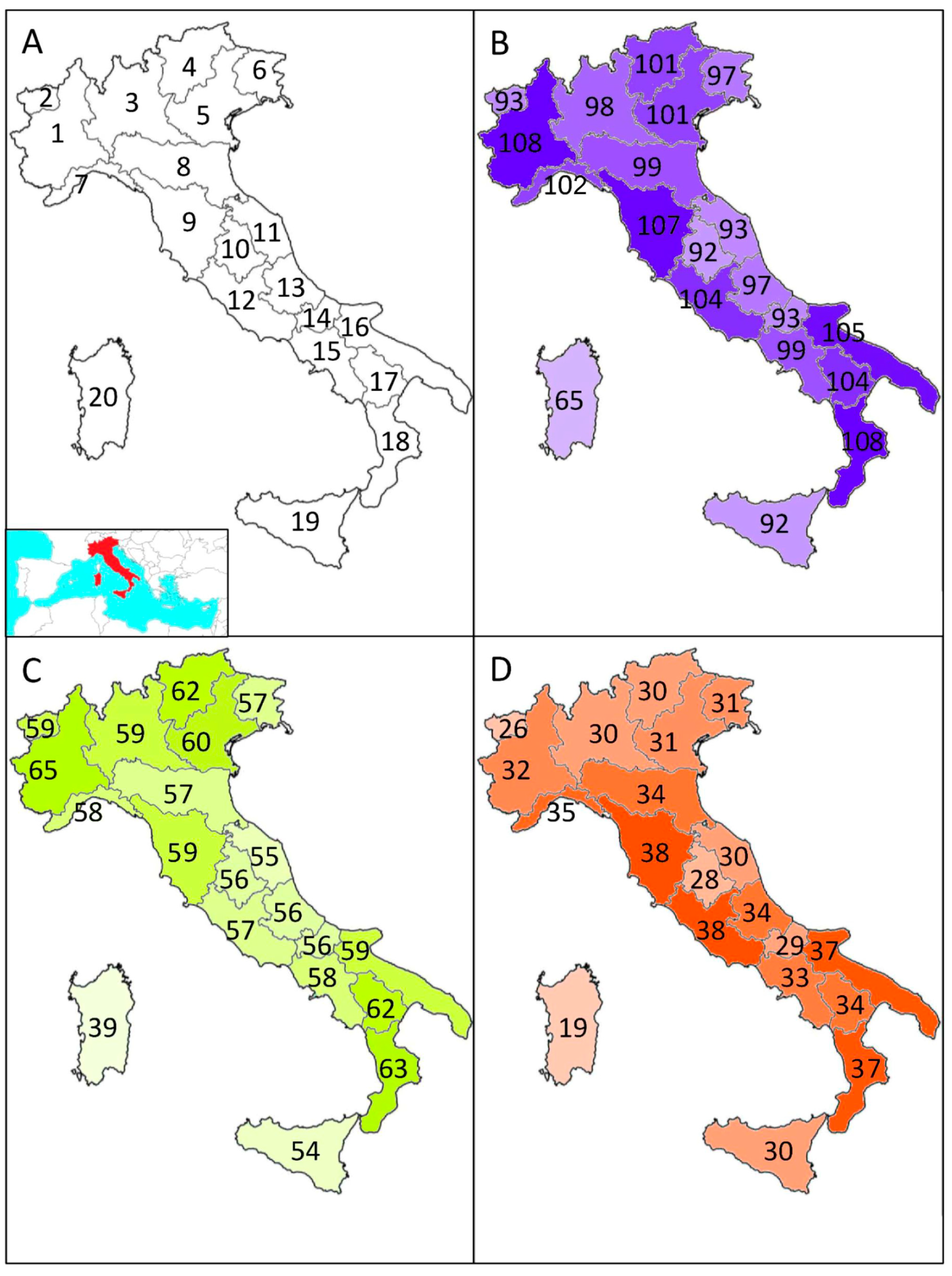
Macroecology of Dung Beetles in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- Species richness increases with area. One of the most universal biogeographical patterns is the species–area relationship (SAR), that is, the increase in species richness with increasing area [62]. Thus, we tested if dung beetle richness in Italy increased with the area of the Italian regions.
- (2)
- Islands have depauperate faunas. The Italian territory is composed of a long peninsula and two main islands (Sicily and Sardinia). Island biotas are known to be impoverished when compared to those of mainland regions of equal size [63,64]. Thus, we tested if island dung beetle faunas were impoverished in comparison with the mainland fauna.
- (3)
- Dung beetles conform to the peninsula effect. The Italian peninsula stretches from the European mainland at the north toward the center of the Mediterranean basin at the south. If the biota of a peninsula results from colonization processes starting from the mainland, species richness should decrease from the base to the tip of the peninsula, a phenomenon recorded for a variety of taxa and peninsulas worldwide [60,61,65,66]. In the case of the Italian peninsula, given its north–south alignment, species richness should decrease from the north (base of the peninsula) to the south (tip of the peninsula) [56,60,61]. To test this hypothesis, we correlated dung beetle richness with the latitude of the regions.
- (4)
- Dung beetles conform to the latitudinal gradient. One of the most widespread macroecological patterns is the latitudinal gradient: species richness tends to decrease from the equator to the poles [53,55,61,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. In the case of the Italian territory, this should translate to a pattern of decreasing richness from south to north, thus leading to a pattern opposite to that predicted by the peninsula effect but which might be explained by the current climate (if species respond positively to increasing temperatures) and the role of southern Italian regions as a Pleistocene refugial center. During the Pleistocene glacials, most European areas northern to the Alps were covered by ice, forcing species to retreat to the southern, ice-free areas; these areas acted as both refugial and speciation centers, from which species recolonized the northern areas after deglaciation [56,60,61]. Within the Italian peninsula, if dung beetles are positively influenced by temperatures and/or southern regions acted as Pleistocene refuges, a negative relationship between latitude and richness is expected [56].
