Duration of Cattle Ranching Affects Dung Beetle Diversity and Secondary Seed Removal in Tropical Dry Forest Landscapes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
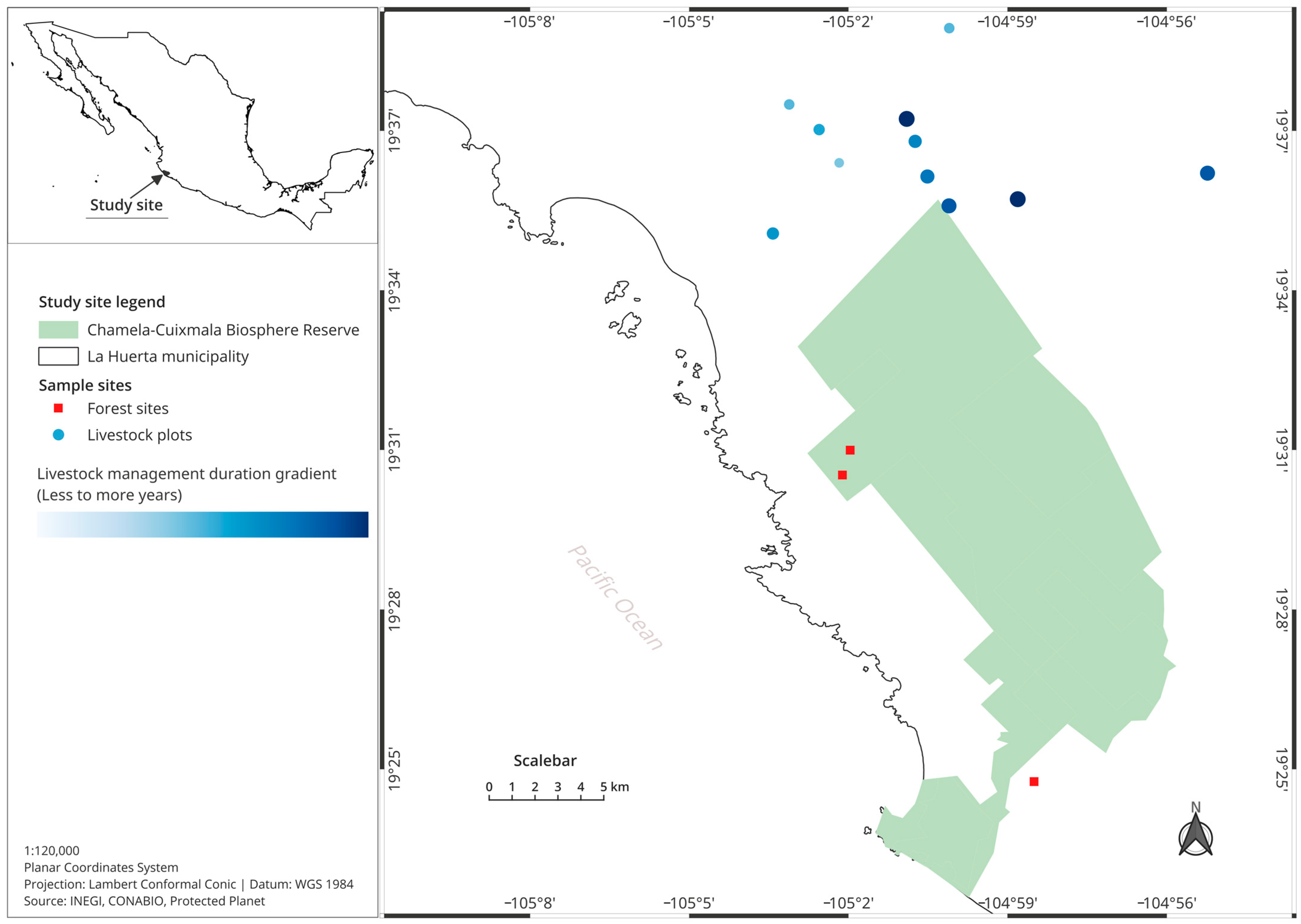
2.1. Study Area
2.2. Selection of Study Plots
2.3. Sampling of Dung Beetles
2.4. Seed Removal
2.5. Data Analysis
2.5.1. Sample Completeness
2.5.2. Beetle Diversity
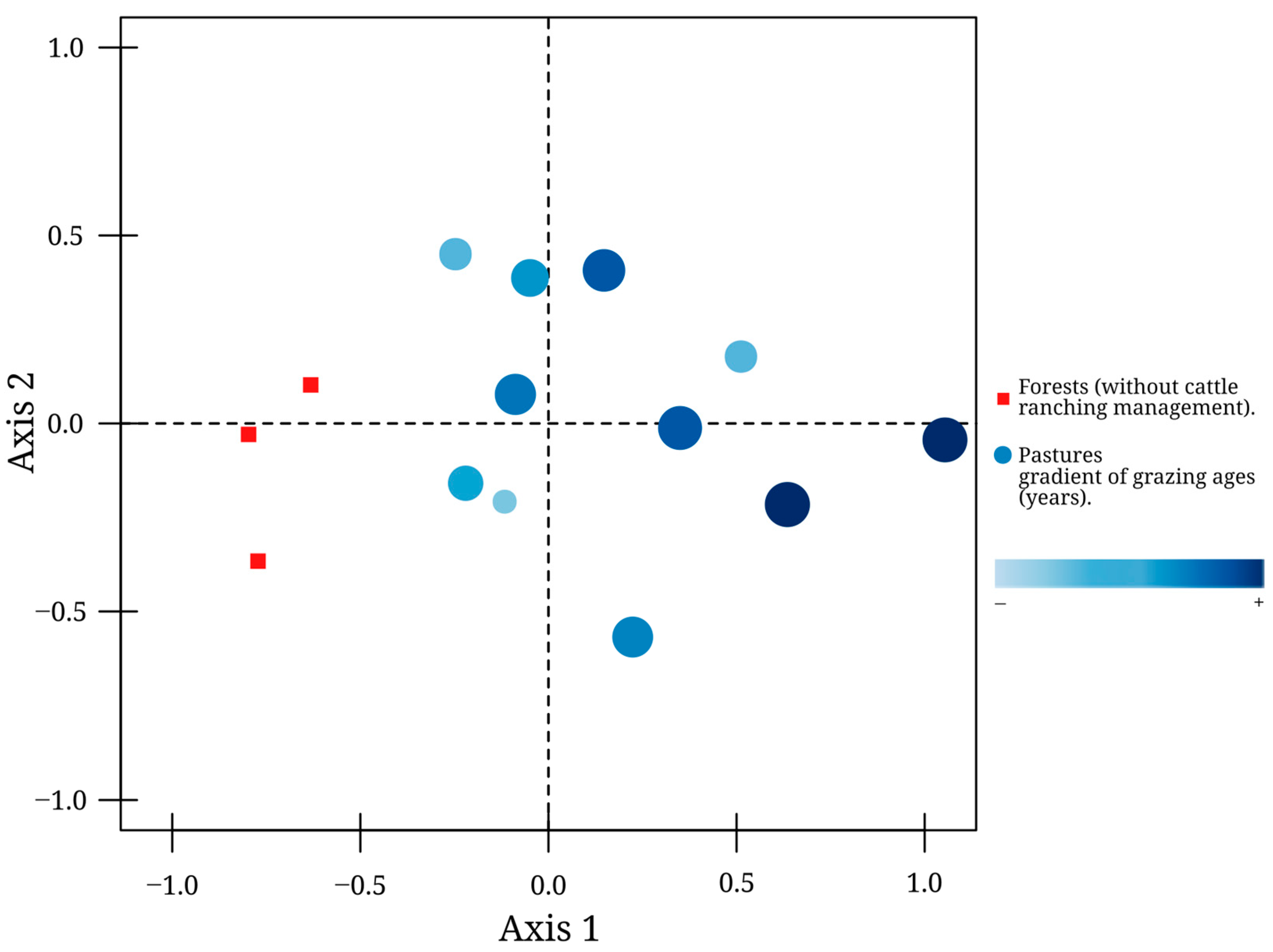
2.5.3. Beetle Assemblage Structure
2.5.4. Relationship of Dung Beetle Abundance and Diversity with Cattle Ranching Duration
2.5.5. Seed Removal by Dung Beetles and Cattle Ranching Duration
3. Results
3.1. Dung Beetle Diversity
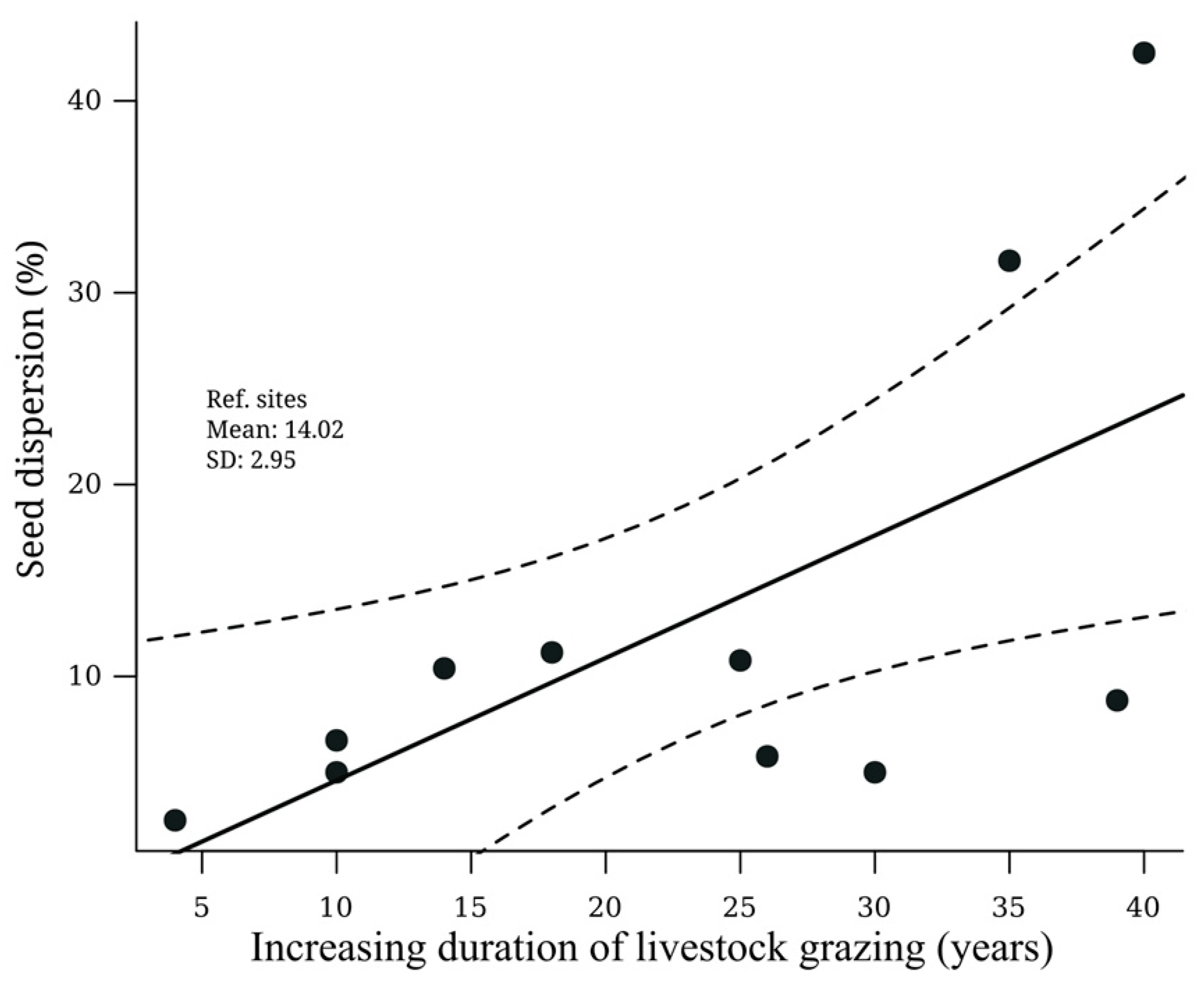
3.2. Relationship between Seed Removal and Cattle Ranching Duration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Azofeifa, A.; Calvo-Alvarado, J.; Espírito-Santo, M.M.; Fernandes, G.W.; Powers, J.; Quesada, M. (Eds.) Tropical Dry Forests in the Americas—Ecology, Conservation, and Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 550. [Google Scholar] [CrossRef]
- Stern, M.; Quesada, M.; Storner, K.E. Changes in composition and structure of a tropical dry forest following intermittent cattle grazing. Rev. Biol. Trop. 2002, 50, 1021–1034. [Google Scholar] [PubMed]
- Trilleras, J.M.; Jaramillo, V.J.; Vega, E.V.; Balvanera, P. Effects of livestock management on the supply of ecosystem services in pastures in a tropical dry region of western Mexico. Agric. Ecosyst. Environ. 2015, 211, 133–144. [Google Scholar] [CrossRef]
