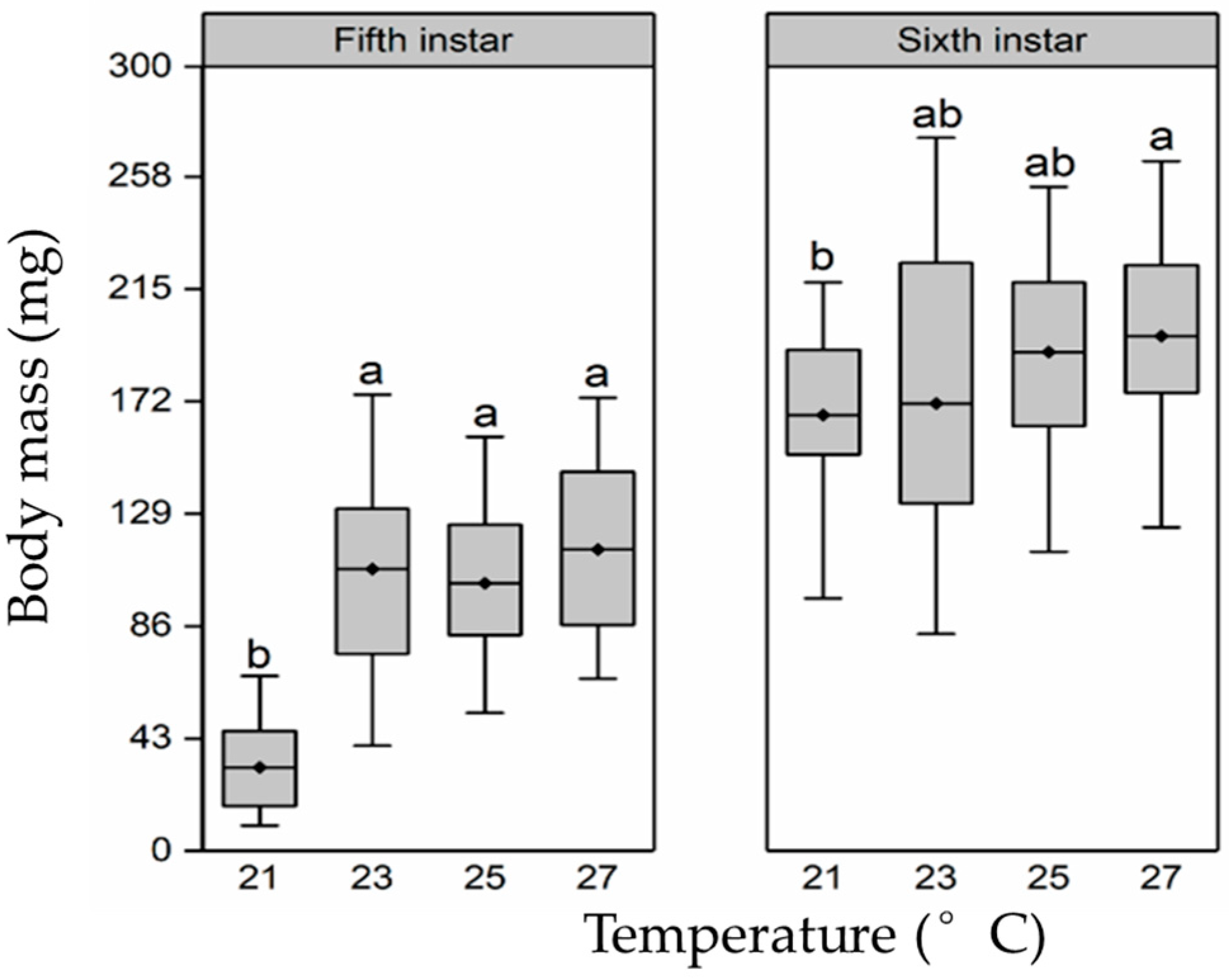


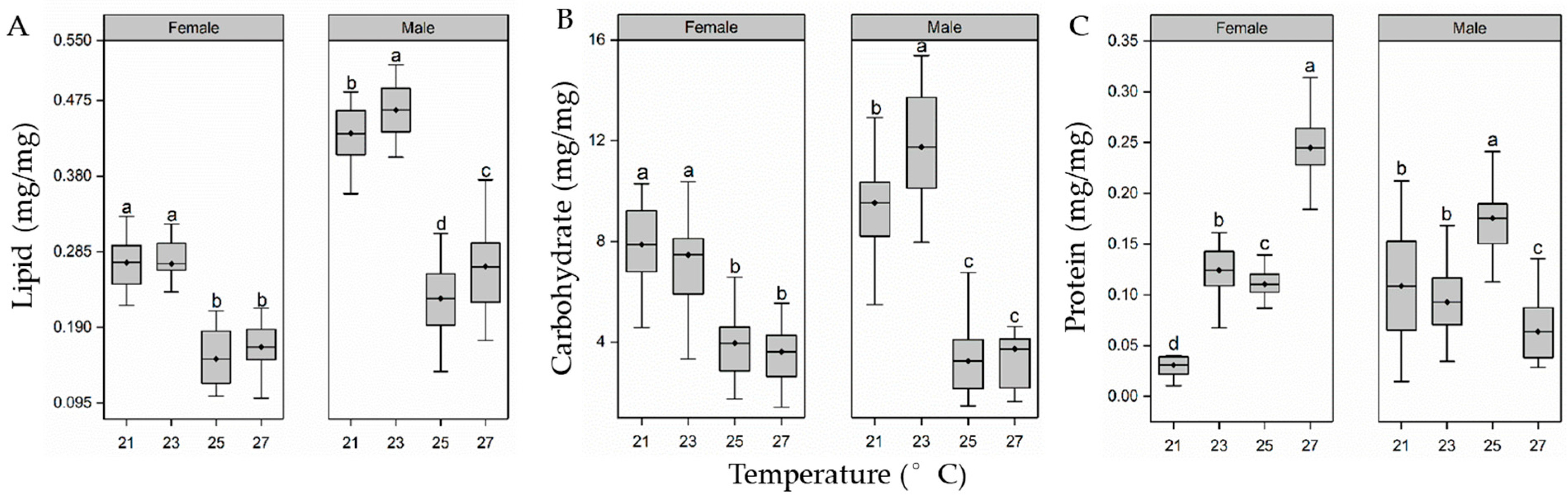
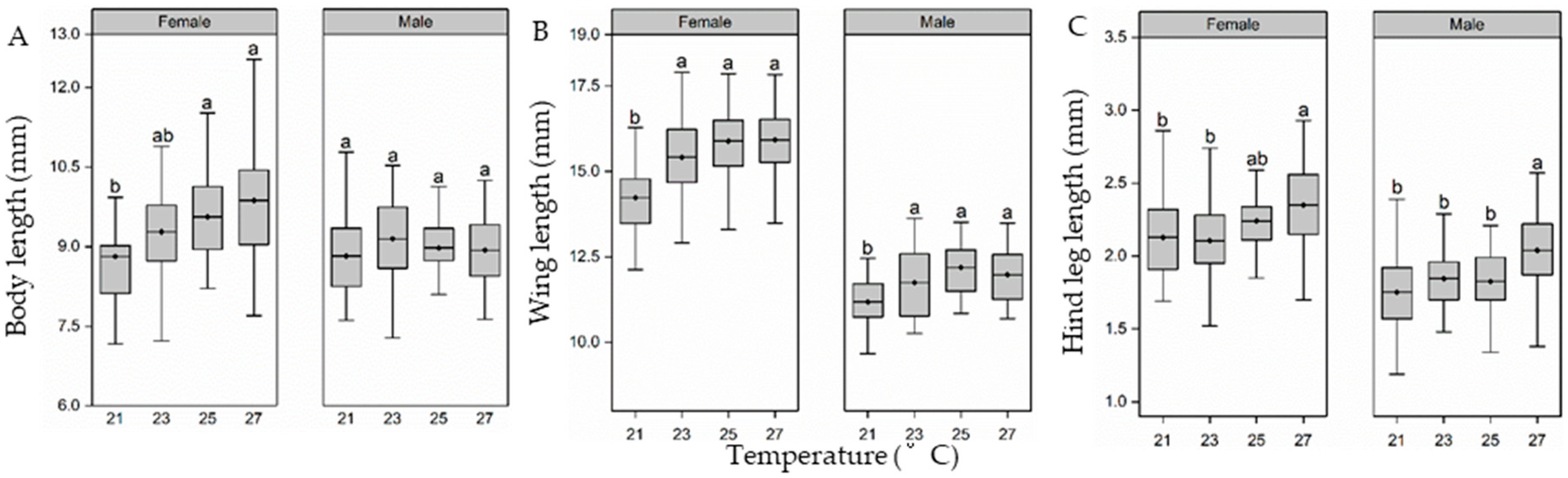

| Rearing Temperature | Development Duration (Days) | |||
|---|---|---|---|---|
| First–Fourth Instar | Fifth Instar | Sixth Instar | Total Larval Stage | |
| 21 °C | 18.54 ± 0.29 a | 7.08 ± 0.23 a | 12.42 ± 0. 3 a | 38.04 ± 0.46 a |
| 23 °C | 15.51 ± 0.17 b | 4.43 ± 0.11 b | 9.70 ± 0.26 a | 29.64 ± 0.28 b |
| 25 °C | 16.13 ± 0.14 b | 4.66 ± 0.08 b | 7.48 ± 0.17 b | 28.27 ± 00.24 c |
| 27 °C | 12.95 ± 0.18 c | 4.02 ± 0.12 b | 7.98 ± 0.25 b | 24.95 ± 0.31 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Xu, M.; Zhao, L. Plasticity of Life-History Traits and Adult Fitness of Fall Webworm in Relation to Climate Change. Insects 2024, 15, 24. https://doi.org/10.3390/insects15010024
Wang K, Xu M, Zhao L. Plasticity of Life-History Traits and Adult Fitness of Fall Webworm in Relation to Climate Change. Insects. 2024; 15(1):24. https://doi.org/10.3390/insects15010024
Chicago/Turabian StyleWang, Kailu, Mingxuan Xu, and Lvquan Zhao. 2024. "Plasticity of Life-History Traits and Adult Fitness of Fall Webworm in Relation to Climate Change" Insects 15, no. 1: 24. https://doi.org/10.3390/insects15010024
APA StyleWang, K., Xu, M., & Zhao, L. (2024). Plasticity of Life-History Traits and Adult Fitness of Fall Webworm in Relation to Climate Change. Insects, 15(1), 24. https://doi.org/10.3390/insects15010024







