Assessment of Nano-Formulated Conventional Insecticide-Treated Sugar Baits on Mosquito Control and the Effect on Non-Target Aphidophagous Coccinella septempunctata
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Attractive Sugar Baits (ASB)
2.2. Preparation of Attractive Toxic Sugar Baits (ATSB)
2.3. Preparation and Characterization of Conventional Insecticides Nano-Formulations
2.4. Preparation of Nano-Formulated Attractive Toxic Sugar Baits (N-ATSB)
2.5. Preparation of ATSB of Insecticides in Conventional/Traditional Form
2.6. Study Design
2.7. Field Collection and Stock Culture of Mosquito
2.8. C. septempunctata Collection and Rearing
2.9. Mosquito Adult Bioassay
2.10. C. septempunctata Bioassay
2.11. Statistical Analysis
3. Results
3.1. Comparative Efficacy of Conventional and Nano-Formulated Pyrethroid-Treated ATSB
3.2. Comparative Efficacy of Conventional and Nano-Formulated Organophosphate-Treated ATSB
3.3. Comparative Efficacy of Conventional and Nano-Formulated Carbamate-Treated ATSB
3.4. Comparative Effect of Commercial and Nano-Formulated Conventional Insecticide-Treated sATSB on C. septempunctata
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, J.-P.; Faucon, F.; Chandor-Proust, A.; Poupardin, R.; Riaz, M.; Bonin, A.; Navratil, V.; Reynaud, S. Comparative analysis of response to selection with three insecticides in the dengue mosquito Aedes aegypti using mRNA sequencing. BMC Genom. 2016, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef]
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjornstad, O.N. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE 2013, 8, e79276. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Rosenfeld, L.C.; Lim, S.S.; Andrews, K.G.; Foreman, K.J.; Haring, D.; Fullman, N.; Naghavi, M.; Lozano, R.; Lopez, A.D. Global malaria mortality between 1980 and 2010: A systematic analysis. Lancet 2012, 379, 413–431. [Google Scholar] [CrossRef]
- Espinosa, M. The Question of Racial Immunity to Yellow Fever in History and Historiography. Soc. Sci. Hist. 2015, 38, 437–453. [Google Scholar] [CrossRef]
- Gibbons, R.V.; Vaughn, D.W. Dengue: An escalating problem. BMJ 2002, 324, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Wolbers, M.; Nguyen, M.N.; Whitehorn, J.; Shi, P.Y.; Young, P.; Petric, R.; Nguyen, V.V.; Farrar, J.; Wills, B. Therapeutics for dengue: Recommendations for design and conduct of early-phase clinical trials. PLoS Negl. Trop. Dis. 2012, 6, e1752. [Google Scholar] [CrossRef]