- Janzen, D.H. Tropical dry forests, the most endangered major tropical ecosystem. In Biodiversity; Wilson, E.O., Ed.; National Academies Press: Washington, DC, USA, 1988; pp. 130–137. [Google Scholar]
- Dirzo, R.; Young, H.S.; Mooney, H.A.; Ceballos, G. (Eds.) Seasonally Dry Tropical Forests: Ecology and Conservation; Island Press: Washington, DC, USA, 2011; p. 392. [Google Scholar]
- García, H.; Corzo, G.; Isaacs, P.; Etter, A. Distribución y estado actual de los remanentes del bioma de bosque seco tropical en Colombia: Insumos para su gestión. In El Bosque Seco Tropical en Colombia; Pizano, C., García, H., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogotá, Colombia, 2014; pp. 229–251. [Google Scholar]
- Banda, K.; Delgado-Salinas, A.; Dexter, K.G.; Linares-Palomino, R.; Oliveira-Filho, A.; Prado, D.; Pullan, M.; Quintana, C.; Riina, R.; Rodríguez, G.; et al. Plant diversity patterns in neotropical dry forests and their conservation implications. Science 2016, 353, 1383. [Google Scholar] [CrossRef]
- Cabrera, G.; Robaina, N.; Ponce de León, D. Riqueza y abundancia de la macrofauna edáfica en cuatro usos de la tierra en las provincias de Artemisa y Mayabeque, Cuba. Pastos Forrajes 2011, 34, 313–330. [Google Scholar]
