The Population Dynamics and Parasitism Rates of Ceratitis capitata, Anastrepha fraterculus, and Drosophila suzukii in Non-Crop Hosts: Implications for the Management of Pest Fruit Flies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Host Fruit Sampling
2.3. Host Fruit Processing
2.4. Fly Puparia Processing and Identification
2.5. Data Analysis
3. Results
3.1. Fly Abundance and Infestation Levels
3.2. Parasitoid Abundance and Parasitism Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Didham, R.K.; Tylianakis, J.M.; Gemmell, N.J.; Rand, T.A.; Ewers, R.M. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. How much information on population biology is needed to manage introduced species? Conserv. Biol. 2003, 17, 83–92. [Google Scholar] [CrossRef]
- Vinatier, F.; Tixier, P.; Duyck, P.F.; Lescourret, F. Factors and mechanisms explaining spatial heterogeneity: A review of methods for insect populations. Methods Ecol. Evol. 2011, 2, 11–22. [Google Scholar] [CrossRef]
- Vasseur, C.; Joannon, A.; Aviron, S.; Burel, F.; Meynard, J.-M.; Baudry, J. The cropping systems mosaic: How does the hidden heterogeneity of agricultural landscapes drive arthropod populations? Agric. Ecosyst. Environ. 2013, 166, 3–14. [Google Scholar] [CrossRef]
- Duyck, P.F.; David, P.; Quilici, S. Can more K-selected species be better invaders? A case study of fruit flies in La reunion. Divers. Distrib. 2007, 13, 535–543. [Google Scholar] [CrossRef]
- Duyck, P.F.; David, P.; Quilici, S. A review of relationships between interspecific competition and invasions in fruit flies (Diptera: Tephritidae). Ecol. Entomol. 2004, 29, 511–520. [Google Scholar] [CrossRef]
- Aluja, M.; Ordano, M.; Guillen, L.; Rull, J. Understanding long-term fruit flies (Diptera: Tephritidae) population dynamics: Implications for area wide management. J. Econ. Entomol. 2012, 105, 823–836. [Google Scholar] [CrossRef]
- Hennig, E.I.; Mazzi, D. Spotted wing Drosophila in sweet cherry orchards in relation to forest characteristics, bycatch, and resource availability. Insects 2018, 9, 118. [Google Scholar] [CrossRef]
- Copeland, R.S.; Wharton, R.A.; Luke, Q.; De Meyer, M. Indigenous hosts of Ceratitis capitata (Diptera: Tephritidae) in Kenya. Ann. Entomol. Soc. Am. 2002, 95, 672–694. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted-wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- De la Vega, G.J.; Corley, J.C. Drosophila suzukii (Diptera: Drosophilidae) distribution modelling improves our understanding of pest range limits. Int. J. Pest Manag. 2019, 65, 217–227. [Google Scholar] [CrossRef]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and fruit fly invasion and expansion: The medfly paradigm. Genetica 2007, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- De Ros, G.; Grassi, A.; Pantezzi, T. Recent trends in the economic impact of Drosophila suzukii. In Drosophila Suzukii Management; Garcia, F.R.M., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 11–28. [Google Scholar]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. R. Soc. B 2014, 281, 20132840. [Google Scholar] [CrossRef] [PubMed]
- CABI, Centre for Agricultural Bioscience International. Invasive Species Compendium, Ceratitis capitata (Mediterranean Fruit Fly). CAB International. 2022. Available online: https://www.cabi.org./isc/datasheet/12367 (accessed on 2 October 2023).
- Norrbom, A.L. Host Plant Database for Anastrepha and Toxotrypana (Diptera: Tephritidae: Toxotrypanini). In Diptera Data Dissemination Disk [CD-ROM] 2; North American Dipterist’s Society: Washington, DC, USA, 2004. [Google Scholar]
- Kenis, M.; Tonina, L.; Eschen, R.; van der Sluis, B.; Sancassani, M.; Mori, N.; Haye, T.; Helsen, H. Non-crop plants used as hosts by Drosophila suzukii in Europe. J. Pest Sci. 2016, 89, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Ims, R.A. Movement patterns related to spatial structures. In Mosaic Landscapes and Ecological Processes; Hansson, L., Fahrig, L., Merriam, G., Eds.; Chapman & Hall: London, UK, 1995; pp. 85–109. [Google Scholar]
- Gutierrez, A.P.; Ponti, L. Assessing the invasive potential of the Mediterranean fruit fly in California and Italy. Biol. Invasions 2011, 13, 2661–2676. [Google Scholar] [CrossRef]
- Kirschbaum, D.S.; Funes, C.F.; Buonocore-Biancheri, M.J.; Suárez, L.; Ovruski, S.M. The biology and ecology of Drosophila suzukii (Diptera: Drosophilidae). In Drosophila Suzukii Management; Garcia, F.R.M., Ed.; Springer Nature: Cham, Switzerland, 2020; Chapter 4; pp. 41–91. [Google Scholar]
- Elsensohn, J.E.; Loeb, G.M. Non-crop host sampling yields insights into small-scale population dynamics of Drosophila suzukii (Matsumura). Insects 2018, 9, 5. [Google Scholar] [CrossRef]
- Lewis, W.J.; van Lenteren, J.C.; Phatak, S.C.; Tumlinson, J.H. A total system approach to sustainable pest management. Proc. Natl. Acad. Sci. USA 1997, 94, 12243–12248. [Google Scholar] [CrossRef]