- Koou, S.-Y.; Chong, C.-S.; Vythilingam, I.; Lee, C.-Y.; Ng, L.-C. Insecticide resistance and its underlying mechanisms in field populations of Aedes aegypti adults (Diptera: Culicidae) in Singapore. Parasites Vectors 2014, 7, 471. [Google Scholar] [CrossRef]
- Chadee, D.D. Resting behaviour of Aedes aegypti in Trinidad: With evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasites Vectors 2013, 6, 255. [Google Scholar] [CrossRef]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef]
- Young, D.S.; Kramer, L.D.; Maffei, J.G.; Dusek, R.J.; Backenson, P.B.; Mores, C.N.; Bernard, K.A.; Ebel, G.D. Molecular epidemiology of eastern equine encephalitis virus, New York. Emerg. Infect. Dis. 2008, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, B.L.; Atkinson, C.T.; LaPointe, D.A.; Hart, P.J.; Spiegel, C.S.; Tweed, E.J.; Henneman, C.; LeBrun, J.; Denette, T.; DeMots, R. Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. Proc. Natl. Acad. Sci. USA 2005, 102, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: A review. Parasitol. Res. 2016, 115, 23–34. [Google Scholar] [CrossRef]
- Brisola Marcondes, C.; Benelli, G. Mosquitoes, Infectious Diseases, and Cancer: A Connection to Study? Int. J. Environ. Res. Public Health 2019, 16, 4859. [Google Scholar] [CrossRef]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef] [PubMed]
- Bkhache, M.; Tmimi, F.Z.; Charafeddine, O.; Filali, O.B.; Lemrani, M.; Labbe, P.; Sarih, M. G119S ace-1 mutation conferring insecticide resistance detected in the Culex pipiens complex in Morocco. Pest. Manag. Sci. 2019, 75, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Palermo, D.; Giunti, G.; Laudani, F.; Palmeri, V.; Campolo, O. Essential Oil-Based Nano-Biopesticides: Formulation and Bioactivity against the Confused Flour Beetle Tribolium confusum. Sustainability 2021, 13, 9746. [Google Scholar] [CrossRef]
- Yousefi, F.; Asadikaram, G.; Karamouzian, S.; Abolhassani, M.; Moazed, V.; Nematollahi, M.H. MGMT methylation alterations in brain cancer following organochlorine pesticides exposure. Environ. Res. Eng. Manag. 2021, 8, 47–53. [Google Scholar] [CrossRef]
- Ghorbani, F.; Vatandoost, H.; Hanafi-Bojd, A.A.; Abai, M.R.; Nikookar, H.; Enayati, A.A. High resistance of vector of West Nile virus, Culex pipiens Linnaeus (Diptera: Culicidae) to different insecticides recommended by WHO in northern Iran. J. Arthropod. Borne Dis. 2018, 12, 24. [Google Scholar]