- (5)
- Climate influences dung beetle richness, with differences between aphodiines and scarabaeines that reflect their main ecological characteristics. To test this hypothesis, we investigated the influence of climatic characteristics of Italian regions on their dung beetle diversity. Precipitation and temperature are expected to influence dung beetle diversity because of their effects on excrement persistence and quality. In cold and humid climates, excrement degradation is slow, while it proceeds faster in hot and arid climates, thus favoring species that can protect the offspring through subterranean pedotrophic nests [1,83,84,85]. Thus, dung beetle communities of temperate areas tend to be dominated by aphodiines (which are mainly dwellers), whereas scarabaeines (which are rollers and tunnellers) predominate in tropical and Mediterranean communities [85,86]. We expect that the amount of precipitation should influence positively the aphodiine richness in Italy and the overall dung beetle richness, given the prevalence of aphodiines in the Italian fauna, but not scarabaeines. We also expect that aridity should affect negatively aphodiines and the total dung beetle richness but not scarabaeines. In general, we expect that, given their different ways of food utilization, aphodiine will be negatively influenced by temperatures, whereas scarabaeines should be positively influenced or not influenced. These contrasting responses should lead to a lack of relationship between total dung beetle richness and temperature. We also expect that climatic variability should have a negative influence on aphodiines and possibly on dung beetles in general, but not on scarabaeines, which should be more adapted to tolerate high temporal variation in temperatures.
- (6)
- Elevation influences dung beetle richness negatively. Dung beetle diversity tends to decline with increasing elevation [1,85,87,88,89,90,91,92,93,94,95,96,97], although mid-elevation peaks [89,91,98] or a lack of relationship have been also reported [85,99]. Not only at higher elevations temperatures might be too low, especially in winter, but the drying effects of increased windiness and insulation might lead to the rapid desiccation of excrements, especially in the summer, when precipitation may be completely absent [85]. Thus, we hypothesize that more mountainous regions should have fewer species of dung beetles than the less mountainous ones, leading to an inverse relationship between dung beetle richness and regional average elevation.
- (7)
- Species distributions may result from three, mutually non-exclusive processes: influences of current (present day) ecological conditions, random processes, and historical (paleogeographical and paleoecological) events. As among current factors, climate and topography are major drivers of species distributions, we expect that inter-regional dissimilarities in species composition (β-diversity) correlate with similarity in these environmental conditions [53,56,60,61,100]. If species distributions result from random processes (from stochastic population dynamics and spatially constrained dispersal), spatial patterns of species similarity among regions should simply reflect their geographical proximity [53,56,60,61,101]. This means that we expect a distance decay of similarity independently from ecological differences among regions. Finally, if current species distributions have been influenced by historical factors (such as the effects of glacials), this would produce regional groupings characterized by distinct biogeographical discontinuities [53,56,60,61].
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanski, I.; Cambefort, Y. (Eds.) Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991; pp. 1–520. [Google Scholar]
- Scholtz, C.H.; Davis, A.L.V.; Kryger, U. Evolutionary Biology and Conservation of Dung Beetles; Pensoft: Sofia, Bulgaria, 2009; pp. 1–567. [Google Scholar]
- Dellacasa, G.; Bordat, P.; Dellacasa, M. A revisional essay of world genus-group taxa of Aphodiinae. Mem. Soc. Entomol. Ital. 2001, 79, 1–482. [Google Scholar]
- Dellacasa, G.; Dellacasa, M. Coleoptera Aphodiidae, Aphodiinae; Fauna d’Italia; Calderini—Il Sole 24 Ore: Milano, Italy, 2006; pp. 1–484. [Google Scholar]
- Halffter, G.; Halffter, V. Why and where coprophagous beetles (Coleoptera: Scarabaeinae) eat seeds, fruits or vegetable detritus. Boletín Soc. Entomológica Aragonesa 2009, 45, 1–22. [Google Scholar]
- Larsen, T.H.; Lopera, A.; Forsyth, A.; Génier, F. From coprophagy to predation: A dung beetle that kills millipedes. Biol. Lett. 2009, 5, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.W.; Ridsdill-Smith, J. Ecology and Evolution of Dung Beetles; John Wiley and Sons: Chichester, UK, 2011; pp. 1–347. [Google Scholar]
- Tonelli, M.; Giménez Gómez, V.C.; Verdú, J.R.; Casanoves, F.; Zunino, M. Dung beetle assemblages attracted to cow and horse dung: The importance of mouthpart traits, body size, and nesting behavior in the community assembly process. Life 2021, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, S.; von Hoermann, C.; Schmitt, T.; Steiger, S.; Ayasse, M. The attraction of the dung beetle Anoplotrupes stercorosus (Coleoptera: Geotrupidae) to volatiles from vertebrate cadavers. Insects 2020, 11, 476. [Google Scholar] [CrossRef]
- Ballerio, A.; Rey, A.; Uliana, M.; Rastelli, M.; Rastelli, S.; Romano, M.; Colacurcio, L. Coleotteri Scarabeoidei d’Italia. Available online: https://www.societaentomologicaitaliana.it/Coleotteri%20Scarabeoidea%20d'Italia%202014/ (accessed on 30 May 2023).