- Farias, P.M.; Arellano, L.; Hernández, M.I.M.; Ortiz, S.L. Response of the copro-necrophagous beetle (Coleoptera: Scarabaeinae) assemblage to a range of soil characteristics and livestock management in a tropical landscape. J. Insect Conserv. 2015, 19, 947–960. [Google Scholar] [CrossRef]
- Noguera-Talavera, Á.; Reyes-Sánchez, N.; Mendieta-Araica, B.; Salgado-Duarte, M.M. Macrofauna edáfica como indicador de conversión agroecológica de un sistema productivo de Moringa oleifera Lam. en Nicaragua. Pastos Forrajes 2017, 40, 184–187. [Google Scholar]
- Giménez-Gómez, V.C.; Verdú, J.R.; Guerra-Alonso, C.B.; Zurita, G.A. Relationship between land uses and diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina: Which are the key factors? Biodivers. Conserv. 2018, 27, 3201–3213. [Google Scholar] [CrossRef]
- Masin, C.; Rodriguez, A.R.; Zalazar, C.; Godoy, J.L. Approach to assess agroecosystem anthropic disturbance: Statistical monitoring based on earthworm populations and edaphic properties. Ecol. Indic. 2020, 111, 105984. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.; Blouin, M.; Brown, G.; Decaëns, T.; Grimaldi, M.; Jiménez, J.J.; McKey, D.; Mathieu, J.; Velasquez, E.; et al. Ecosystem engineers in a self-organized soil: A review of concepts and future research questions. Soil Sci. 2016, 181, 91–109. [Google Scholar] [CrossRef]
- Halffter, G.; Edmonds, W.D. The Nesting Behavior of Dung Beetles (Scarabaeinae): An Ecological and Evolutive Approach; Instituto de Ecologia, A.C.: Mexico City, Mexico, 1982; p. 176. [Google Scholar]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E.; Network, T.S.R. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Cons. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Halffter, G.; Favila, M.E.; Halffter, V. A comparative study of the structure of the scarab guild in Mexican tropical rain forests and derived ecosystems. Folia Entomol. Mex. 1992, 84, 131–156. [Google Scholar]
- Favila, M.E.; Halffter, G. The use of indicator groups for measuring biodiversity as related to community structure and function. Acta Zool. Mex. (Nueva Ser.) 1997, 72, 1–25. [Google Scholar] [CrossRef]
- Lobo, J.M.; Lumaret, J.P.; Jay-Robert, P. Sampling dung beetles in the French Mediterranean area: Effects of abiotic factors and farm practices. Pedobiologia 1998, 42, 252–266. [Google Scholar] [CrossRef]
- Slade, E.M.; Mann, D.J.; Villanueva, J.F.; Lewis, O.T. Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. J. Anim. Ecol. 2007, 76, 1094–1104. [Google Scholar] [CrossRef]
- Gómez, V.C.G.; Verdú, J.R.; Zurita, G.A. Thermal niche helps to explain the ability of dung beetles to exploit disturbed habitats. Sci. Rep. 2020, 10, 13364. [Google Scholar] [CrossRef]
- Andresen, E. The Role of Dung Beetles in the Regeneration of Rainforest Plants in Central Amazonia. Ph.D. Thesis, Florida University, Gainesville, FL, USA, 2000. Available online: https://www.proquest.com/openview/d0f11cf3870e1d4e67d9e4a7b9360498/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 15 May 2020).