- Bhattacharyya, A.; Bhaumik, A.; Rani, P.U.; Mandal, S.; Epidi, T.T. Nano-particles-A recent approach to insect pest control. Afr. J. Biotechnol. 2010, 9, 3489–3493. [Google Scholar]
- Sujitha, V.; Murugan, K.; Dinesh, D.; Pandiyan, A.; Aruliah, R.; Hwang, J.S.; Kalimuthu, K.; Panneerselvam, C.; Higuchi, A.; Aziz, A.T.; et al. Green-synthesized CdS nano-pesticides: Toxicity on young instars of malaria vectors and impact on enzymatic activities of the non-target mud crab Scylla serrata. Aquat. Toxicol. 2017, 612, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Dinesh, D.; Nataraj, D.; Subramaniam, J.; Amuthavalli, P.; Madhavan, J.; Rajasekar, A.; Rajan, M.; Thiruppathi, K.P.; Kumar, S.; et al. Iron and iron oxide nanoparticles are highly toxic to Culex quinquefasciatus with little non-target effects on larvivorous fishes. Environ. Sci. Pollut. Res. Int. 2018, 25, 10504–10514. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Kalaitzaki, A.; Karamaouna, F.; Michaelakis, A.; Papadimitriou, V.; Dourtoglou, V.; Papachristos, D.P. Nano-formulation enhances insecticidal activity of natural pyrethrins against Aphis gossypii (Hemiptera: Aphididae) and retains their harmless effect to non-target predators. Environ. Sci. Pollut. Res. Int. 2018, 25, 10243–10249. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Madhavan, J.; Murugan, K.; Samidoss, C.M.; Kumar, S.; Higuchi, A.; Benelli, G. Flower-Like Copper Sulfide Nanocrystals are Highly Effective Against Chloroquine-Resistant Plasmodium falciparum and the Malaria Vector Anopheles stephensi. J. Clust. Sci. 2016, 28, 581–594. [Google Scholar] [CrossRef]
- Pavela, R.; Murugan, K.; Canale, A.; Benelli, G. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Ind. Crops Prod. 2017, 97, 338–344. [Google Scholar] [CrossRef]
- Mishra, P.; Balaji, A.P.B.; Dhal, P.K.; Suresh Kumar, R.S.; Magdassi, S.; Margulis, K.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Stability of nano-sized permethrin in its colloidal state and its effect on the physiological and biochemical profile of Culex tritaeniorhynchus larvae. Bull. Entomol. Res. 2017, 107, 676–688. [Google Scholar] [CrossRef]
- Rajaganesh, R.; Murugan, K.; Panneerselvam, C.; Jayashanthini, S.; Aziz, A.T.; Roni, M.; Suresh, U.; Trivedi, S.; Rehman, H.; Higuchi, A.; et al. Fern-synthesized silver nanocrystals: Towards a new class of mosquito oviposition deterrents? Res. Vet. Sci. 2016, 109, 40–51. [Google Scholar] [CrossRef]