- Grandi, G. Introduzione allo Studio dell’Entomologia, Vol. II. Pterigoti; Edizioni Agricole: Bologna, Italy, 1951; Volume XLI, pp. 1–1332. [Google Scholar]
- Goidanich, A. Secoli di umanità nella entomologia italiana (per il centenario della S.E.I., Firenze 31 Ottobre 1869-Genova 31 Ottobre 1969). Mem. Soc. Entomol. Ital. 1969, 48, XXV–LXXXIV. [Google Scholar]
- Ratcliffe, B.C. Scarab beetles in human culture. In Scarabaeoidea in the 21st Century: A Festschrift Honoring Henry F. Howden; Jameson, M.L., Ratcliffe, B.C., Eds.; The Coleopterists Society Monographs: Washington, DC, USA, 2006; Volume 5, pp. 85–101. [Google Scholar]
- Govorushko, S. Human-Insect Interactions; CRC Press: Boca Raton, FI, USA, 2018; pp. 1–442. [Google Scholar]
- Fabre, J.H. Souvenirs Entomologiques: Études sur l’Instinct et les Moeurs des Insectes. Série 1; Ch. Delagrave: Paris, France, 1879; pp. 1–324. [Google Scholar]
- Fabre, J.H. Souvenirs Entomologiques: Études sur l’Instinct et les Moeurs des Insectes. Série 5; Ch. Delagrave: Paris, France, 1897; pp. 1–383. [Google Scholar]
- Ben-Tor, D. The Scarab: A Reflection of Ancient Egypt, 1st ed.; The Israel Museum: Jerusalem, Israel, 1993; pp. 1–84. [Google Scholar]
- Poole, F. Scarabs from the necropolis of Pontecagnano. In Atti Sesto Congresso Internazionale di Egittologia; Tipografia Torinese: Torino, Italy, 1993; Volume 2, pp. 407–414. [Google Scholar]
- Halffter, G.; Edmonds, W.D. The Nesting Behaviour of Dung Beetles (Scarabaeinae): An Ecological and Evolutive Approach; Instituto de Ecologìa: México City, Mexico, 1982; pp. 1–176. [Google Scholar]
- Doube, B.M. A functional classification for the analysis of dung beetle assemblages. Ecol. Entomol. 1990, 15, 371–383. [Google Scholar] [CrossRef]
- Tonelli, M. Some considerations on the terminology applied to dung beetle functional groups. Ecol. Entomol. 2021, 46, 772–776. [Google Scholar] [CrossRef]
- Yokoyama, K.; Kai, H.; Koga, T.; Kawaguchi, S. Effect of dung beetle, Onthophagus lenzii H. on nitrogen transformation in cow dung and dung balls. Soil Sci. Plant Nutr. 1991, 37, 341–345. [Google Scholar] [CrossRef]
- Fowler, F.; Denning, S.; Hu, S.; Watson, W. Carbon neutral: The failure of dung beetles (Coleoptera: Scarabaeidae) to affect dung-generated greenhouse gases in the pasture. Environ. Entomol. 2000, 49, 1105–1116. [Google Scholar] [CrossRef]
- Andresen, E.; Feer, F. The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. In Seed Fate: Predation, Dispersal and Seedling Establishment; Forget, P.-M., Lambert, J.E., Hulme, P.E., Vander Wall, S.B., Eds.; CAB International: Wallingford, UK, 2005; pp. 331–349. [Google Scholar]
- Bang, H.S.; Lee, J.-H.; Kwon, O.S.; Na, Y.E.; Jang, Y.S.; Kim, W.H. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil Ecol. 2005, 29, 165–171. [Google Scholar] [CrossRef]
- Bishop, A.L.; McKenzie, H.J.; Spohr, L.J.; Barchia, I.M. Interactions between dung beetles (Coleoptera: Scarabaeidae) and the arbovirus vector Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae). Aust. J. Entomol. 2005, 44, 89–96. [Google Scholar] [CrossRef]
- Yamada, D.; Imura, O.; Shi, K.; Shibuya, T. Effect of tunneler dung beetles on cattle dung decomposition, soil nutrients and herbage growth. Graesslia 2007, 53, 121–129. [Google Scholar] [CrossRef]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Penttilä, A.; Slade, E.M.; Simojoki, A.; Riutta, T.; Minkkinen, K.; Roslin, T. Quantifying beetle-mediated effects on gas fluxes from dung pats. PLoS ONE 2013, 8, e71454. [Google Scholar] [CrossRef]
- Nichols, E.; Gómez, A. Dung beetles and fecal helminth transmission: Patterns, mechanisms, and questions. Parasitology 2014, 141, 614–623. [Google Scholar] [CrossRef]
- Verdú, J.R.; Sánchez-Piñero, F.; Lobo, J.M.; Cortez, V. Evaluating long-term ivermectin use and the role of dung beetles in reducing short-term CH4 and CO2 emissions from livestock faeces: A mesocosm design under Mediterranean conditions. Ecol. Entomol. 2019, 45, 109–120. [Google Scholar] [CrossRef]
- Stanbrook, R.; King, J.R. Dung beetle community composition affects dung turnover in subtropical US grasslands. Ecol. Evol. 2022, 12, e8660. [Google Scholar] [CrossRef]
- Jargalsaikhan, P.; Altangerel, G.; Enkhchimeg, T.; Aibek, U.; Bayartogtokh, B. Variation in dung removal rates by dung beetles (Coleoptera: Scarabaeoidea) in a temperate, dry steppe ecosystem. Diversity 2023, 15, 91. [Google Scholar] [CrossRef]
- Ma, L.; Weeraratne, N.; Gurusinghe, S.; Aktar, J.; Haque, K.M.S.; Eberbach, P.; Gurr, G.G.; Weston, L.A. Dung beetle activity is soil-type-dependent and modulates pasture growth and associated soil microbiome. Agronomy 2023, 13, 325. [Google Scholar] [CrossRef]
- de Andrade, R.B.D.; Barlow, J.; Louzada, J.; Vaz-de-Mello, F.Z.; Souza, M.; Silveira, J.M.; Cochrane, M.A. Quantifying responses of dung beetles to fire disturbance in tropical forests: The importance of trapping method and seasonality. PLoS ONE 2011, 6, e26208. [Google Scholar] [CrossRef]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.-P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Numa, C.; Tonelli, M.; Lobo, J.M.; Verdú, J.R.; Lumaret, J.-P.; Sánchez-Piñero, F.; Ruiz, J.L.; Dellacasa, M.; Ziani, S.; Arriaga, A.; et al. The Conservation Status and Distribution of Mediterranean Dung Beetles; IUCN: Gland, Switzerland; Málaga, Spain, 2020. [Google Scholar]
- Palusci, E.; Mantoni, C.; Strona, G.; Fattorini, S. Wildfire does not affect the dung beetle diversity of high-altitude Mediterranean habitats. Int. J. Wildland Fire 2021, 30, 636–642. [Google Scholar] [CrossRef]
- Noriega, J.A.; March-Salas, M.; Castillo, S.; García-Q, H.; Hortal, J.; Santos, A.M.C. Human perturbations reduce dung beetle diversity and dung removal ecosystem function. Biotropica 2021, 53, 753–766. [Google Scholar] [CrossRef]
- Lumaret, J.-P.; Kadiri, N.; Martínez, M.I. The global decline of dung beetles. In Imperiled: The Encyclopedia of Conservation; DellaSala, D.A., Goldstein, M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 3, pp. 553–562. [Google Scholar]