- Andresen, E.; Feer, F. The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. In Seed Fate Predation, Dispersal Seed. Establ; Forget, P.M., Lambert, J.E., Hulme, P.E., Vander Wall, S.B., Eds.; CAB Publishing: Wallingford, UK, 2005; pp. 331–349. [Google Scholar] [CrossRef]
- Vander Wall, S.B.; Kuhn, K.M.; Beck, M.J. Seed removal, seed predation, and secondary dispersal. Ecology 2005, 86, 801–806. [Google Scholar] [CrossRef]
- Andresen, E.; Urrea-Galeano, L.A. Effects of dung beetle activity on tropical forest plants. Front. Ecol. Evol. 2022, 10, 979676. [Google Scholar] [CrossRef]
- Zunino, M. Food relocation behaviour: A multivalent strategy of Coleoptera. Adv. Coleopterol. 1991, 1991, 297–314. [Google Scholar]
- Feer, F. Effects of dung beetles (Scarabaeidae) on seeds dispersed by howler monkeys (Alouatta seniculus) in the French Guianan rain forest. J. Trop. Ecol. 1999, 15, 129–142. [Google Scholar] [CrossRef]
- Holter, P.; Scholtz, C.H. Are ball-rolling (Scarabaeini, Gymnopleurini, Sisyphini) and tunnelling scarabaeine dung beetles equally choosy about the size of ingested dung particles? Ecol. Entomol. 2005, 30, 700–705. [Google Scholar] [CrossRef]
- Doube, B.M. A functional classification for analysis of the structure of dung beetle assemblages. Ecol. Entomol. 1990, 15, 371–383. [Google Scholar] [CrossRef]
- Estrada, A.; Coates-Estrada, R. Howler monkeys (Alouatta palliata), dung beetles (Scarabaeidae) and seed dispersal: Ecological interactions in the tropical rain forest of Los Tuxtlas, Mexico. J. Trop. Ecol. 1991, 7, 459–474. [Google Scholar] [CrossRef]
- Andresen, E. Dung beetles in a Central Amazonian rainforest and their ecological role as secondary seed dispersers. Ecol. Entomol. 2002, 27, 257–270. [Google Scholar] [CrossRef]
- Kunz, B.K.; Krell, F.T. Habitat differences in dung beetle assemblages in an African savanna–forest ecotone: Implications for secondary seed dispersal. Integr. Zool. 2011, 6, 81–96. [Google Scholar] [CrossRef] [PubMed]
- D’hondt, B.; Bossuyt, B.; Hoffmann, M.; Bonte, D. Dung beetles as secondary seed dispersers in a temperate grassland. Basic Appl. Ecol. 2008, 9, 542–549. [Google Scholar] [CrossRef]
- Cambefort, Y.; Hanski, I. Dung beetle population biology. In Dung Beetle Ecology; Hanski, I., Cambefort, Y., Eds.; Princeton University Press: Princeton, NJ, USA, 1991; pp. 36–50. [Google Scholar] [CrossRef]
- Senra, A. Impacto del manejo del ecosistema del pastizal en la fertilidad natural y sostenibilidad del suelo. Av. Investig. Agropecu 2009, 13, 3–16. [Google Scholar]
- Negro, M.; Rolando, A.; Palestrini, C. The impact of overgrazing on dung beetle diversity in the Italian Maritime Alps. Environ. Entomol. 2011, 40, 1081–1092. [Google Scholar] [CrossRef]
- Arellano, L.; Noriega, J.A.; Ortega-Martínez, I.J.; Rivera, J.D.; Correa, C.M.A.; Gómez-Cifuentes, A.; Ramírez-Hernández, A.; Barragán, F. Dung Beetles (Coleoptera: Scarabaeidae) in Grazing Lands of the Neotropics: A Review of Patterns and Research Trends of Taxonomic and Functional Diversity, and Functions. Front. Ecol. Evol. 2023, 11, 1084009. [Google Scholar] [CrossRef]
- Demeza-Deara, A.; Arellano, L. Remoción de estiércol por escarabajos coprófagos (Coleoptera: Scarabaeidae: Scarabaeinae) en un gradiente de duración de la actividad ganadera, en Chamela, Jalisco. Entomol. Mex. 2013, 12, 611–616. [Google Scholar]
- Demeza-Deara, A. Efectos del Régimen de Manejo Ganadero en la Magnitud de la Función de Remoción de Estiércol y en la Estructura Funcional de Escarabajos (Coleoptera: Scarabaeinae) en un Paisaje de Bosque Tropical Seco en Jalisco. Bachelor’s Thesis, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mexico, 2014. [Google Scholar]
- Mesquita-Filho, W.; Flechtmann, C.A.; Godoy, W.A.; Bjornstad, O.N. The impact of the introduced Digitonthophagus gazella on a native dung beetle community in Brazil during 26 years. Biol. Invasions 2018, 20, 963–979. [Google Scholar] [CrossRef]
- Halffter, G.; Arellano, L. Response of dung beetle diversity to human-induced changes in a tropical landscape. Biotropica 2002, 34, 144–154. [Google Scholar] [CrossRef]
- Amézquita, S.; Favila, M.E. Removal rates of native and exotic dung by dung beetles (Scarabaeidae: Scarabaeinae) in a fragmented tropical rain forest. Environ. Entomol. 2010, 39, 328–336. [Google Scholar] [CrossRef]