- Fiorenzano, J.M.; Koehler, P.G.; Xue, R.D. Attractive Toxic Sugar Bait (ATSB) For Control of Mosquitoes and Its Impact on Non-Target Organisms: A Review. Int. J. Environ. Res. Public Health 2017, 14, 4. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef]
- Foster, W.A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef]
- Allan, S.A. Susceptibility of adult mosquitoes to insecticides in aqueous sucrose baits. J. Vector. Ecol. 2011, 36, 59–67. [Google Scholar] [CrossRef]
- Xue, R.D.; Ali, A.; Kline, D.L.; Barnard, D.R. Field evaluation of boric acid- and fipronil-based bait stations against adult mosquitoes. J. Am. Mosq. Control Assoc. 2008, 24, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.; Hemingway, J. Malaria management: Past, present, and future. Annu. Rev. Entomol. 2010, 55, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Qualls, W.A.; Muller, G.C.; Revay, E.E.; Allan, S.A.; Arheart, K.L.; Beier, J.C.; Smith, M.L.; Scott, J.M.; Kravchenko, V.D.; Hausmann, A.; et al. Evaluation of attractive toxic sugar bait (ATSB)-Barrier for control of vector and nuisance mosquitoes and its effect on non-target organisms in sub-tropical environments in Florida. Acta. Trop. 2014, 131, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Benelli, M.; Leather, S.R.; Francati, S.; Marchetti, E.; Dindo, M.L. Effect of two temperatures on biological traits and susceptibility to a pyrethroid insecticide in an exotic and native coccinellid species. Bull. Insectology 2015, 68, 23–29. [Google Scholar]
- Zappala, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arno, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pestic. Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Farahi, S.; Sadeghi, H. Species diversity of aphids and ladybird Mashhad district (Khorasan razavi province). J. Plant Prot. Res. 2009, 23, 89–95. [Google Scholar]
- Naranjo, S.; Ellsworth, P.; Chu, C.C.; Henneberry, T. Conservation of predatory arthropods in cotton: Role of action thresholds for Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 2002, 95, 682–691. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Müller, G.C.; Beier, J.C.; Traore, S.F.; Toure, M.B.; Traore, M.M.; Bah, S.; Doumbia, S.; Schlein, Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar. J. 2010, 9, 210. [Google Scholar] [CrossRef]
- Azari-Hamidian, S.; Harbach, R.E. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae). Zootaxa 2009, 2078, 1–33. [Google Scholar] [CrossRef]