- Aluja, M. Fruit fly (Diptera: Tephritidae) research in Latin America: Myths, realities, and dreams. An. Soc. Entomol. Bras. 1999, 28, 565–594. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Aluja, M.; Rull, J. Managing pestiferous fruit flies (Diptera: Tephritidae) through environmental manipulation. In Biorational Tree Fruit Pest Management; Aluja, M., Leskey, T., Vincent, C., Eds.; CAB International: Wallingford, UK, 2009; pp. 214–252. [Google Scholar]
- Thomson, L.J.; Hoffmann, A.A. Natural enemy responses and pest control: Importance of local vegetation. Biol. Control 2010, 52, 160–166. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Veres, A.; Petit, S.; Conord, C.; Lavigne, C. Does landscape composition affect pest abundance and their control by natural enemies? A review. Agric. Ecosyst. Environ. 2013, 166, 110–117. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Whitehouse, T.S.; Green, K.; Krehenwinkel, H.; Schmidt-Jeffris, R.; Sial, A.A. Local and landscape-scale heterogeneity shape spotted wing drosophila (Drosophila suzukii) activity and natural enemy abundance: Implications for trophic interactions. Agric. Ecosyst. Environ. 2019, 272, 86–94. [Google Scholar] [CrossRef]
- Aluja, M.; Sivinski, J.; van Driesche, R.; Anzures-Dadda, A.; Guillén, L. Pest management through tropical tree conservation. Biodivers. Conserv. 2014, 23, 831–853. [Google Scholar] [CrossRef]
- Ehler, L.E. Parasitoid communities, parasitoid guilds, and biological control. In Parasitoid Community Ecology; Hawkins, B.A., Sheehan, W., Eds.; Oxford University Press: Oxford, UK, 1994; pp. 418–436. [Google Scholar]
- Holland, J.M.; Bianchi, F.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef]
- Markó, V.; Elek, Z.; Kovács-Kostyánszki, A.; Kőrösi, Á.; László, S.; Földesi, R.; Varga, Á.; Iván, Á.; Báldi, A. Landscapes, orchards, pesticides—Abundance of beetles (Coleoptera) in apple orchards along pesticide toxicity and landscape complexity gradients. Agric. Ecosyst. Environ. 2017, 247, 246–254. [Google Scholar] [CrossRef]
- Vargas, G.; Rivera-Pedroza, L.-F.; Cracía, L.F.; Mundstock Jahnke, S. Conservation biological control as an important tool in the Neotropical region. Neotrop. Entomol. 2023, 52, 134–151. [Google Scholar] [CrossRef]
- Brown, A.D.; Grau, H.R.; Malizia, L.R.; Grau, A. Argentina. In Bosques Nublados del Neotrópico; Kappelle, M., Brown, A.D., Eds.; Instituto Nacional de Biodiversidad: San José, Costa Rica, 2001; pp. 623–659. [Google Scholar]
- Grau, H.R.; Aragon, R. Arboles invasores de la Sierra de San Javier, Tucumán, Argentina. In Ecologıa de Árboles Exóticos en las Yungas Argentinas; Grau, H.R., Aragon, R., Eds.; Laboratorio de Investigaciones Ecológicas de las Yungas, Universidad Nacional de Tucumán: San Miguel de Tucuman, Argentina, 2020; pp. 5–20. [Google Scholar]
- Schliserman, P.; Aluja, M.; Rull, J.; Ovruski, S.M. Temporal diversity and abundance patterns of parasitoids of fruit-infesting Tephritidae (Diptera) in the Argentinean Yungas: Implications for biological control. Environ. Entomol. 2016, 45, 1184–1198. [Google Scholar] [CrossRef]
- Buonocore-Biancheri, M.J.; Suárez, L.; Kirschbaum, D.S.; Garcia, F.R.M.; Funes, C.F.; Ovruski, S.M. Natural parasitism influences biological control strategies against both global invasive pests Ceratitis capitata (Diptera: Tephritidae) and Drosophila suzukii (Diptera: Drosophilidae), and the Neotropical-native pest Anastrepha fraterculus (Diptera: Tephritidae). Environ. Entomol. 2022, 51, 1120–1135. [Google Scholar] [CrossRef]
- Buonocore-Biancheri, M.J.; Kirschbaum, D.S.; Suárez, L.; Ponssa, M.D.; Ovruski, S.M. Drosophila suzukii in Argentina: State of the art and further perspectives. Neotrop. Entomol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Guillén, D.; Sánchez, R. Expansion of the national fruit fly control programme in Argentina. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 653–660. [Google Scholar]
- Buonocore-Biancheri, M.J.; Núñez-Campero, S.R.; Suárez, L.; Ponssa, M.D.; Kirschbaum, D.S.; Garcia, F.R.M.; Ovruski, S.M. Implications of the niche partitioning and coexistence of two resident parasitoids for Drosophila suzukii management in non-crop areas. Insects 2023, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.D. Las selvas pedemontanas de las Yungas: Manejo sustentable y conservación de la biodiversidad de un ecosistema prioritario del noroeste argentino. In Selva Pedemontana de las Yungas: Historia Natural, Ecología y Manejo de un Ecosistema en Peligro; Brown, A.D., Blendinger, P.G., Lomáscolo, T., García Bes, P., Eds.; Ediciones del Subtrópico, Yerba Buena: Tucumán, Argentina, 2009; pp. 13–36. [Google Scholar]
- Schliserman, P.; Aluja, M.; Rull, J.; Ovruski, S.M. Habitat degradation and introduction of exotic plants favor persistence of invasive species and population growth of native polyphagous fruit fly pests in a Northwestern Argentinean mosaic. Biol. Invasions 2014, 16, 2599–2613. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International-ACIAR, Redwood Press Ltd.: Melksham, UK, 1992. [Google Scholar]
- Hauser, M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Anguage and Environment for Statistical Computing; R. Foundat Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 5 October 2023).