- Stoch, F. How many endemic species? Species richness assessment and conservation priorities in Italy. Belg. J. Entomol. 2000, 2, 125–133. [Google Scholar]
- Ruffo, S.; Stoch, F. (Eds.) Checklist and Distribution of the Italian Fauna. 10,000 Terrestrial and Freshwater Species, 2 Serie, Sez. Scienze della Vita, 2nd revised ed.; Memorie del Museo Civico di Storia Naturale di Verona: Verona, Italy, 2007; Volume 17. [Google Scholar]
- Blasi, C.; Biondi, E. La flora in Italia; Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Sapienza Università Editrice: Rome, Italy, 2017; pp. 1–704. [Google Scholar]
- Stinca, A.; Musarella, C.M.; Rosati, L.; Laface, V.L.A.; Licht, W.; Fanfarillo, E.; Wagensommer, R.P.; Galasso, G.; Fascetti, S.; Esposito, A.; et al. Italian vascular flora: New findings, updates and exploration of floristic similarities between regions. Diversity 2021, 13, 600. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Médail, F.; Myers, N. Mediterranean Basin. In Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; Mittermeier, R.A., Gil, P.R., Hoffman, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J., Da Fonseca, G.A.B., Eds.; CEMEX: Agrupación Sierra Madre, Mexico, 2004; pp. 144–147. [Google Scholar]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Heidelberg, Germany, 2011; pp. 2–22. [Google Scholar]
- Hewitt, G.M. Mediterranean peninsulas: The evolution of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Heidelberg, Germany, 2011; pp. 123–147. [Google Scholar]
- Birdlife International. Ecosystem Profile. Basin Biodiversity Hotspot. Available online: https://www.cepf.net/sites/default/files/mediterranean-basin-2017-ecosystem-profile-english_0.pdf (accessed on 25 November 2023).
- Dennis, R.L.H.; Williams, W.R.; Shreeve, T.G. Faunal structures among European butterflies: Evolutionary implications of bias for geography, endemism and taxonomic affiliation. Ecography 1998, 21, 181–203. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK, 1999; pp. 1–328. [Google Scholar]
- Dapporto, L.; Habel, J.C.; Dennis, R.L.H.; Schmitt, T. The biogeography of the western Mediterranean: Elucidating contradictory distribution patterns of differentiation in Maniola jurtina (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 2011, 103, 571–577. [Google Scholar] [CrossRef]
- Fattorini, S.; Baselga, A. Species richness and turnover patterns in European tenebrionid beetles. Insect Conserv. Diver. 2012, 5, 331–345. [Google Scholar] [CrossRef]
- Fattorini, S.; Ulrich, W. Spatial distributions of European Tenebrionidae point to multiple postglacial colonization trajectories. Biol. J. Linn. Soc. 2012, 105, 318–329. [Google Scholar] [CrossRef]
- Fattorini, S.; Ulrich, W. Drivers of species richness in European Tenebrionidae (Coleoptera). Acta Oecol. 2012, 43, 22–28. [Google Scholar] [CrossRef]
- Fattorini, S. Tenebrionid beetle distributional patterns in Italy: Multiple colonisation trajectories in a biogeographical crossroad. Insect Conserv. Divers. 2014, 7, 144–160. [Google Scholar] [CrossRef]
- Dapporto, L.; Fattorini, S.; Vodă, R.; Dincă, V.; Vila, R. Biogeography of western Mediterranean butterflies: Combining turnover and nestedness components of faunal dissimilarity. J. Biogeogr. 2014, 41, 1639–1650. [Google Scholar] [CrossRef]
- Fattorini, S. Conservation biogeography of tenebrionid beetles: Insights from Italian reserves. Diversity 2020, 12, 348. [Google Scholar] [CrossRef]
- Schmitt, T.; Fritz, U.; Delfino, M.; Ulrich, W.; Habel, J.C. Biogeography of Italy revisited: Genetic lineages confirm major phylogeographic patterns and a pre-Pleistocene origin of its biota. Front. Zool. 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Odonate diversity patterns in Italy disclose intricate colonization pathways. Biology 2022, 11, 886. [Google Scholar] [CrossRef]
- Fattorini, S. Biogeographical patterns of earwigs in Italy. Insects 2023, 14, 235. [Google Scholar] [CrossRef]
- Matthews, T.; Triantis, K.; Whittaker, R. (Eds.) The Species-Area Relationship: Theory and Application; Cambridge University Press: Cambridge, UK, 2020; pp. 1–481. [Google Scholar]
- Heiser, M.; Dapporto, L.; Schmitt, T. Coupling impoverishment analysis and partitioning of beta diversity allows a comprehensive description of Odonata biogeography in the Western Mediterranean. Org. Divers. Evol. 2014, 14, 203–214. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M.; Matthews, T.J. Island Biogeography: Geo-Environmental Dynamics, Ecology, Evolution, Human Impact, and Conservation; Oxford University Press: Oxford, UK, 2023; pp. 1–476. [Google Scholar]
- Battisti, C. ‘Peninsula effect’ and Italian peninsula: Matherials for a review and implications in applied biogeography. Biogeographia 2006, 27, 153–188. [Google Scholar] [CrossRef]
- Battisti, C. Peninsular patterns in biological diversity: Historical arrangement, methodological approaches and causal processes. J. Nat. Hist. 2014, 48, 43–44. [Google Scholar] [CrossRef]
- Pianka, E.R. Latitudinal gradients in species diversity: A review of concepts. Am. Nat. 1966, 100, 33–46. [Google Scholar] [CrossRef]
- Fattorini, S. Global patterns of earwig species richness. Diversity 2022, 14, 890. [Google Scholar] [CrossRef]
- Rohde, K. Latitudinal gradients in species diversity: The search for the primary cause. Oikos 1992, 65, 514–527. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Ashton, K.G. Are ecological and evolutionary rules being dismissed prematurely? Divers. Distrib. 2001, 7, 289–295. [Google Scholar] [CrossRef]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef]
- Cancello, E.M.; Silva, R.R.; Vasconcellos, A.; Reis, Y.T.; Oliveira, L.M. Latitudinal variation in termite species richness and abundance along the Brazilian Atlantic forest hotspot. Biotropica 2014, 46, 441–450. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Schemske, D.W.; Mittelbach, G.G. “Latitudinal gradients in species diversity”: Reflections on Pianka’s 1966 article and a look forward. Am. Nat. 2017, 189, 599–603. [Google Scholar] [CrossRef]