- Akram, M.I. comparative efficacy of pyrethroids on Aedes aegypti, Aedes albopictus and Culex quinquefasciatus collected from different ecological zones of Punjab, Pakistan. Pak. J. Agric. 2017, 54, 567–578. [Google Scholar]
- Desheesh, M.A.; El-Masry, D.M.; Fargand, M.M.; Youssef, H.M. Larvicidal Activity of Nano- Encapsulated Lambda—Cyhalothrin against Susceptible Mosquito Larvae (Culex Pipiens) in Comparison with Conventional form. Alex. Sci. Exch. 2019, 40, 568–573. [Google Scholar] [CrossRef]
- Jamal, K. Comparative efficacy of conventional insecticide and eco-friendly biopesticide in the management of Anopheles stephensi mosquito (Vector of Plasmodium parasite): A synthesis of child outcomes. Child. Dev. Perspect. 2018, 10, 172–183. [Google Scholar]
- Yewhalaw, D.; Balkew, M.; Shililu, J.; Suleman, S.; Getachew, A.; Ashenbo, G.; Chibsa, S.; Dissanayake, G.; George, K.; Dengela, D.; et al. Determination of the residual efficacy of carbamate and organophosphate insecticides used for indoor residual spraying for malaria control in Ethiopia. Malar. J. 2017, 16, 471. [Google Scholar] [CrossRef]
- Curtis, C.F.; Miller, J.E.; Hodjati, M.H.; Kolaczinski, J.H.; Kasumba, I. Can anything be done to maintain the effectiveness of pyrethroid-impregnated bednets against malaria vectors? Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 353, 1769–1775. [Google Scholar] [CrossRef][Green Version]
- Kolaczinski, J.; Fanello, C.; Hervé, J.-P.; Conway, D.; Carnevale, P.; Curtis, C. Experimental and molecular genetic analysis of the impact of pyrethroid and non-pyrethroid insecticide impregnated bednets for mosquito control in an area of pyrethroid resistance. Bull. Entomol. 2000, 90, 125–132. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.; Swale, D.; Carlier, P.R.; Hartsel, J.A.; Ma, M.; Ekström, F.; Bloomquist, J.R. Evaluation of novel carbamate insecticides for neurotoxicity to non-target species. Pestic. Biochem. Physiol. 2013, 106, 156–161. [Google Scholar] [CrossRef]
- Asidi, A.N.; N’Guessan, R.; Koffi, A.A.; Curtis, C.F.; Hougard, J.M.; Chandre, F.; Corbel, V.; Darriet, F.; Zaim, M.; Rowland, M.W. Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes. Malar. J. 2005, 4, 25. [Google Scholar] [CrossRef][Green Version]
