Can a Mixture of Farnesene Isomers Avert the Infestation of Aphids in Sugar Beet Crops?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
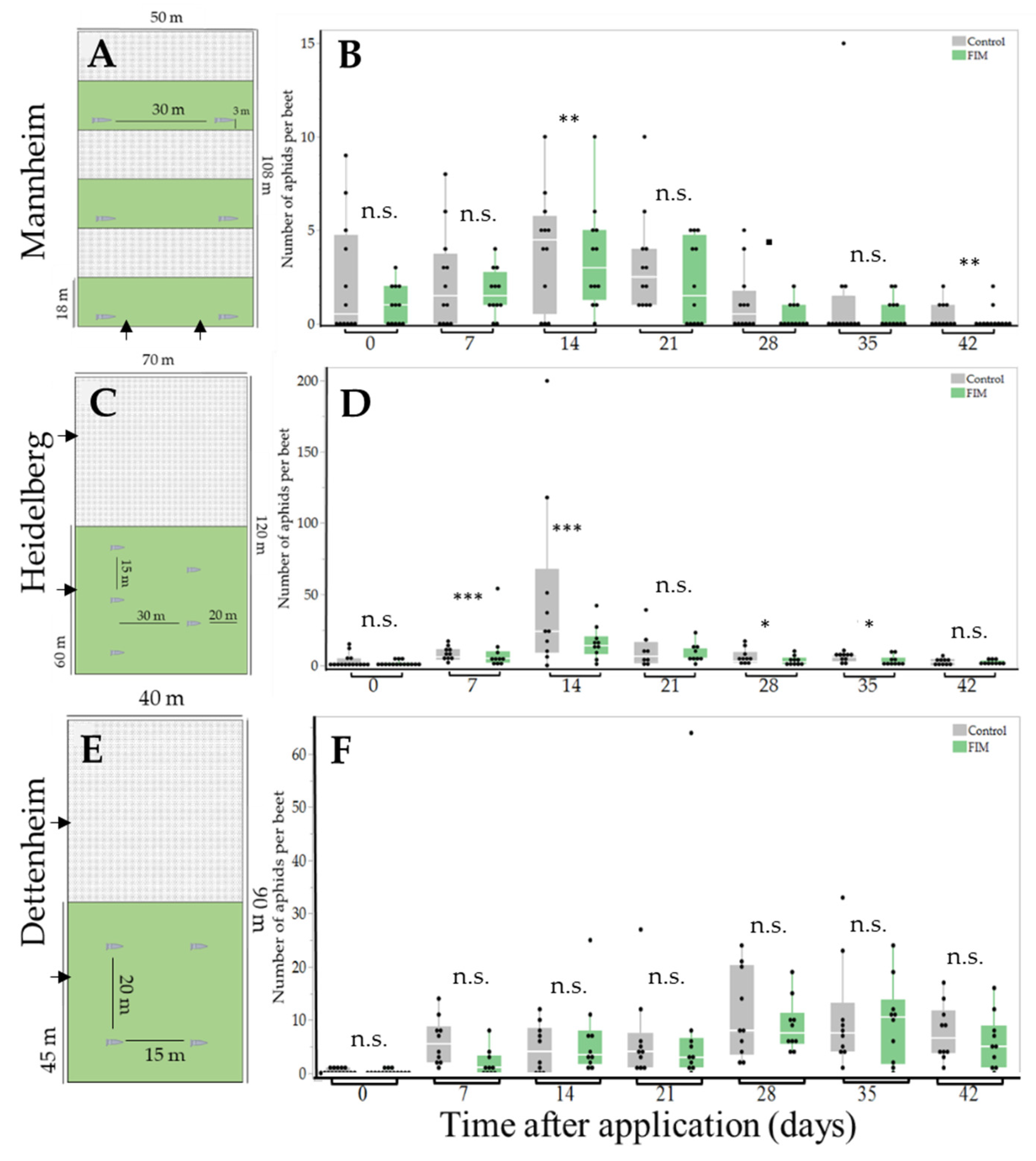
2.1. Experimental Sites
2.2. Dispenser Preparation and Application
2.3. Sampling
2.4. Experimental Setups
2.5. Preparation of Samples
2.6. Statistical Analyses
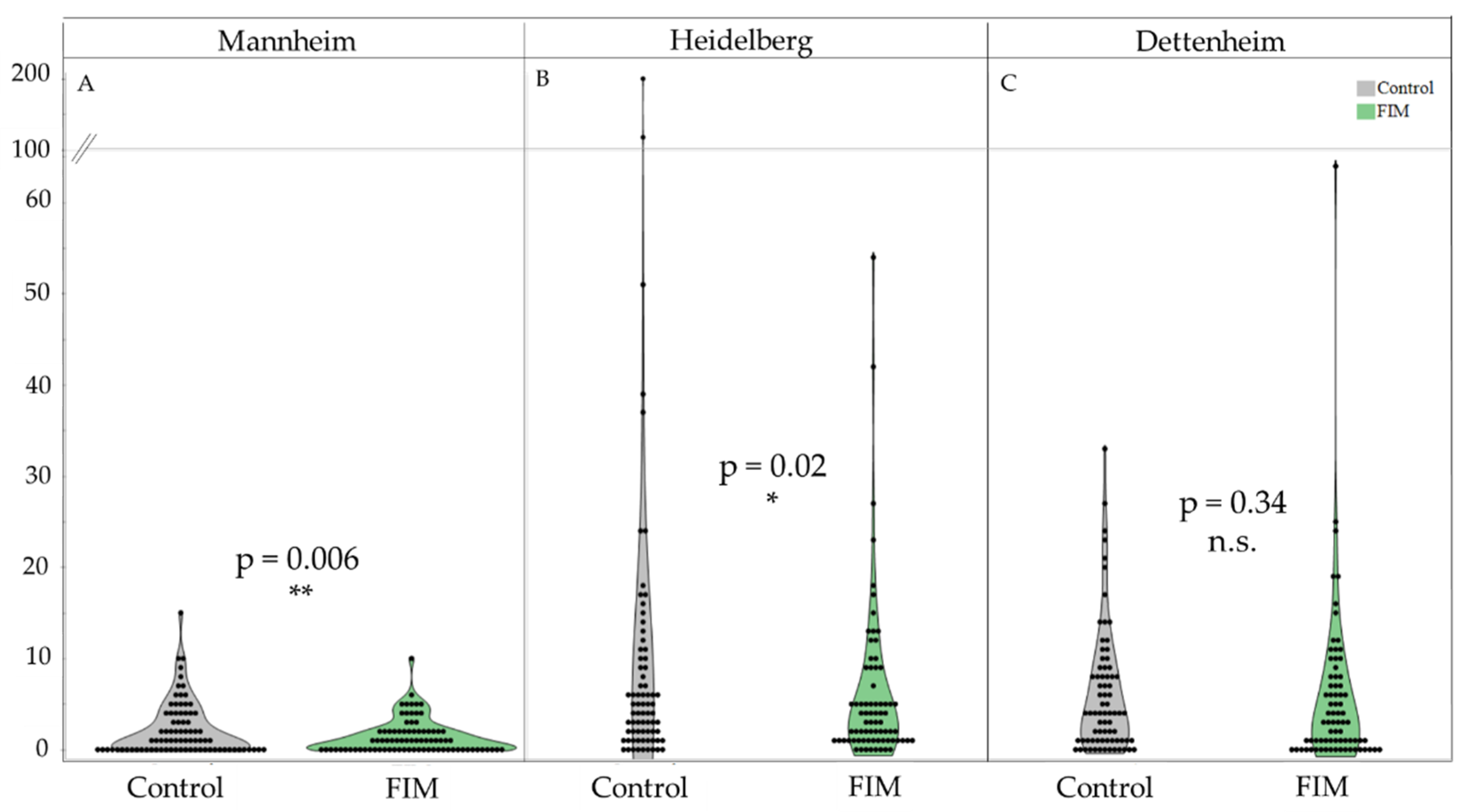
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting Agriculturally Driven Global Environmental Change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod Decline in Grasslands and Forests Is Associated with Landscape-Level Drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Baskaran, S.; Kennedy, I.R. Ecological Relative Risk (EcoRR): Another Approach for Risk Assessment of Pesticides in Agriculture. Agric. Ecosyst. Environ. 2002, 91, 37–57. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Insecticides Mode of Action in Relation to Their Toxicity to Non-Target Organisms. J. Environ. Anal. Toxicol. 2012, s4, 2. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal Efficacy of Microbial-Mediated Synthesized Copper Nano-Pesticide against Insect Pests and Non-Target Organisms. Int. J. Environ. Res. Public Health 2021, 18, 10536. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-Effects of Pesticides on Non-Target Insects in Agriculture: A Mini-Review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Aradottir, G.I.; Smart, L.E.; Martin, J.L.; Caulfield, J.C.; Doherty, A.; Sparks, C.A.; Woodcock, C.M.; Birkett, M.A.; Napier, J.A.; et al. The First Crop Plant Genetically Engineered to Release an Insect Pheromone for Defence. Sci. Rep. 2015, 5, 11183. [Google Scholar] [CrossRef]
- Norin, T. Semiochemicals for Insect Pest Management. Pure Appl. Chem. 2007, 79, 2129–2136. [Google Scholar] [CrossRef]
- Karlson, P.; Butenandt, A. Pheromones (Ectohormones) in Insects. Annu. Rev. Entomol. 1959, 4, 39–58. [Google Scholar] [CrossRef]
- Whittaker, R.H. Communities and Ecosystems; Macmillan: London, UK; University of California: Los Angeles, CA, USA, 1970; ISBN 978-0-02-427400-7. [Google Scholar]
- Hoffmann, C.; Doye, E. How to Measure Mating Disruption Efficacy of Pheromone Products for Registration Purposes in the Field? Pheromones Semiochem. Integr. Prod. 2017, 126, 59–70. [Google Scholar]