- Kinlock, N.L.; Prowant, L.; Herstoff, E.M.; Foley, C.M.; Akin-Fajiye, M.; Bender, N.; Umarani, M.; Ryu, H.Y.; Sen, H.Y.; Gurevitch, J.; et al. Explaining global variation in the latitudinal diversity gradient: Meta-analysis confirms known patterns and uncovers new ones. Glob. Ecol. Biogeogr. 2018, 27, 125–141. [Google Scholar] [CrossRef]
- Beaugrand, G.; Kirby, R.; Goberville, E. The mathematical influence on global patterns of biodiversity. Ecol. Evol. 2020, 10, 6494–6511. [Google Scholar] [CrossRef] [PubMed]
- Cushman, J.; Lawton, J.; Manly, B. Latitudinal patterns in European ant assemblages: Variation in species richness and body size. Oecologia 1993, 95, 30–37. [Google Scholar] [CrossRef]
- Baselga, A. Determinants of species richness, endemism and turnover in European longhorn beetles. Ecography 2008, 31, 263–271. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T. Environmental and historical effects on richness and endemism patterns of carabid beetles in the western Palaearctic. Ecography 2009, 32, 705–714. [Google Scholar] [CrossRef]
- Ulrich, W.; Fiera, C. Environmental correlates of species richness of European springtails (Hexapoda: Collembola). Acta Oecol. 2009, 35, 45–52. [Google Scholar] [CrossRef]
- Bąkowski, M.; Ulrich, W.; Laštůvka, Z. Environmental correlates of species richness of Sesiidae (Lepidoptera) in Europe. Eur. J. Entomol. 2010, 107, 563–570. [Google Scholar] [CrossRef]
- Finn, J.A.; Gittings, T. A review of competition in north temperate dung beetle communities. Ecol. Entomol. 2003, 28, 1–13. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Piattella, E. Competizione interspecifica e andamento stagionale di una comunità coprofaga in un’area verde urbana di Roma (Coleoptera, Scarabaeoidea). Boll. Ass. Romana Entomol. 1990, 44, 67–79. [Google Scholar]
- Mantoni, C.; Tsafack, N.; Palusci, E.; Di Pietro, S.; Fattorini, S. Diversity patterns of dung beetles along a Mediterranean elevational gradient. Insects 2021, 12, 781. [Google Scholar] [CrossRef]
- Martín-Piera, F.; Veiga, C.M.; Lobo, J.M. Ecology and biogeography of dung-beetle communities (Coleoptera, Scarabaeoidea) in an Iberian mountain range. J. Biogeogr. 1992, 19, 677–691. [Google Scholar] [CrossRef]
- Lumaret, J.P.; Stiernet, N. Montane dung beetles. In Dung Beetle Ecology; Hanski, I., Cambefort, Y., Eds.; Princeton University Press: Princeton, NJ, USA, 1991; pp. 242–254. [Google Scholar]
- Lumaret, J.P.; Stiernet, N. Biogeography of dung beetle communities in the western and central Alps (Coleoptera, Scarabaeoidea). Biogeographia 1992, 16, 425–436. [Google Scholar]
- Jay-Robert, P.; Lobo, J.M.; Lumaret, J.P. Altitudinal turnover and species richness variation in European montane dung beetle assemblages. Arct. Antarct. Alp. Res. 1997, 29, 196–205. [Google Scholar] [CrossRef]
- Avila, J.M.; Pascual, F. Contribución al conocimiento de los escarabeidos coprófagos (Coleoptera, Scarabaeoidea) de Sierra Nevada: III. Distribución altitudinal y temporal. Boll. Mus. Reg. Sci. Nat. Torino 1988, 6, 217–240. [Google Scholar]
- Lobo, J.M.; Halffter, G. Biogeographical and ecological factors affecting the altitudinal variation of mountainous communities of coprophagous beetles (Coleoptera: Scarabaeoidea): A comparative study. Ann. Entomol. Soc. Am. 2000, 93, 115–126. [Google Scholar] [CrossRef]
- Errouissi, F.; Jay-Robert, P.; Lumaret, J.P.; Piau, O. Composition and structure of dung beetle (Coleoptera: Aphodiidae, Geotrupidae, Scarabaeidae) assemblages in mountain grasslands of the Southern Alps. Ann. Entomol. Soc. Am. 2004, 97, 701–709. [Google Scholar] [CrossRef]
- Escobar, F.; Halffter, G.; Arellano, L. From forest to pasture: An evaluation of the influence of environment and biogeography on the structure of beetle (Scarabaeinae) assemblages along three altitudinal gradients in the Neotropical region. Ecography 2007, 30, 193–208. [Google Scholar] [CrossRef]
- Herzog, S.K.; Hamel-Leigue, A.C.; Larsen, T.H.; Mann, D.J.; Soria-Auza, R.W.; Gill, B.D.; Edmonds, W.D.; Spector, S. Elevational distribution and conservation biogeography of Phanaeine dung beetles (Coleoptera: Scarabaeinae) in Bolivia. PLoS ONE 2013, 8, e64963. [Google Scholar] [CrossRef]
- Nunes, C.A.; Braga, R.F.; Figueira, J.E.C.; Neves, F.D.S.; Fernandes, G.W. Dung beetles along a tropical altitudinal gradient: Environmental filtering on taxonomic and functional diversity. PLoS ONE 2016, 11, e0157442. [Google Scholar] [CrossRef]
- Şenyüz, Y.; Lobo, J.M.; Dindar, K. Altitudinal gradient in species richness and composition of dung beetles (Coleoptera: Scarabaeidae) in an eastern Euro-Mediterranean locality: Functional, seasonal and habitat influences. Eur. J. Entomol. 2019, 116, 309–319. [Google Scholar] [CrossRef]
- Stanbrook, R.; Wheater, C.P.; Harris, W.E.; Jones, M. Habitat type and altitude work in tandem to drive the community structure of dung beetles in Afromontane forest. J. Insect Conserv. 2021, 25, 159–173. [Google Scholar] [CrossRef]
- Lobo, J.M.; Guéorguiev, B.; Chehlarov, E. Convergences and divergences between two European mountain dung beetle assemblages (Coleoptera, Scarabaeoidea). Anim. Biodivers. Conserv. 2007, 30.1, 83–96. [Google Scholar] [CrossRef]
- Romero-Alcaraz, E.; Ávila, J.M. Effect of elevation and type of habitat on the abundance and diversity of Scarabaeoid dung beetle (Scarabaeoidea) assemblages in a Mediterranean area from Southern Iberian Peninsula. Zool. Stud. 2000, 39, 351–359. [Google Scholar]
- Keddy, P.; Laughlin, D. A Framework for Community Ecology: Species Pools, Filters and Traits; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Hubbell, S.P. The Unified Theory of Biogeography and Biodiversity; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Forum Entomologi Italiani. Available online: http://www.entomologiitaliani.net/public/forum/phpBB3/index.php (accessed on 30 November 2023).