- Favila, M.E. Historical, biogeographical and ecological factors explain the success of some native dung beetles after the introduction of cattle in Mexico. Pastos 2012, 42, 161–181. [Google Scholar]
- Ocampo-Castillo, J.; Andresen, E. Interacciones entre semillas y escarabajos del estiércol (Scarabaeinae) en un bosque tropical seco. Tip 2018, 21, 24–33. [Google Scholar] [CrossRef]
- Kohlmann, B. A preliminary study of the invasion and dispersal of Digitonthophagus gazella (Fabricius, 1797) in Mexico (Coleoptera: Scarabaeidae: Scarabaeinae). Acta Zool. Mex. (Nueva Ser.) 1994, 61, 35–42. [Google Scholar] [CrossRef]
- Montes de Oca, E.; Halffter, G. Invasion of Mexico by two dung beetles previously introduced into the United States. Stud. Neotrop. Fauna Environ. 1998, 33, 37–45. [Google Scholar] [CrossRef]
- Noriega, J.A.; Moreno, J.; Otavo, S. Quince años del arribo del escarabajo coprófago Digitonthophagus gazella (Fabricius, 1787) (Coleoptera: Scarabaeidae) a Colombia: Proceso de invasión y posibles efectos de su establecimiento. Biota Colomb. 2011, 12, 35–44. [Google Scholar]
- Arellano, L. Escarabajos del estiércol: Invasores o exóticos. Cienc. Y Desarro. 2012, Sep–Oct, 64–69. [Google Scholar]
- Lee, J.M.; Peng, Y.S. Influence of manure availability and nesting density on the progeny size of Onthophagus gazella. Environ. Entomol. 1982, 11, 38–41. [Google Scholar] [CrossRef]
- Anduaga, S. Impact of the activity of dung beetles (Coleoptera: Scarabaeidae: Scara-baeinae) inhabiting pasture land in Durango, Mexico. Environ. Entomol. 2004, 33, 1306–1312. [Google Scholar] [CrossRef]
- Anderson, J.R.; Loomis, E.C. Exotic dung beetles in pasture and range land ecosystems. Calif. Agric. 1978, 32, 31–32. [Google Scholar]
- Blume, R.R. Euoniticellus intermedius (Coleoptera: Scarabaeidae): Description of adults and immatures and biology of adults. Environ. Entomol. 1984, 13, 1064–1068. [Google Scholar] [CrossRef]
- Blume, R.R.; Aga, A. Onthophagus gazella F.: Progress of experimental release in South Texas [USA]. Folia Entomol. Mex. 1978, 39–40, 190–191. [Google Scholar]
- Pickett, P.S.; White, S.T.A. The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: New York, NY, USA, 1985; p. 472. [Google Scholar] [CrossRef]
- Halffter, G.; Favila, M.E. The Scarabaeinae (Insecta: Coleoptera) an animal group for analysing, inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biol. Int. 1993, 27, 15–21. [Google Scholar]
- Barragán, F.; Moreno, C.E.; Escobar, F.; Halffter, G.; Navarrete, D. Negative impacts of human land use on dung beetle functional diversity. PLoS ONE 2011, 6, e17976. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.; Korasaki, V.; Andresen, E.; Louzada, J. Dung Beetle Community and functions along a habitat-disturbance gradient in the Amazon: A rapid assessment of ecological functions associated to biodiversity. PLoS ONE 2013, 8, e57786. [Google Scholar] [CrossRef]
- Arellano, L.; León-Cortés, J.L.; Halffter, G.; Montero, J. Acacia woodlots, cattle and dung beetles (Coleoptera: Scarabaeinae) in a Mexican silvopastoral landscape. Rev. Mex. Biodivers. 2013, 84, 650–660. [Google Scholar] [CrossRef]
- Garcia, E. Modificaciones al Sistema de Clasificación Climática de Koppen, (Para Adaptarlo a las Condiciones de la República Mexicana), 4th ed.; Offset Larios: Mexico City, Mexico, 1988; 217p. [Google Scholar]
- García-Oliva, F.; Camou, A.; Maass, J.M. El clima de la región central de la costa del Pacífico mexicano. In Historia Natural De Chamela, 1st ed.; Noriega, F., Vega, J., García-Aldrete, A., Quesada, M., Eds.; UNAM: Mexico City, Mexico, 2002; pp. 3–10. [Google Scholar]
- CONAGUA/SMN. Información Climatológica. Normales Climatológicas por Estado. Available online: http://smn.conagua.gob.mx/es/climatologia/informacion-climatologica (accessed on 25 January 2020).
- Ceballos, G.; Szekely, A.; García, A.; Rodríguez, P.; Noguera, F. Programa de Manejo de la Reserva de la Biosfera Chamela-Cuixmala; Instituto Nacional de Ecología, SEMARNAT: Mexico City, Mexico, 1999; 141p. [Google Scholar]
- Ayuntamiento de la Huerta, J. Plan Municipal de Desarrollo y Gobernanza 2018–2021. 37p. Available online: https://transparencia.info.jalisco.gob.mx/sites/default/files/Plan%20Municipal%20de%20Desarrollo%202018-2021_8.pdf (accessed on 13 June 2020).