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Diamantidis, A.D.; Carey, J.R.; Nakas, C.T.; Papadopoulos, N.T. Population-specific demography and invasion potential in medfly. Ecol. Evol. 2011, 1, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Stacconi, M.V.; Kaur, R.; Mazzoni, V.; Ometto, L.; Grassi, A.; Gottardello, A.; Rota-Stabelli, O.; Anfora, G. Multiple lines of evidence for reproductive winter diapause in the invasive pest Drosophila suzukii. J. Pest Sci. 2016, 89, 689–700. [Google Scholar] [CrossRef]
- Grassi, A.; Gottardello, A.; Dalton, D.T.; Tait, G.; Rendon, D.; Ioriatti, C.; Gibeaut, D.; Rossi-Stacconi, M.V.; Walton, V.M. Seasonal reproductive biology of Drosophila suzukii (Diptera: Drosophilidae) in temperate climates. Environ. Entomol. 2018, 47, 166–174. [Google Scholar] [CrossRef]
- Stockton, D.; Wallingford, A.; Loeb, G. Phenotypic plasticity promotes overwintering survival in a globally invasive crop pest, Drosophila suzukii. Insects 2018, 9, 105. [Google Scholar] [CrossRef]
- Kovaleski, A.; Sugayama, R.L.; Malavasi, A. Movement of Anastrepha fraterculus from native breeding sites into apple orchards in Southern Brazil. Entomol. Exp. Appl. 1999, 91, 457–467. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Lasa, R.; Funes, C.F.; Buzzetti, K. Drosophila suzukii management in Latin America: Current status and perspectives. J. Econ. Entomol. 2022, 115, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, F.E.; Reche, V.A.; Margaría, C.B.; Aquino, D.A.; Ansa, M.A.; Dettler, M.A.; Vazquez, F.; Barrientos, G.; Santadino, M.; Martínez, E.; et al. Survey of potential parasitoids (Hymenoptera) of Drosophila suzukii (Diptera: Drosophilidae) in Buenos Aires province. Argentina. Rev. Soc. Entomol. Arg. 2022, 81, 71–78. [Google Scholar] [CrossRef]
- Wang, X.G.; Kaçar, G.; Daane, K.M. Temporal dynamics of host use by Drosophila suzukii in California’s San Joaquin Valley: Implications for area-wide pest management. Insects 2019, 10, 206. [Google Scholar] [CrossRef]
- Markow, T.A.; O’Grady, P. Reproductive ecology of Drosophila. Funct. Ecol. 2008, 22, 747–759. [Google Scholar] [CrossRef]
- Funes, C.F.; Escobar, L.I.; Dadda, G.E.; Villagrán, M.E.; Olivera, G.I.; Gastaminza, G.G.; Kirschbaum, D.S. Occurrence and population fuctuations of Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops of subtropical Argentina. Acta Hortic. 2023, 1357, 257–264. [Google Scholar] [CrossRef]
- Wollmann, J.; Schlesener, D.C.H.; Ferreira, M.S.; Kruger, A.P.; Bernardi, D.; Garcia, J.A.B.; Nunes, A.M.; Garcia, M.S.; Garcia, F.R.M. Population dynamics of Drosophila suzukii (Diptera: Drosophilidae) in berry crops in southern Brazil. Neotrop. Entomol. 2019, 48, 699–705. [Google Scholar] [CrossRef]
- Arnó, J.; Sola, M.; Riudavets, J.; Gabarra, R. Population dynamics, non-crop hosts, and fruit susceptibility of Drosophila suzukii in Northeast Spain. J. Pest Sci. 2016, 89, 713–723. [Google Scholar] [CrossRef]
- Bal, H.K.; Adams, C.; Grieshop, M. Evaluation of off-season potential breeding sources for spotted wing Drosophila (Drosophila suzukii Matsumura) in Michigan. J. Econ. Entomol. 2017, 110, 2466–2470. [Google Scholar] [CrossRef]