- Pinkert, S.; Barve, V.; Guralnick, R.; Jetz, W. Global geographical and latitudinal variation in butterfly species richness captured through a comprehensive country-level occurrence database. Glob. Ecol. Biogeogr. 2022, 31, 830–839. [Google Scholar] [CrossRef]
- Hortal, J. Uncertainty and the measurement of terrestrial biodiversity gradients. J. Biogeogr. 2008, 35, 1202–1214. [Google Scholar] [CrossRef]
- Heino, J.; Alahuhta, J.; Fattorini, S.; Schmera, D. Predicting beta diversity of terrestrial and aquatic beetles using ecogeographical variables: Insights from the replacement and richness difference components. J. Biogeogr. 2019, 46, 304–315. [Google Scholar] [CrossRef]
- Konvicka, M.; Fric, Z.; Benes, J. Butterfly extinctions in European states: Do socioeconomic conditions matter more than physical geography? Glob. Ecol. Biogeogr. 2006, 15, 82–92. [Google Scholar] [CrossRef]
- Dapporto, L.; Dennis, R.L.H. Conservation biogeography of large Mediterranean islands. Butterfly impoverishment, conservation priorities and inferences for an ecological island paradigm. Ecography 2009, 32, 169–179. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Williams, W.R.; Shreeve, T.G. A multivariate approach to the determination of faunal units among European butterfly species (Lepidoptera: Rhopalocera). Zool. J. Linn. Soc. 1991, 101, 1–49. [Google Scholar] [CrossRef]
- Keil, P.; Hawkins, B.A. Grids versus regional species lists: Are broad-scale patterns of species richness robust to the violation of constant grain size? Biodiv. Conserv. 2009, 18, 3127–3137. [Google Scholar] [CrossRef]
- ISTAT—Istituto Nazionale di Statistica. Available online: https://www.istat.it/it/archivio/222527 (accessed on 3 October 2023).
- Worldclim—Maps, Graphs, Tables, and Data of the Global Climate. Available online: https://www.worldclim.org/data/worldclim21.html (accessed on 3 October 2023).
- ESRI. ArcGis Desktop: Version 10.4; Environmental Systems Research Institute (ESRI): Redlands, CA, USA, 2016. [Google Scholar]
- Tarquini, S.; Isola, I.; Favalli, M.; Battistini, A.; Dotta, G. TINITALY, a Digital Elevation Model of Italy with a 10 Meters Cell Size, Version 1.1. Istituto Nazionale di Geofisica e Vulcanologia (INGV). 2023. Available online: https://tinitaly.pi.ingv.it/ (accessed on 3 October 2023).
- Triantis, K.A.; Guilhaumon, F.; Whittaker, R.J. The island species–area relationship: Biology and statistics. J. Biogeogr. 2012, 39, 215–231. [Google Scholar] [CrossRef]
- Matthews, T.J.; Guilhaumon, F.; Triantis, K.A.; Borregaard, M.K.; Whittaker, R.J. On the form of species–area relationships in habitat islands and true islands. Glob. Ecol. Biogeogr. 2016, 25, 847–858. [Google Scholar] [CrossRef]
- Fattorini, S.; Borges, P.V.A.; Dapporto, L.; Strona, G. What can the parameters of the species–area relationship (SAR) tell us? Insights from Mediterranean islands. J. Biogeogr. 2017, 44, 1018–1028. [Google Scholar] [CrossRef]
- Sturba, L.; Fattorini, N.; Liberatori, G.; Vannuccini, M.L.; Nannoni, F.; Protano, G.; Tursi, A.; Corsi, I. Multi-model inference analysis of toxicological responses and levels of heavy metals in soft tissue of land snail Cornu aspersum caged in proximity to an industrial setting. Ecol. Indic. 2020, 117, 106688. [Google Scholar] [CrossRef]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. A framework for delineating biogeographical regions based on species distributions. J. Biogeogr. 2010, 37, 2029–2053. [Google Scholar] [CrossRef]
- Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Baselga, A.; Leprieur, F. Comparing methods to separate components of beta diversity. Methods Ecol. Evol. 2015, 6, 1069–1079. [Google Scholar] [CrossRef]
- Castro-Insua, A.; Gómez-Rodríguez, C.; Baselga, A. Dissimilarity measures affected by richness differences yield biased delimitations of biogeographic realms. Nat. Commun. 2018, 9, 5084. [Google Scholar] [CrossRef]
- Sánchez-González, L.A.; Morrone, J.J.; Navarro-Sigüenza, A.G. Distributional patterns of the Neotropical humid montane forest avifaunas. Biol. J. Linn. Soc. 2008, 94, 175–194. [Google Scholar] [CrossRef]
- Fattorini, S. Biogeography of the tenebrionid beetles (Coleoptera, Tenebrionidae) on the Aegean Islands (Greece). J. Biogeogr. 2002, 29, 49–67. [Google Scholar] [CrossRef]
- Graham, C.H.; Smith, T.B.; Languy, M. Current and historical factors influencing patterns of species richness and turnover of birds in the Gulf of Guinea highlands. J. Biogeogr. 2005, 32, 1371–1384. [Google Scholar] [CrossRef]
- Guerrero, J.C.; Vargas, J.; Real, R. A hypothetico-deductive analysis of the environmental factors involved in the current reptile distribution pattern in the Canary Islands. J. Biogeogr. 2005, 32, 1343–1351. [Google Scholar] [CrossRef]