- Weerasooriya, M.V.; Munasinghe, C.; Mudalige, M.P.; Curtis, C.; Samarawickrema, W. Comparative efficacy of house curtains impregnated with permethrin, lambdacyhalothrin or bendiocarb against the vector of bancroftian filariasis, Culex quinquefasciatus, in Matara, Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 103–104. [Google Scholar] [CrossRef]
- Curtis, C.F.; Myamba, J.; Wilkes, T.J. Comparison of different insecticides and fabrics for anti-mosquito bednets and curtains. Med. Vet. Entomol. 1996, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- N’Guessan, R.; Corbel, V.; Bonnet, J.; Yates, A.; Asidi, A.; Boko, P.; Odjo, A.; Akogbéto, M.; Rowland, M. Evaluation of indoxacarb, an oxadiazine insecticide for the control of pyrethroid-resistant Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Guillet, P.; N’guessan, R.; Darriet, F.; Traore-Lamizana, M.; Chandre, F.; Carnevale, P. Combined pyrethroid and carbamate ‘two-in-one’treated mosquito nets: Field efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus. Med. Vet. Entomol. 2001, 15, 105–112. [Google Scholar] [CrossRef]
- Yeebiyo, Y.; Dengela, D.; Tesfaye, A.G.; Anshebo, G.Y.; Kolyada, L.; Wirtz, R.; Chibsa, S.; Fornadel, C.; George, K.; Belemvire, A.; et al. Short persistence of bendiocarb sprayed on pervious walls and its implication for the indoor residual spray program in Ethiopia. Parasites Vectors 2016, 9, 266. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Kraiss, H.; Cullen, E.M. Efficacy and nontarget effects of reduced-risk insecticides on Aphis glycines (Hemiptera: Aphididae) and its biological control agent Harmonia axyridis (Coleoptera: Coccinellidae). J. Econ. Entomol. 2014, 101, 391–398. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, F.; Khan, I.; Khan, M.A.; Khan, S.Z.; Khan, M.A.; Khan, I.A.; Iqbal, T. Toxicity of selected insecticides against the zig zag ladybird beetle Menochilus Sexmaculatus. J. Zool. Stud. 2015, 3, 143–147. [Google Scholar]
- Mughal, T.K.; Ullah, Z.; Sabri, M.A.; Ahmad, S.; Hussain, D. In vitro comparative toxicity of different insecticides against adults of seven spotted beetle, Coccinella septempunctata L.(Coleoptera: Coccinellidae). J. Entomol. Zool. Stud. 2017, 5, 498–502. [Google Scholar]
- Ahmad, M.; Rafiq, M.; Arif, M.I.; Sayyed, A.H. Toxicity of some commonly used insecticides against Coccinella undecimpunctata (Coleoptera: Coccinellidae). Pak. J. Zool. 2011, 43, 1161–1165. [Google Scholar]
- Khatun, F.; Latif, M.; Hossain, M. Effectiveness of some chemicals and botanicals against jassid (Amrasca biguttula biguttula Ishida) of brinjal and their impact on spiders and lady bird beetles. J. Sher-e-Bangia Agric. Univ. 2009, 5, 15–21. [Google Scholar]