- Park, S.-W.; Kaimoyo, E.; Kumar, D.; Mosher, S.; Klessig, D.F. Methyl Salicylate Is a Critical Mobile Signal for Plant Systemic Acquired Resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, L.; Liu, Y.; Chen, J.; Francis, F. Use of Slow-Release Plant Infochemicals to Control Aphids: A First Investigation in a Belgian Wheat Field. Sci. Rep. 2016, 6, 31552. [Google Scholar] [CrossRef] [PubMed]
- Herrbach, E. Effect of Dodecanoic Acid on the Colonisation of Sugar Beet by Aphids and the Secondary Spread of Virus Yellows. Ann. Appl. Biol. 1987, 111, 477–482. [Google Scholar] [CrossRef]
- Ninkovic, V.; Glinwood, R.; Ünlü, A.G.; Ganji, S.; Unelius, C.R. Effects of Methyl Salicylate on Host Plant Acceptance and Feeding by the Aphid Rhopalosiphum padi. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Cui, L.-L.; Francis, F.; Heuskin, S.; Lognay, G.; Liu, Y.-J.; Dong, J.; Chen, J.-L.; Song, X.-M.; Liu, Y. The Functional Significance of E-β-Farnesene: Does It Influence the Populations of Aphid Natural Enemies in the Fields? Biol. Control 2012, 60, 108–112. [Google Scholar] [CrossRef]
- Bowers, W.S.; Nault, L.R.; Webb, R.E.; Dutky, S.R. Aphid Alarm Pheromone: Isolation, Identification, Synthesis. Science 1972, 177, 1121–1122. [Google Scholar] [CrossRef]
- Nault, L.R.; Edwards, L.J.; Styer, W.E. Aphid Alarm Pheromones: Secretion and Reception. Environ. Entomol. 1973, 2, 101–105. [Google Scholar] [CrossRef]
- Francis, F.; Vandermoten, S.; Verheggen, F.; Lognay, G.; Haubruge, E. Is the (E)-β-Farnesene Only Volatile Terpenoid in Aphids? J. Appl. Entomol. 2004, 129, 6–11. [Google Scholar] [CrossRef]
- Vandermoten, S.; Mescher, M.C.; Francis, F.; Haubruge, E.; Verheggen, F.J. Aphid Alarm Pheromone: An Overview of Current Knowledge on Biosynthesis and Functions. Insect Biochem. Mol. Biol. 2012, 42, 155–163. [Google Scholar] [CrossRef]
- Edwards, J.S. Defence by Smear: Supercooling in the Cornicle Wax of Aphids. Nature 1966, 211, 73–74. [Google Scholar] [CrossRef]
- Strong, F.E. Observations on Aphid Cornicle Secretions. Ann. Entomol. Soc. Am. 1967, 60, 668–673. [Google Scholar] [CrossRef]
- Montgomery, M.E.; Nault, L.R. Comparative Response of Aphids to the Alarm Pheromone, (E)-ß-Farnsene. Entomol. Exp. Appl. 1977, 22, 236–242. [Google Scholar] [CrossRef]