- Smith, S.A.; Bermingham, E. The biogeography of lower Mesoamerican freshwater fishes. J. Biogeogr. 2005, 32, 1835–1854. [Google Scholar] [CrossRef]
- Holt, B.; Lessard, J.P.; Borregaard, M.K.; Fritz, S.A.; Araujo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jonsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the world. Science 2013, 339, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.J.; Tsai, P.; Wong, P.S.; Cheung, A.K.L.; Basher, Z.; Chaudhary, C. Marine biogeographic realms and species endemicity. Nat. Commun. 2017, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Historical biogeography of earwigs. Biology 2022, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Podani, J. On the sensitivity of ordination and classification methods to variation in the input order of data. J. Veg. Sci. 1997, 8, 153–156. [Google Scholar] [CrossRef]
- Dapporto, L.; Ramazzotti, M.; Fattorini, S.; Talavera, G.; Vila, R.; Dennis, R.L.H. Recluster: An unbiased clustering procedure for beta-diversity turnover. Ecography 2013, 36, 1070–1075. [Google Scholar] [CrossRef]
- Fattorini, S. Influence of recent geography and paleogeography on the structure of reptile communities in a land-bridge archipelago. J. Herpetol. 2010, 44, 242–252. [Google Scholar] [CrossRef]
- Moulpied, M.; Smith, C.H.; Robertson, C.R.; Johnson, N.A.; Lopez, R.; Randklev, C.R. Biogeography of freshwater mussels (Bivalvia: Unionida) in Texas and implications on conservation biology. Divers. Distrib. 2022, 28, 1458–1474. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.r-project.org/ (accessed on 15 March 2022).
- Bartoń, K. MuMIn: Multi-Model Inference. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 15 March 2022).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E. Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 April 2022).
- Dapporto, L.; Ramazzotti, M.; Fattorini, S.; Vila, R.; Talavera, G.; Dennis, R.L.H. recluster: Ordination Methods for the Analysis of Beta-Diversity Indices. Available online: https://CRAN.R-project.org/package=recluster (accessed on 15 March 2022).
- Lomolino, M.V. Ecology’s most general, yet protean 1 pattern: The species-area relationship. J. Biogeogr. 2000, 27, 17–26. [Google Scholar] [CrossRef]
- Lobo, J.M.; Fermín, M.-P. Between-group differences in the Iberian dung beetle species–area relationship (Coleoptera: Scarabaeidae). Acta Oecologica 1999, 20, 587–597. [Google Scholar] [CrossRef]
- Connor, E.F.; Mccoy, E.D. The statistics and biology of the species-area relationship. Am. Nat. 1979, 113, 791–833. [Google Scholar] [CrossRef]
- Fattorini, S. Beetle species–area relationships and extinction rates in protected areas. Insects 2020, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Noriega, J.A.; Realpe, E. Altitudinal turnover of species in a Neotropical peripheral mountain system: A case study with dung beetles (Coleoptera: Aphodiinae and Scarabaeinae). Environ. Entomol. 2018, 47, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, V.R.; Noriega, J.A. Diversity of the dung beetles (Coleoptera: Scarabaeinae) in an altitudinal gradient in the east slope of los Andes, Napo province, Ecuador. Neotrop. Biodivers. 2018, 4, 145–151. [Google Scholar] [CrossRef]
- Lobo, J.M.; Lumaret, J.-P.; Jay-Robert, P. Modelling the species richness distribution of French dung beetles (Coleoptera, Scarabaeidae) and delimiting the predictive capacity of different groups of explanatory variables. Glob. Ecol. Biogeogr. 2002, 11, 265–277. [Google Scholar] [CrossRef]
- Lobo, J.M.; Lumaret, J.R.P. Modelling the species richness distribution for French Aphodiidae (Coleoptera, Scarabaeoidea). Ecography 2010, 27, 145–156. [Google Scholar] [CrossRef]
- Fattorini, S.; Di Biase, L.; Chiarucci, A. Recognizing and interpreting vegetational belts: New wine in the old bottles of a von Humboldt’s legacy. J. Biogeogr. 2019, 46, 1643–1651. [Google Scholar] [CrossRef]
- Di Biase, L.; Fattorini, S.; Cutini, M.; Bricca, A. The role of inter- and intraspecific variations in grassland plant functional traits along an elevational gradient in a Mediterranean mountain area. Plants 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Pace, L.; Mantoni, C.; Fattorini, S. Variations in plant richness, biogeographical composition, and life forms along an elevational gradient in a Mediterranean mountain. Plants 2021, 10, 2090. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Dellacasa, M.; Lobo, J.M. Ancient human colonization explains dung beetle species richness in the Mediterranean and Macaronesian islands. J. Biogeogr. 2023, 50, 2095–2108. [Google Scholar] [CrossRef]
- Baroni Urbani, C.; Ruffo, S.; Vigna Taglianti, A. Materiali per una biogeogeografia italiana fondata su alcuni generi di Coleotteri Cicindelidi, Carabidi e Crisomelidi. Mem. Soc. Entomol. Ital. 1978, 56, 35–92. [Google Scholar]