- Ceballos, G.; Miranda, A. Guía de Campo de los Mamíferos de la Costa de Jalisco, México, 1st ed.; Fundación Ecológica de Cuixmala, A. C. and Universidad Nacional Autónoma de México: Mexico City, Mexico, 2000; 502p. [Google Scholar]
- Larsen, T.H.; Forsyth, A. Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 2005, 37, 322–325. [Google Scholar] [CrossRef]
- Arellano, L. A novel method for measuring dung removal by tunneler dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) in pastures. Coleopt. Bull. 2016, 70, 1–4. [Google Scholar] [CrossRef]
- Andresen, E. Seed dispersal by monkeys and the fate of dispersed seeds in a Peruvian rain forest. Biotropica 1999, 31, 145–158. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 10 February 2018).
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Moreno, C.E.; Barragán, F.; Pineda, E.; Pavón, N. Reanálisis de la diversidad alfa: Alternativas para interpretar y comparar información sobre comunidades ecológicas. Rev. Mex. Biodiv. 2011, 82, 1249–1261. [Google Scholar] [CrossRef]
- Shepard, R.N. Metric structures in ordinal data. J. Math. Psychol. 1966, 3, 287–315. [Google Scholar] [CrossRef]
- Oksanen, J. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. 2015. p. 42. Available online: https://john-quensen.com/wp-content/uploads/2018/10/Oksanen-Jari-vegantutor.pdf (accessed on 18 July 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-3. Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 June 2018).
- Ewers, R.M.; Didham, R.K. Continuous response functions for quantifying the strength of edge effects. J. App. Ecol. 2006, 43, 527–536. [Google Scholar] [CrossRef]
- Zurita, G.; Péer, G.; Bellocq, M.I.; Hansbauer, M.M. Edge effects and their influence on habitat suitability calculations: A continuous approach applied to birds of the Atlantic forest. J. App. Ecol. 2012, 49, 503–512. [Google Scholar] [CrossRef]
- Villada-Bedoya, S.; Cultid-Medina, C.A.; Escobar, F.; Guevara, R.; Zurita, G. Edge effects on dung beetle assemblages in an Andean mosaic of forest and coffee plantations. Biotropica 2017, 49, 195–205. [Google Scholar] [CrossRef]
- Nummelin, M.; Hanski, I. Dung beetles of the Kibale Forest, Uganda; comparison between virgin and managed forests. J. Trop. Ecol. 1989, 5, 349–352. [Google Scholar] [CrossRef]
- Davis, A.J. Species richness of dung-feeding beetles (Coleoptera: Aphodiidae, Scarabaeidae, Hybosoridae) in tropical rainforest at Danum Valley, Sabah, Malaysia. Coleopt. Bull. 2000, 54, 221–231. [Google Scholar] [CrossRef]
- Davis, A.J.; Holloway, J.D.; Huijbregts, H.; Krikken, J.; Kirk-Spriggs, A.H.; Sutton, S.L. Dung beetles as indicators of change in the forests of northern Borneo. J. App. Ecol. 2001, 38, 593–616. [Google Scholar] [CrossRef]
- Estrada, A.; Coates-Estrada, R. Dung beetles in continuous forest, forest fragments and in an agricultural mosaic habitat island at Los Tuxtlas, Mexico. Biodiv. Conserv. 2002, 11, 1903–1918. [Google Scholar] [CrossRef]
- Vulinec, K. Dung Beetle Communities and Seed Dispersal in Primary Forest and Disturbed Land in Amazonia. Biotropica 2006, 34, 297–309. [Google Scholar] [CrossRef]
- Escobar, F. Diversity and composition of dung beetle (Scarabaeinae) assemblages in a heterogeneous Andean landscape. Trop. Zool. 2004, 17, 123–136. [Google Scholar] [CrossRef]
- Davis, A.L.; Philips, T.K. Effect of deforestation on a southwest Ghana dung beetle assemblage (Coleoptera: Scarabaeidae) at the periphery of Ankasa conservation area. Environ. Entomol. 2005, 34, 1081–1088. [Google Scholar] [CrossRef]
- Quintero, I.; Roslin, T. Rapid recovery of dung beetle communities following habitat fragmentation in Central Amazonia. Ecology 2005, 86, 3303–3311. [Google Scholar] [CrossRef]
- Scheffler, P.Y. Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. J. Trop. Ecol. 2005, 21, 9–19. [Google Scholar] [CrossRef]
- Pineda, E.; Moreno, C.E.; Escobar, F.; Halffter, G. Frog, bat, and dung beetle diversity in the cloud forest and coffee agroecosystems of Veracruz, Mexico. Conserv. Biol. 2005, 19, 400–410. [Google Scholar] [CrossRef]