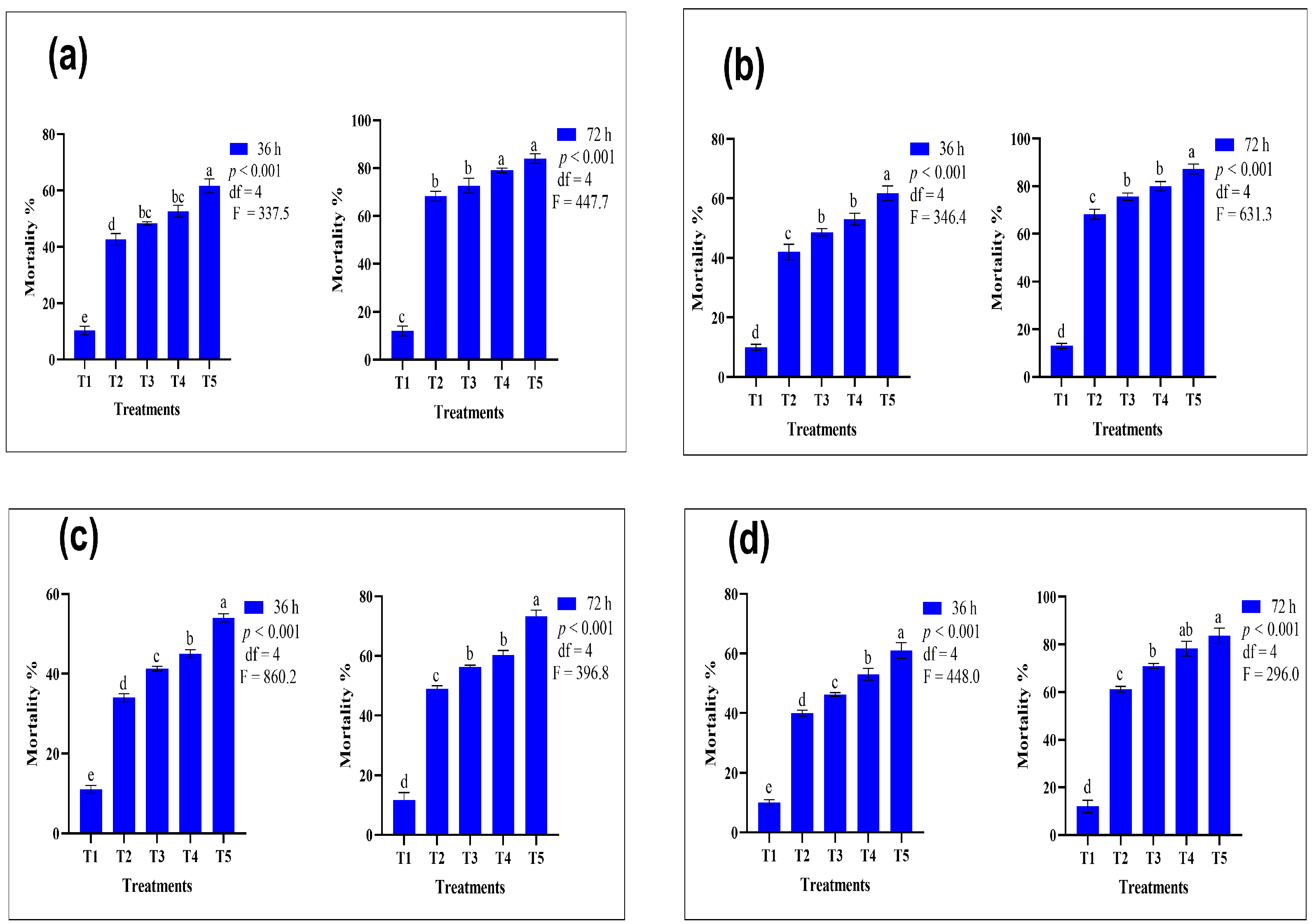
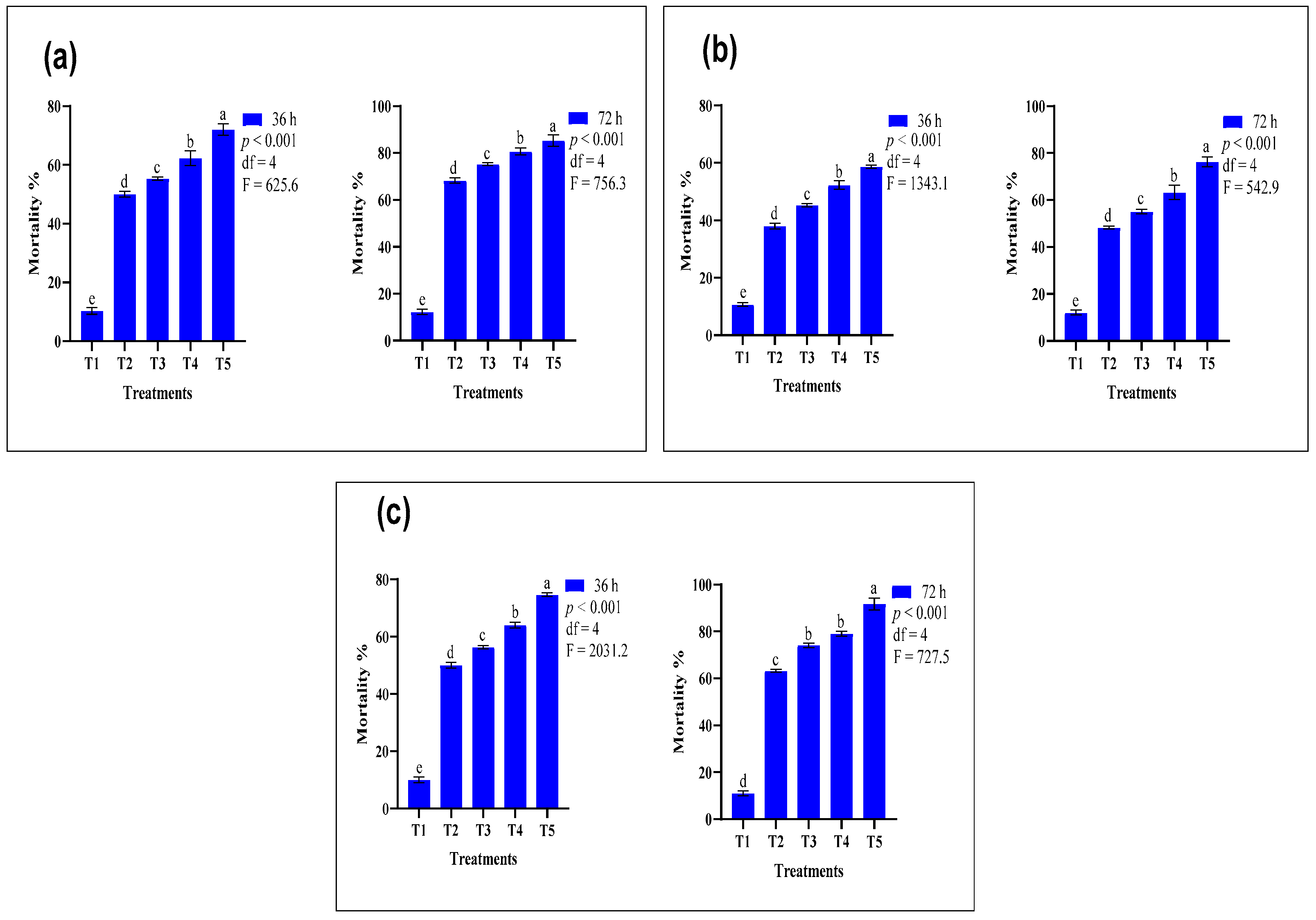
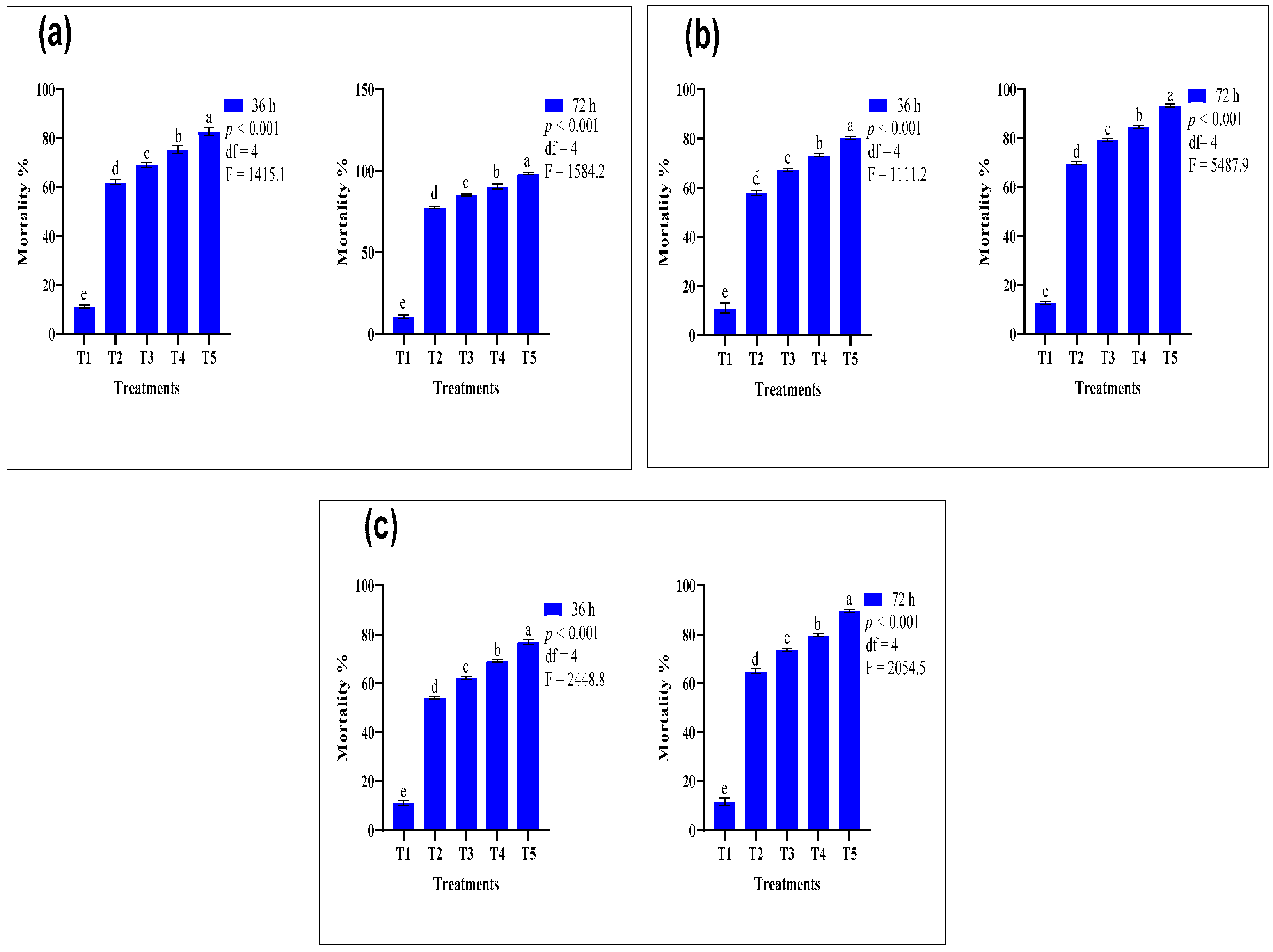
| Insecticides | Treatments | Mortality after 36 h (%) | Mortality after 72 h (%) | GLM Test Results |
|---|---|---|---|---|
| Lambda-cyhalothrin | T1 | 0 | 0 | Treatment: F = 2678.9, p < 0.001 Time: F = 66.7, p < 0.001 Treatment × time: F = 9.4, p < 0.001 |
| T2 | 67.67 ± 2.05 b | 81.00 ± 2.94 a | ||
| T3 | 76.00 ± 1.41 a | 81.67 ± 2.36 a | ||
| T4 | 9.33 ± 0.47 d | 13.67 ± 0.47 b | ||
| T5 | 12.67 ± 0.47 c | 14.33 ± 0.47 b | ||
| Cypermethrin | T1 | 0 | 0 | Treatment: F = 1294.1, p < 0.001 Time: F = 21.3, p < 0.001 Treatment × time: F = 6.3, p < 0.001 |
| T2 | 75.00 ± 4.08 a | 84.00 ± 1.41 a | ||
| T3 | 79.33 ± 0.94 a | 90.00 ± 4.08 a | ||
| T4 | 13.67 ± 0.47 b | 14.67 ± 0.47 b | ||
| T5 | 15.00 ± 0.82 b | 15.67 ± 0.47 b | ||
| Deltamethrin | T1 | 0 | 0 | Treatment: F = 1973.3, p < 0.001 Time: F = 52.1, p < 0.001 Treatment × time: F = 8.6, p < 0.001 |
| T2 | 56.67 ± 2.36 b | 70.67 ± 0.94 a | ||
| T3 | 65.00 ± 4.08 a | 73.33 ± 2.36 a | ||
| T4 | 7.33 ± 0.94 c | 9.67 ± 0.47 b | ||
| T5 | 8.67 ± 0.47 c | 10.67 ± 0.47 b | ||
| Bifenthrin | T1 | 0 | 0 | Treatment: F = 1682.2, p < 0.001 Time: F = 37.8, p < 0.001 Treatment × time: F = 6.2, p < 0.001 |
| T2 | 63.33 ± 2.36 a | 76.33 ± 3.09 a | ||
| T3 | 70.00 ± 4.08 a | 78.00 ± 1.41 a | ||
| T4 | 7.67 ± 0.94 b | 10.00 ± 0.82 b | ||
| T5 | 9.00 ± 0.82 b | 11.00 ± 0.82 b |
| Insecticides | Treatments | Mortality after 36 h (%) | Mortality after 72 h (%) | GLM Test Results |
|---|---|---|---|---|
| Triazofos | T1 | 0 | 0 | Treatment: F = 2448.7, p < 0.001 Time: F = 57.4, p < 0.001 Treatment × time: F = 6.3, p < 0.001 |
| T2 | 66.00 ± 1.41 a | 76.33 ± 1.70 a | ||
| T3 | 71.67 ± 2.36 a | 80.67 ± 2.49 a | ||
| T4 | 8.33 ± 1.25 b | 11.33 ± 0.94 b | ||
| T5 | 10.67 ± 1.25 b | 13.67 ± 0.47 b | ||
| Profenofos | T1 | 0 | 0 | Treatment: F = 3183.2, p < 0.001 Time: F = 153.4, p < 0.001 Treatment × time: F = 21.0, p < 0.001 |
| T2 | 73.67 ± 1.70 a | 85.67 ± 1.70 b | ||
| T3 | 77.00 ±1.41 a | 92.00 ± 2.16 a | ||
| T4 | 9.67 ± 0.47 b | 14.33 ± 0.47 c | ||
| T5 | 12.00 ± 0.82 b | 16.67 ± 0.47 c | ||
| Chlorpyrifos | T1 | 0 | 0 | Treatment: F = 5833.4, p < 0.001 Time: F = 312.5 p < 0.001 Treatment × time: F = 32.7, p < 0.001 |
| T2 | 77.33 ± 0,47 b | 86.67 ± 0.94 b | ||