- Balletto, E.; Cassulo, L.A.; Toso, G.G. Contributo alla biogeografia degli Zigenidi delle Alpi Liguri (Lepidoptera: Zygaenidae). Biogeographia 1984, 9, 489–565. [Google Scholar] [CrossRef]
- Fratianni, S.; Acquaotta, F. The climate of Italy. In Landscapes and Landforms of Italy; Soldati, M., Marchetti, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 29–38. [Google Scholar]




| Group | Equation | R2 | p-Value |
|---|---|---|---|
| Islands included | |||
| All dung beetle species | ln(S) = 0.004 × ln(A) + 4.570 | <0.001 | 0.933 |
| Aphodiinae | ln(S) = −0.019 × ln(A) + 4.096 | 0.013 | 0.638 |
| Scarabaeinae | ln(S) = 0.032 × ln(A) +3.368 | 0.299 | 0.608 |
| Islands excluded | |||
| All dung beetle species | ln(S) = 0.056 × ln(A) + 4.464 | 0.412 | 0.004 |
| Aphodiinae | ln(S) = 0.029 × ln(A) + 4.001 | 0.143 | 0.123 |
| Scarabaeinae | ln(S) = 0.098 × ln(A) + 3.234 | 0.295 | 0.020 |
| Group | Equation | R2 | p-Value |
|---|---|---|---|
| All dung beetle species | Richness = −0.592 × Latitude + 25.748 | 0.103 | 0.193 |
| Aphodiinae | Richness = −0.035 × Latitude − 1.471 | <0.001 | 0.904 |
| Scarabaeinae | Richness = −0.830 × Latitude + 36.095 | 0.370 | 0.007 |
| Group | Parameter | Estimate | SE | p-Value |
|---|---|---|---|---|
| Total | ||||
| Intercept | 20.535 | 19.111 | 0.294 | |
| Full average | ||||
| Temperature Annual Range | −0.859 | 0.668 | 0.211 | |
| Annual Precipitation | 0.010 | 0.010 | 0.355 | |
| Precipitation of Driest Quarter | −0.045 | 0.048 | 0.356 | |
| Mean Elevation | −0.0002 | 0.001 | 0.783 | |
| Conditional average | ||||
| Temperature Annual Range | −1.158 | 0.506 | 0.031 | |
| Annual Precipitation | 0.018 | 0.007 | 0.012 | |
| Precipitation of Driest Quarter | −0.085 | 0.319 | 0.013 | |
| Mean Elevation | −0.002 | 0.002 | 0.289 | |
| Aphodiinae | ||||
| Intercept | 6.576 | 9.413 | 0.495 | |
| Full average | ||||
| Annual Mean Temperature | −0.139 | 0.237 | 0.562 | |
| Annual Precipitation | 0.004 | 0.005 | 0.468 | |
| Precipitation of Driest Quarter | −0.026 | 0.030 | 0.392 | |
| Mean Elevation | 0.001 | 0.002 | 0.397 | |
| Temperature Annual Range | −0.216 | 0.336 | 0.530 | |
| Conditional average | ||||
| Annual Mean Temperature | −0.433 | 0.217 | 0.059 | |
| Annual Precipitation | 0.009 | 0.004 | 0.036 | |
| Precipitation of Driest Quarter | −0.049 | 0.023 | 0.042 | |
| Mean Elevation | 0.003 | 0.001 | 0.042 | |
| Temperature Annual Range | −0.598 | 0.292 | 0.058 | |
| Scarabaeinae | ||||
| Intercept | 15.639 | 22.463 | 0.491 | |
| Full average | ||||
| Temperature Annual Range | −0.854 | 0.445 | 0.063 | |
| Mean Elevation | −0.001 | 0.005 | 0.868 | |
| Annual Mean Temperature | 0.488 | 0.678 | 0.476 | |
| Annual Precipitation | 0.002 | 0.003 | 0.517 | |
| Conditional average | ||||
| Temperature Annual Range | −0.977 | 0.326 | 0.005 | |
| Mean Elevation | −0.001 | 0.006 | 0.829 | |
| Annual Mean Temperature | 0.936 | 0.680 | 0.175 | |
| Annual Precipitation | 0.004 | 0.003 | 0.140 |
| Matrix Correlation | Biogeographical Distances | ||||
|---|---|---|---|---|---|
| Matrix A × Matrix B | Matrix C (Controlling) | Sørensen | Simpson | ||
| r | p-Value | r | p-Value | ||
| Total dung beetle fauna | |||||
| Environmental distances | - | 0.308 | 0.059 | 0.411 | 0.026 |
| Centroids | - | 0.572 | <0.001 | 0.712 | <0.001 |
| Environmental distances | Centroids | −0.021 | 0.443 | 0.017 | 0.332 |
| Centroids | Environmental distances | 0.507 | <0.001 | 0.638 | <0.001 |
| Aphodiinae | |||||
| Environmental distances | - | 0.235 | 0.099 | 0.395 | 0.027 |
| Centroids | - | 0.613 | <0.001 | 0.724 | <0.001 |
| Environmental distances | Centroids | −0.169 | 0.914 | −0.024 | 0.447 |
| Centroids | Environmental distances | 0.598 | <0.001 | 0.666 | <0.001 |
| Scarabaeinae | |||||
| Environmental distances | - | 0.474 | 0.003 | 0.168 | 0.097 |
| Centroids | - | 0.621 | <0.001 | 0.484 | <0.001 |
| Environmental distances | Centroids | 0.192 | 0.0804 | −01.45 | 0.862 |
| Centroids | Environmental distances | 0.486 | <0.001 | 0.478 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattorini, S.; Vitozzi, A.; Di Biase, L.; Bergamaschi, D. Macroecology of Dung Beetles in Italy. Insects 2024, 15, 39. https://doi.org/10.3390/insects15010039
Fattorini S, Vitozzi A, Di Biase L, Bergamaschi D. Macroecology of Dung Beetles in Italy. Insects. 2024; 15(1):39. https://doi.org/10.3390/insects15010039
Chicago/Turabian StyleFattorini, Simone, Alessia Vitozzi, Letizia Di Biase, and Davide Bergamaschi. 2024. "Macroecology of Dung Beetles in Italy" Insects 15, no. 1: 39. https://doi.org/10.3390/insects15010039
APA StyleFattorini, S., Vitozzi, A., Di Biase, L., & Bergamaschi, D. (2024). Macroecology of Dung Beetles in Italy. Insects, 15(1), 39. https://doi.org/10.3390/insects15010039