- Panel, A.D.C.; Zeeman, L.; van der Sluis, B.J.; van Elk, P.; Pannebakker, B.A.; Wertheim, B.; Helsen, H.H.M. Overwintered Drosophila suzukii are the main source for infestations of the first fruit crops of the season. Insects 2018, 9, 145. [Google Scholar] [CrossRef]
- Drummond, F.; Ballman, E.; Collins, J. Population dynamics of spotted wing Drosophila (Drosophila suzukii (Matsumura)) in Maine wild blueberry (Vaccinium angustifolium Aiton). Insects 2019, 10, 205. [Google Scholar] [CrossRef]
- Gavriel, S.; Gazit, Y.; Leach, A.; Mumford, J.; Yuval, B. Spatial patterns of sterile Mediterranean fruit fly dispersal. Entomol. Exp. Appl. 2012, 142, 17–26. [Google Scholar] [CrossRef]
- De Villiers, M.; Manrakhan, A.; Addison, P.; Hattingh, V. The distribution, relative abundance, and seasonal phenology of Ceratitis capitata, Ceratitis rosa, and Ceratitis cosyra (Diptera: Tephritidae) in South Africa. Environ. Entomol. 2013, 42, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Araujo, E.S.; Lino, B.; Monteiro, R.S.; Nishimura, G.; Franck, P.; Lavigne, C. Impact of native forest remnants and wild host plants on the abundance of the South American fruit fly, Anastrepha fraterculus in Brazilian apple orchards. Agric. Ecosyst. Environ. 2019, 275, 93–99. [Google Scholar] [CrossRef]
- Aluja, M.; Piñero, J.; Jácome, I.; Diaz-Fleischer, F.; Sivinski, J. Behavior of flies in the genus Anastrepha (Trypetinae: Toxotrypanini). In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press LLC., Corporate Blvd.: Boca Raton, FL, USA, 2000; pp. 375–406. [Google Scholar]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Thies, C.; Steffan-Dewenter, I.; Tscharntke, T. Effects of landscape context on herbivory and parasitism at different spatial scales. Oikos 2003, 101, 18–25. [Google Scholar] [CrossRef]
- Santadino, M.V.; Riquelme-Virgala, M.B.; Ansa, M.A.; Bruno, M.; Di Silvestro, G.; Lunazzi, E.G. Primer registro de Drosophila suzukii (Diptera: Drosophilidae) asociado al cultivo de arándanos (Vaccinium spp.) de Argentina. Rev. Soc. Entomol. Arg. 2015, 74, 183–185. [Google Scholar]
- Dagatti, C.V.; Marcucci, B.; Herrera, M.E.; Becerra, V.C. First record of Drosophila suzukii (Diptera: Drosophilidae) associated to blackberry in Mendoza, Argentina. Rev. Soc. Entomol. Arg. 2018, 7, 26–29. [Google Scholar] [CrossRef]
- Dettler, M.A.; Barrientos, G.N.; Martinez, E.; Ansa, M.A.; Santadino, M.V.; Coviella, C.E.; Riquelme-Virgala, M.B. Field infestation level of Zaprionus indianus (Gupta) and Drosophíla suzukii (Matsumura) (Díptera: Drosophilidae) in Ficus carica L. (Rosales: Moraceae) and Rubus idaeus L. (Rosales: Rosaceae) in the Northeast of Buenos Aires province. Rev. Soc. Entomol. Argent. 2021, 80, 43–47. [Google Scholar] [CrossRef]
- Cichón, L.I.; Garrido, S.; Lago, J. Drosophila suzukii: Una Nueva Plaga Presente en la Norpatagonia. Ediciones-INTA, Instituto Nacional de Tecnología Agropecuaria, Río Negro, Argentina. 2016. Available online: https://repositorio.inta.gob.ar/bitstream/handle/20.500.12123/3119/INTA_CRPatagoniaNorte_EEAAltoValle_Cichon_LI_Drosophila_suzukii_nueva_plaga_presente_Norpatagonia.pdf (accessed on 10 July 2023).