- Wohlers, P. Escape responses of pea aphids Acyrthosiphon pisum to alarm pheromones and additional stimuli. Entomol. Exp. Appl. 1980, 27, 156–168. [Google Scholar] [CrossRef]
- Sloggett, J.J.; Weisser, W.W. Parasitoids Induce Production of the Dispersal Morph of the Pea Aphid, Acyrthosiphon pisum. Oikos 2002, 98, 323–333. [Google Scholar] [CrossRef]
- Kunert, G.; Otto, S.; Röse, U.S.R.; Gershenzon, J.; Weisser, W.W. Alarm Pheromone Mediates Production of Winged Dispersal Morphs in Aphids. Ecol. Lett. 2005, 8, 596–603. [Google Scholar] [CrossRef]
- Podjasek, J.O.; Bosnjak, L.M.; Brooker, D.J.; Mondor, E.B. Alarm Pheromone Induces a Transgenerational Wing Polyphenism in the Pea Aphid, Acyrthosiphon pisum. Can. J. Zool. 2005, 83, 1138–1141. [Google Scholar] [CrossRef]
- Su, J.; Zhu, S.; Zhang, Z.; Ge, F. Effect of Synthetic Aphid Alarm Pheromone (E)-β-Farnesene on Development and Reproduction of Aphis gossypii (Homoptera: Aphididae). J. Econ. Entomol. 2006, 99, 1636–1640. [Google Scholar] [CrossRef]
- de Vos, M.; Cheng, W.Y.; Summers, H.E.; Raguso, R.A.; Jander, G. Alarm Pheromone Habituation in Myzus persicae Has Fitness Consequences and Causes Extensive Gene Expression Changes. Proc. Natl. Acad. Sci. USA 2010, 107, 14673–14678. [Google Scholar] [CrossRef]
- Francis, F.; Lognay, G.; Haubruge, E. Olfactory Responses to Aphid and Host Plant Volatile Releases: (E)-β-Farnesene an Effective Kairomone for the Predator Adalia bipunctata. J. Chem. Ecol. 2004, 30, 741–755. [Google Scholar] [CrossRef]
- Al Abassi, S.; Birkett, M.A.; Pettersson, J.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Response of the Seven-Spot Ladybird to an Aphid Alarm Pheromone and an Alarm Pheromone Inhibitor Is Mediated by Paired Olfactory Cells. J. Chem. Ecol. 2000, 26, 1765–1771. [Google Scholar] [CrossRef]
- Francis, F.; Martin, T.; Lognay, G.; Haubruge, E. Role of (E)-ß-Farnesene in Systematic Aphid Prey Location by Episyrphus balteatus Larvae (Diptera: Syrphidae). Eur. J. Entomol. 2005, 102. [Google Scholar] [CrossRef]
- Micha, S.G.; Wyss, U. Aphid Alarm Pheromone (E)-β-Farnesene: A Host Finding Kairomone for the Aphid Primary Parasitoid Aphidius uzbekistanicus (Hymenoptera: Aphidiinae). Chemoecology 1996, 7, 132–139. [Google Scholar] [CrossRef]
- Joachim, C.; Vosteen, I.; Weisser, W.W. The Aphid Alarm Pheromone (E)-β-Farnesene Does Not Act as a Cue for Predators Searching on a Plant. Chemoecology 2015, 25, 105–113. [Google Scholar] [CrossRef]