| T3 | 80.33 ± 0.47 a | 94.33 ± 1.70 a | ||
| T4 | 10.33 ± 0.47 d | 17.67 ± 0.47 c | ||
| T5 | 13.00 ± 082 c | 20.00 ± 0.82 c |
| Insecticides | Treatments | Mortality after 36 h (%) | Mortality after 72 h (%) | GLM Test Results |
|---|---|---|---|---|
| Carbosulfan | T1 | 0 | 0 | Treatment: F = 4376.3, p < 0.001 Time: F = 112.3, p < 0.001 Treatment × time: F = 15.2, p < 0.001 |
| T2 | 81.00 ± 0.82 b | 86.00 ± 0.82 b | ||
| T3 | 86.67 ± 1.25 a | 96.00 ± 1.41 a | ||
| T4 | 13.67 ± 0.94 d | 20.00 ± 0.82 c | ||
| T5 | 18.00 ± 0.82 c | 22.67 ± 0.94 c | ||
| Propoxur | T1 | 0 | 0 | Treatment: F = 2833.5, p < 0.001 Time: F = 63.9, p < 0.001 Treatment × time: F = 6.1, p = 0.002 |
| T2 | 73.67 ± 2.7 b | 83.67 ± 2.36 b | ||
| T3 | 81.33 ± 1.89 a | 88.33 ± 1.25 a | ||
| T4 | 11.33 ± 0.47 c | 15.00 ± 0.82 c | ||
| T5 | 13.67 ± 0.47 c | 18.33 ± 1.25 c | ||
| Methomyl | T1 | 0 | 0 | Treatment: F = 3606.1, p < 0.001 Time: F = 114.3, p < 0.001 Treatment × time: F = 12.1, p < 0.001 |
| T2 | 71.00 ± 0.82 b | 84.00 ± 0.82 a | ||
| T3 | 82.67 ± 2.05 a | 87.33 ± 2.49 a | ||
| T4 | 10.00 ± 0.82 d | 17.33 ± 0.47 b | ||
| T5 | 14.00 ± 0.82 c | 19.00 ± 0.82 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, M.; Zhao, C.; Akhtar, S.; Ahmad, I.; Jilong, P.; Zhang, S. Assessment of Nano-Formulated Conventional Insecticide-Treated Sugar Baits on Mosquito Control and the Effect on Non-Target Aphidophagous Coccinella septempunctata. Insects 2024, 15, 70. https://doi.org/10.3390/insects15010070
Farhan M, Zhao C, Akhtar S, Ahmad I, Jilong P, Zhang S. Assessment of Nano-Formulated Conventional Insecticide-Treated Sugar Baits on Mosquito Control and the Effect on Non-Target Aphidophagous Coccinella septempunctata. Insects. 2024; 15(1):70. https://doi.org/10.3390/insects15010070
Chicago/Turabian StyleFarhan, Muhammad, Chenchen Zhao, Sohail Akhtar, Ishtiaq Ahmad, Pan Jilong, and Shuai Zhang. 2024. "Assessment of Nano-Formulated Conventional Insecticide-Treated Sugar Baits on Mosquito Control and the Effect on Non-Target Aphidophagous Coccinella septempunctata" Insects 15, no. 1: 70. https://doi.org/10.3390/insects15010070
APA StyleFarhan, M., Zhao, C., Akhtar, S., Ahmad, I., Jilong, P., & Zhang, S. (2024). Assessment of Nano-Formulated Conventional Insecticide-Treated Sugar Baits on Mosquito Control and the Effect on Non-Target Aphidophagous Coccinella septempunctata. Insects, 15(1), 70. https://doi.org/10.3390/insects15010070