- Tait, G.; Cabianca, A.; Grassi, A.; Pfab, F.; Oppedisano, T.; Puppato, S.; Mazzoni, V.; Anfora, G.; Walton, V.M. Drosophila suzukii daily dispersal between distinctly different habitats. Entomol. Gen. 2020, 40, 25–37. [Google Scholar] [CrossRef]
- Wiman, N.G.; Walton, V.M.; Dalton, D.T.; Gianfranco Anfora, G.; Burrack, H.J.; Chiu, J.C.; Daane, K.M.; Grassi, A.; Miller, B.; Tochen, S.; et al. Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS ONE 2014, 9, e106909. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological control of tephritid fruit flies in the Americas and Hawaii: A review of the use of parasitoids and predators. Insects 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Aluja, M.; Ovruski, S.M.; Guillén, L.; Oroño, L.E.; Sivinski, J. Comparison of the host searching and oviposition behaviors of the tephritid (Diptera) parasitoids Aganaspis pelleranoi and Odontosema anastrephae (Hymenoptera: Figitidae, Eucoilinae). J. Insect Behav. 2009, 22, 423–451. [Google Scholar] [CrossRef]
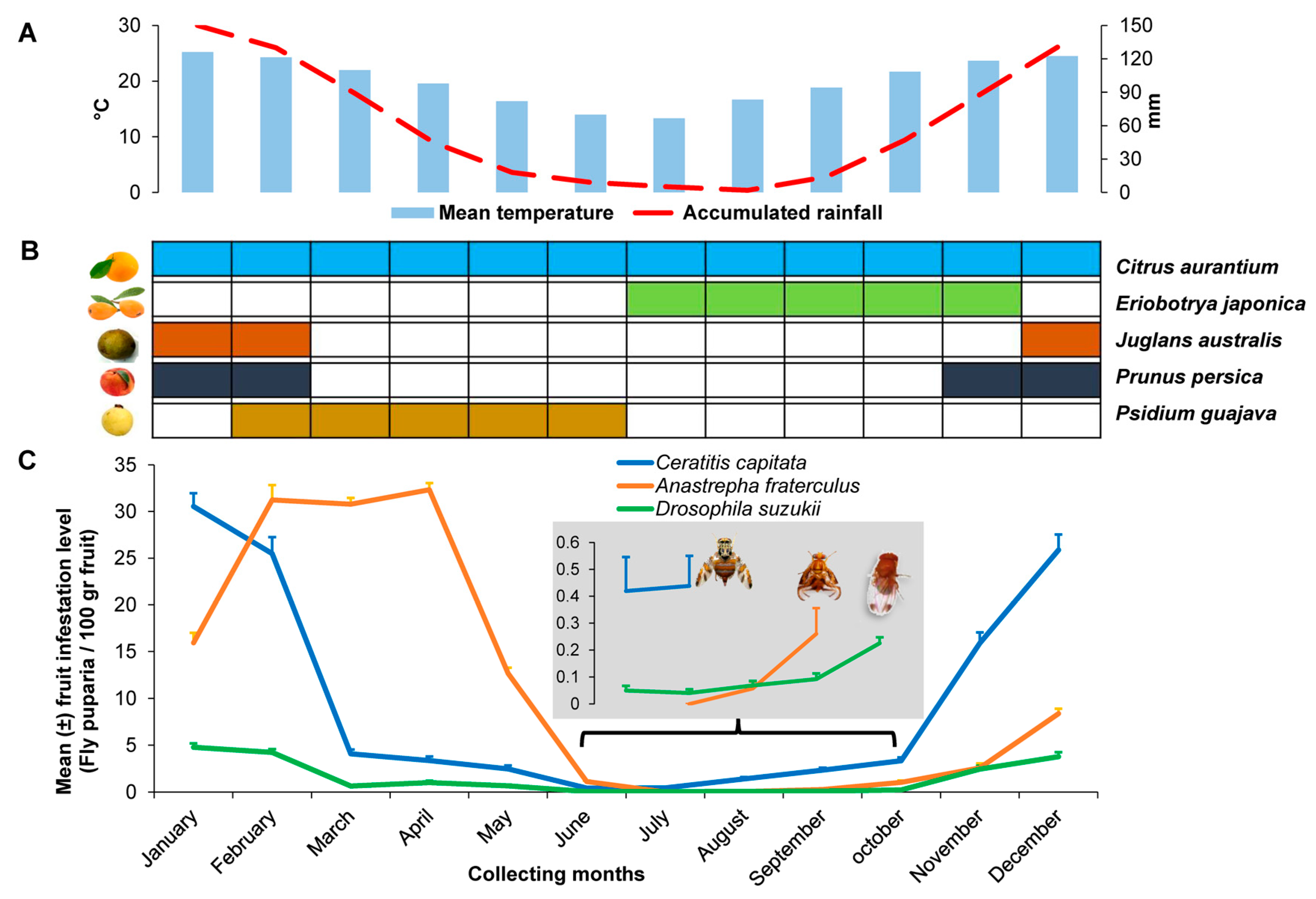

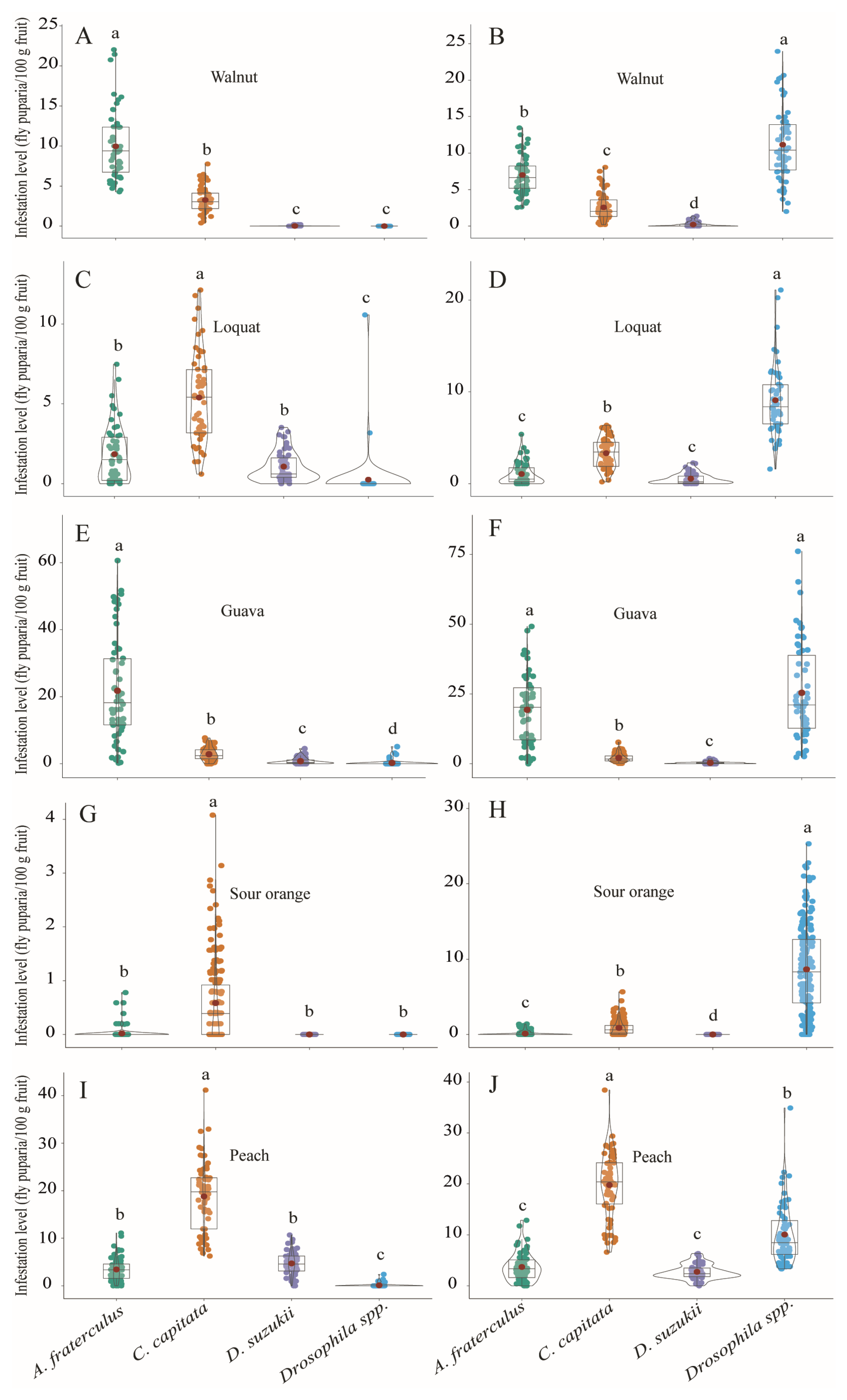
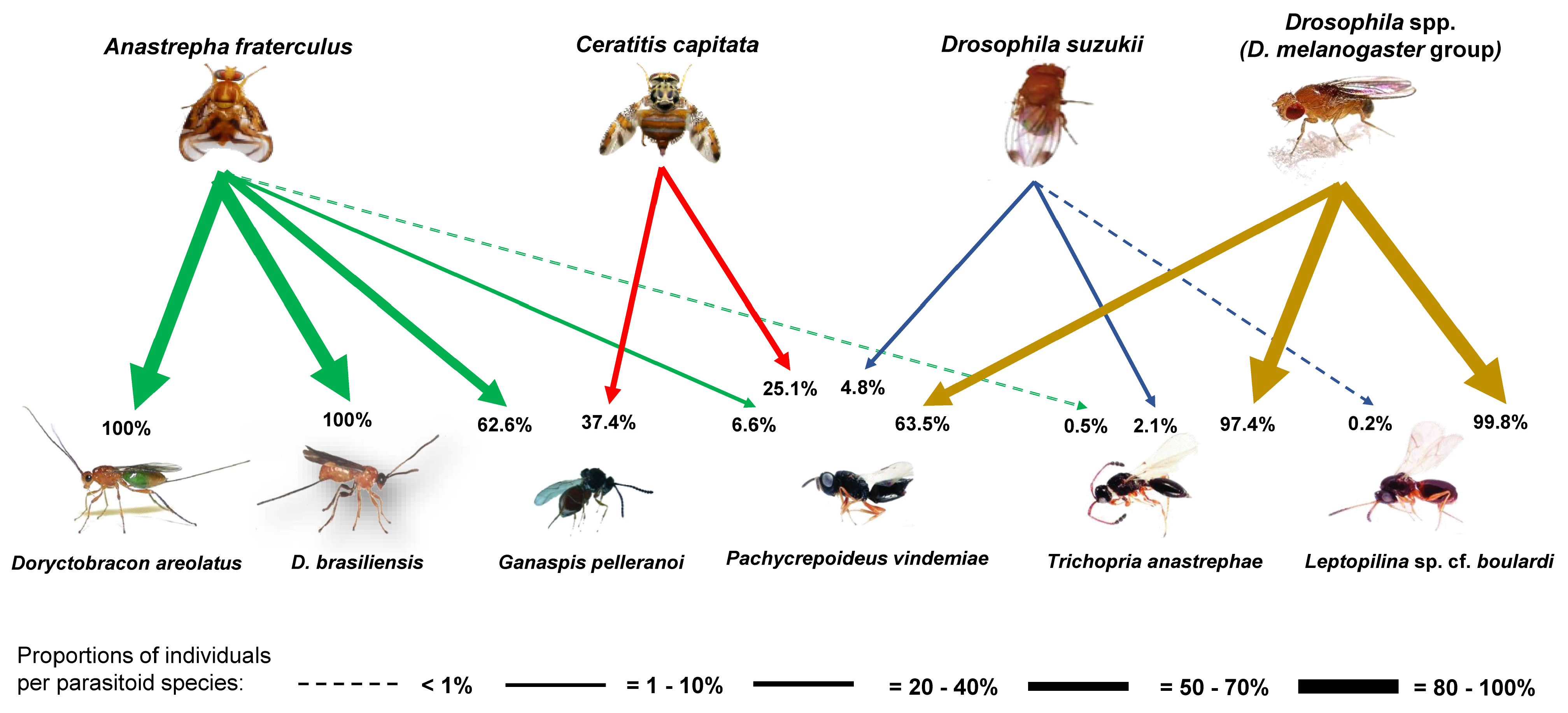