- Joachim, C.; Weisser, W.W. Does the Aphid Alarm Pheromone (E)-β-Farnesene Act as a Kairomone under Field Conditions? J. Chem. Ecol. 2015, 41, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Vosteen, I.; Weisser, W.W.; Kunert, G. Is There Any Evidence That Aphid Alarm Pheromones Work as Prey and Host Finding Kairomones for Natural Enemies? Ecol. Entomol. 2016, 41, 1–12. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Ibbotson, A.; Booth, C.O. The Distribution of Aphid Infestation in Relation to the Leaf Age: Myzus persicae (Sulz.) and Aphis fabae (Scop.) on Spindle Trees and Sugar Beet Plants. Ann. Appl. Biol. 1950, 37, 651–679. [Google Scholar] [CrossRef]
- Sémétey, O.; Bressan, A.; Richard-Molard, M.; Boudon-Padieu, E. Monitoring of Proteobacteria and Phytoplasma in Sugar Beets Naturally or Experimentally Affected by the Disease Syndrome ‘Basses Richesses’. Eur. J. Plant Pathol. 2007, 117, 187–196. [Google Scholar] [CrossRef]
- Smith, H.G.; Hallsworth, P.B. The Effects of Yellowing Viruses on Yield of Sugar Beet in Field Trials, 1985 and 1987. Ann. Appl. Biol. 1990, 116, 503–511. [Google Scholar] [CrossRef]
- Dusi, A.N.; Peters, D. Beet Mosaic Virus: Its Vector and Host Relationships. J. Phytopathol. 1999, 147, 293–298. [Google Scholar] [CrossRef]
- Hossain, R.; Menzel, W.; Lachmann, C.; Varrelmann, M. New Insights into Virus Yellows Distribution in Europe and Effects of Beet Yellows Virus, Beet Mild Yellowing Virus, and Beet Chlorosis Virus on Sugar Beet Yield Following Field Inoculation. Plant Pathol. 2021, 70, 584–593. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio PBC: Boston, MA, USA, 2020. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Knudson, C.; Geyer, C.J.; Benson, S. Glmm: Generalized Linear Mixed Models via Monte Carlo Likelihood Approximation. 2022. [Google Scholar]
- Sachs, L. Angewandte Statistik. Anwendung Statistischer Methoden, 7th ed.; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Bushra, S.; Tariq, M. How Aphid Alarm Pheromone Can Control Aphids: A Review. Arch. Phytopathol. Plant Prot. 2014, 47, 1563–1573. [Google Scholar] [CrossRef]
- Xu, Q.; Hatt, S.; Lopes, T.; Zhang, Y.; Bodson, B.; Chen, J.; Francis, F. A Push–Pull Strategy to Control Aphids Combines Intercropping with Semiochemical Releases. J. Pest Sci. 2018, 91, 93–103. [Google Scholar] [CrossRef]