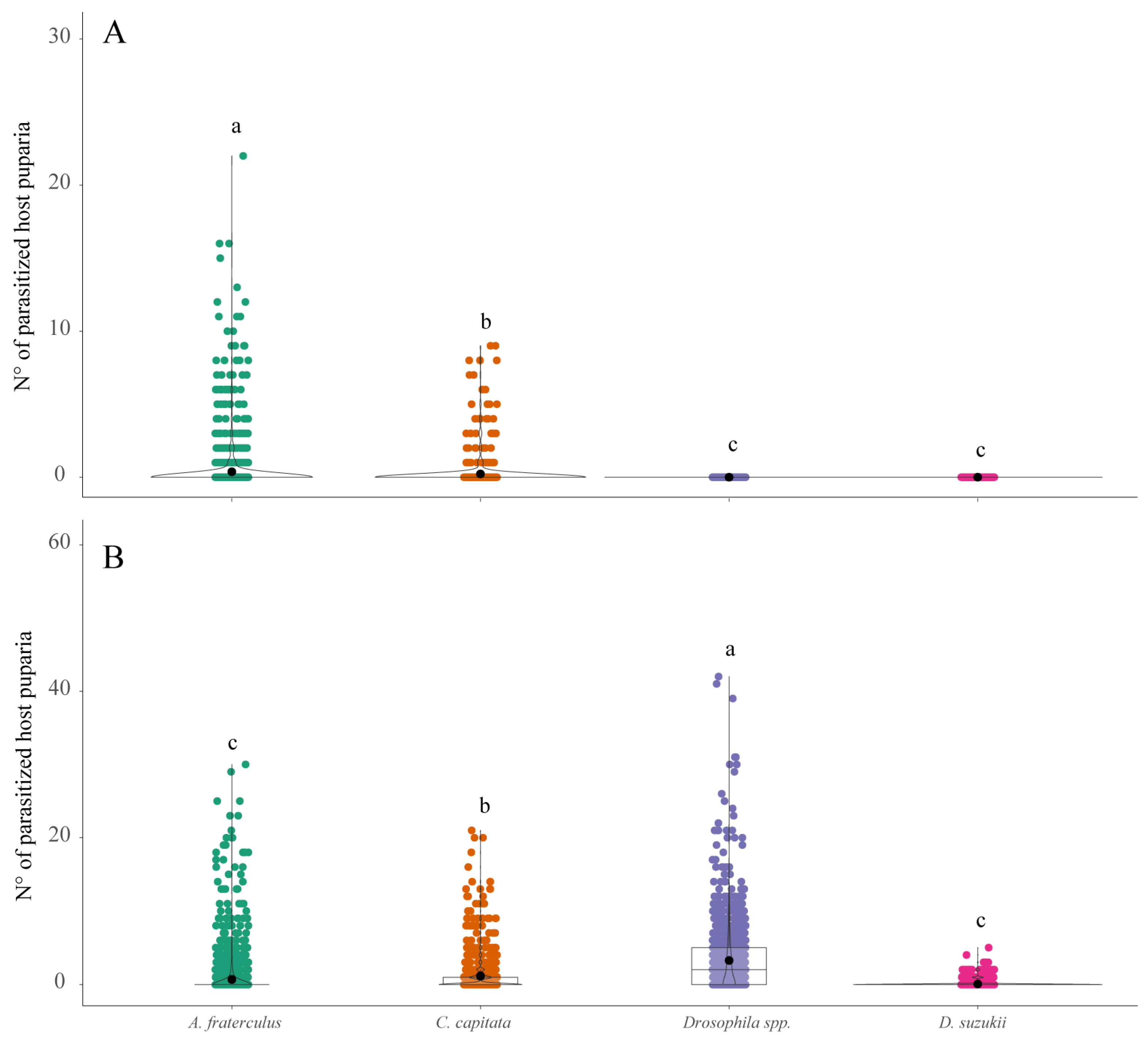

| Fruit Origin | Fruit Species | No. of Collected Fruit (Weight, Kg) | Total Numbers | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Af Puparia | Af Adults | Cc Puparia | Cc Adults | Ds Puparia | Ds Adults | Dspp Puparia | Dspp Adults | |||
| Canopy | Ca | 692 (87.4) | 17 | 8 | 514 | 203 | 0 | 0 | 0 | 0 |
| Ej | 2700 (26.9) | 492 | 245 | 1442 | 763 | 286 | 144 | 16 | 11 | |
| Ja | 672 (28.3) | 2819 | 1437 | 923 | 493 | 4 | 2 | 0 | 0 | |
| Pp | 960 (32.6) | 1122 | 550 | 6120 | 2948 | 1537 | 725 | 36 | 23 | |
| Pg | 580 (29.1) | 6321 | 3059 | 824 | 358 | 224 | 86 | 73 | 47 | |
| Ground | Ca | 92 (87.8) | 108 | 45 | 767 | 301 | 0 | 0 | 7595 | 3158 |
| Ej | 2700 (27.1) | 291 | 94 | 895 | 336 | 148 | 41 | 2458 | 1015 | |
| Ja | 672 (28.1) | 1974 | 832 | 724 | 299 | 61 | 23 | 3145 | 1209 | |
| Pp | 960 (32.3) | 1195 | 543 | 6376 | 3009 | 887 | 374 | 3249 | 1339 | |
| Pg | 580 (29.2) | 5650 | 2350 | 612 | 228 | 95 | 25 | 7427 | 2857 | |
| Fruit Origin: Fruit Species | Statistical Results | |||
|---|---|---|---|---|
| df | n | χ2 | p | |
| Canopy: | ||||
| Citrus aurantium | 3 | 348 | 316.90 | <0.0001 |
| Eriobotrya japonica | 3 | 108 | 145.38 | <0.0001 |
| Juglans australis | 3 | 112 | 209.24 | <0.0001 |
| Prunus persica | 3 | 128 | 208.25 | <0.0001 |
| Psidium guajava | 3 | 116 | 164.67 | <0.0001 |
| Ground: | ||||
| Citrus aurantium | 3 | 348 | 485.34 | <0.0001 |
| Eriobotrya japonica | 3 | 108 | 155.39 | <0.0001 |
| Juglans australis | 3 | 112 | 179.20 | <0.0001 |
| Prunus persica | 3 | 128 | 181.80 | <0.0001 |
| Psidium guajava | 3 | 116 | 173.21 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonocore-Biancheri, M.J.; Wang, X.; Núñez-Campero, S.R.; Suárez, L.; Schliserman, P.; Ponssa, M.D.; Kirschbaum, D.S.; Garcia, F.R.M.; Ovruski, S.M. The Population Dynamics and Parasitism Rates of Ceratitis capitata, Anastrepha fraterculus, and Drosophila suzukii in Non-Crop Hosts: Implications for the Management of Pest Fruit Flies. Insects 2024, 15, 61. https://doi.org/10.3390/insects15010061
Buonocore-Biancheri MJ, Wang X, Núñez-Campero SR, Suárez L, Schliserman P, Ponssa MD, Kirschbaum DS, Garcia FRM, Ovruski SM. The Population Dynamics and Parasitism Rates of Ceratitis capitata, Anastrepha fraterculus, and Drosophila suzukii in Non-Crop Hosts: Implications for the Management of Pest Fruit Flies. Insects. 2024; 15(1):61. https://doi.org/10.3390/insects15010061
Chicago/Turabian StyleBuonocore-Biancheri, María Josefina, Xingeng Wang, Segundo Ricardo Núñez-Campero, Lorena Suárez, Pablo Schliserman, Marcos Darío Ponssa, Daniel Santiago Kirschbaum, Flávio Roberto Mello Garcia, and Sergio Marcelo Ovruski. 2024. "The Population Dynamics and Parasitism Rates of Ceratitis capitata, Anastrepha fraterculus, and Drosophila suzukii in Non-Crop Hosts: Implications for the Management of Pest Fruit Flies" Insects 15, no. 1: 61. https://doi.org/10.3390/insects15010061
APA StyleBuonocore-Biancheri, M. J., Wang, X., Núñez-Campero, S. R., Suárez, L., Schliserman, P., Ponssa, M. D., Kirschbaum, D. S., Garcia, F. R. M., & Ovruski, S. M. (2024). The Population Dynamics and Parasitism Rates of Ceratitis capitata, Anastrepha fraterculus, and Drosophila suzukii in Non-Crop Hosts: Implications for the Management of Pest Fruit Flies. Insects, 15(1), 61. https://doi.org/10.3390/insects15010061