- Ester, A.; Gut, J.; Van Oosten, A.M.; Pijnenburg, H.C.H. Controlling Aphids in Iceberg Lettuce by Alarm Pheromone in Combination with an Insecticide. J. Appl. Entomol. 1993, 115, 432–440. [Google Scholar] [CrossRef]
- Beale, M.H.; Birkett, M.A.; Bruce, T.J.A.; Chamberlain, K.; Field, L.M.; Huttly, A.K.; Martin, J.L.; Parker, R.; Phillips, A.L.; Pickett, J.A.; et al. Aphid Alarm Pheromone Produced by Transgenic Plants Affects Aphid and Parasitoid Behavior. Proc. Natl. Acad. Sci. USA 2006, 103, 10509–10513. [Google Scholar] [CrossRef]
- Agelopoulos, N.G.; Chamberlain, K.; Pickett, J.A. Factors Affecting Volatile Emissions of Intact Potato Plants, Solanum Tuberosum: Variability of Quantities and Stability of Ratios. J. Chem. Ecol. 2000, 26, 497–511. [Google Scholar] [CrossRef]
- van Emden, H.F.; Dingley, J.; Dewhirst, S.Y.; Pickett, J.A.; Woodcock, C.M.; Wadhams, L.J. The Effect of Artificial Diet on the Production of Alarm Pheromone by Myzus persicae. Physiol. Entomol. 2014, 39, 285–291. [Google Scholar] [CrossRef]
- Jing-Gong, X.; Feng, Z.; Yu-Ling, F.; Wei, K.; Guang-Xue, Z.; Zhong-Ning, Z. Behavioural Response of Aphids to the Alarm Pheromone Component (E)-β-Farnesene in the Field. Physiol. Entomol. 2002, 27, 307–311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, D.; Nägele, N.; Tolasch, T.; Petschenka, G.; Steidle, J.L.M. Can a Mixture of Farnesene Isomers Avert the Infestation of Aphids in Sugar Beet Crops? Insects 2024, 15, 736. https://doi.org/10.3390/insects15100736
Kuhn D, Nägele N, Tolasch T, Petschenka G, Steidle JLM. Can a Mixture of Farnesene Isomers Avert the Infestation of Aphids in Sugar Beet Crops? Insects. 2024; 15(10):736. https://doi.org/10.3390/insects15100736
Chicago/Turabian StyleKuhn, Denise, Nils Nägele, Till Tolasch, Georg Petschenka, and Johannes L. M. Steidle. 2024. "Can a Mixture of Farnesene Isomers Avert the Infestation of Aphids in Sugar Beet Crops?" Insects 15, no. 10: 736. https://doi.org/10.3390/insects15100736
APA StyleKuhn, D., Nägele, N., Tolasch, T., Petschenka, G., & Steidle, J. L. M. (2024). Can a Mixture of Farnesene Isomers Avert the Infestation of Aphids in Sugar Beet Crops? Insects, 15(10), 736. https://doi.org/10.3390/insects15100736