- Arellano, L.; Favila, M.E.; Huerta, C. Diversity of dung and carrion beetles in a disturbed Mexican tropical montane cloud forest and on shade coffee plantations. Biodiv. Conserv. 2005, 14, 601–615. [Google Scholar] [CrossRef]
- Lee, J.M.; Peng, Y.S. Influence of adult size of Onthophagus gazella on manure pat degradation, nest construction and progeny size. Environ. Entomol. 1981, 10, 626–630. [Google Scholar] [CrossRef]
- Janzen, D.H. Seasonal change in abundance of large nocturnal dung beetles (Scarabaeidae) in a Costa Rican deciduous forest and adjacent horse pasture. Oikos 1983, 41, 274–283. [Google Scholar] [CrossRef]
- Escobar, F. Estudios de la comunidad de coleópteros coprófagos (Scarabaeidae) en un remanente de bosque seco al norte del Tolima, Colombia. Caldasia 1997, 19, 419–430. [Google Scholar] [CrossRef]
- Walker, B.; Kinzig, A.; Langridge, J. Plant attribute diversity, resilience, and ecosystem function: The nature and significance of dominant and minor species. Ecosystems 1999, 2, 95–113. [Google Scholar] [CrossRef]
- Cosyns, E.; Claerbout, S.; Lamoot, I.; Hoffmann, M. Endozoochorous seed dispersal by cattle and horse in a spatially heterogeneous landscape. Plant Ecol. 2005, 178, 149–162. [Google Scholar] [CrossRef]
- Miranda, C.H.B.; Do Santos, J.C.C.; Bianchin, I. Contribution of Onthophagus gazella to soil fertility improvement by bovine fecal mass incorporation into the soil. 1: Greenhouse studies. Rev. Bras. Zootec. 1998, 27, 681–685. [Google Scholar]
- Wicklow, D.T.; Kumar, R.; Lloyd, J.E. Germination of blue grama r seeds buried by dung beetles (Coleoptera: Scarabaeidae). Environ. Entomol. 1984, 13, 878–881. [Google Scholar] [CrossRef]
- Andresen, E. Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography 2003, 26, 87–97. [Google Scholar] [CrossRef]
- Bang, H.S.; Lee, J.H.; Kwon, O.S.; Na, Y.E.; Jang, Y.S.; Kim, W.H. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil Ecol. 2005, 29, 165–171. [Google Scholar] [CrossRef]
- Anduaga, S.; Huerta, C. Importance of dung incorporation activity by three species of coprophagous beetle (Coleoptera: Scarabaeidae: Scarabaeinae) macrofauna in pastureland on “La Michilía” Biosphere Reserve in Durango, Mexico. Environ. Entomol. 2007, 36, 555–559. [Google Scholar] [CrossRef]
- Nervo, B.; Caprio, E.; Celi, L.; Lonati, M.; Lombardi, G.; Falsone, G.; Iussig, G.; Palestrini, C.; Said-Pullicino, D.; Rolando, A. Ecological functions provided by dung beetles are interlinked across space and time: Evidence from 15N isotope tracing. Ecology 2017, 98, 433–446. [Google Scholar] [CrossRef]
- Arellano, L.; Douterlungne, D.; Torres, A.; Ramírez-Hernández, A.; Barragán, F. Escarabajos estercoleros: Adaptación y mitigación al cambio climático en sistemas agroforestales pecuarios. In Tecnologías Agroforestales Para la Adaptación y Mitigación al -Cambio Climático—Opciones y Perspectivas; Palma, J.M., Torres Rivera, J.A., Valdés-Velarde, E., Coordinador; VI. 3; Dirección General de Publicaciones de la Universidad de Colima: Colima, Mexico, 2022; pp. 237–259. Available online: http://ww.ucol.mx/publicacionesenlinea/?docto=527 (accessed on 10 January 2023).
- Pedersen, K.M.; Blüthgen, N. Seed size and pubescence facilitate secondary dispersal by dung beetles. Biotropica 2022, 54, 215–225. [Google Scholar] [CrossRef]
- Laverde Angarita, L.J.; Castellanos, M.C.; Stevenson, P. Dispersión sencundaria de semillas por escarabajos coprófagos (Scarabaeidae) a partir de heces de churucos (Lagothrix lagothricha) en el Parque Nacional Tinigua, Colombia. Univ. Sci. 2002, 7, 17–29. [Google Scholar]
- Andresen, E.; Levey, D.J. Effects of dung and seed size on secondary dispersal, seed predation, and seedling establishment of rain forest trees. Oecologia 2004, 139, 45–54. [Google Scholar] [CrossRef]
- Andresen, E. Effects of season and vegetation type on community organization of dung beetles in a tropical dry forest. Biotropica 2005, 37, 291–300. [Google Scholar] [CrossRef]
- Andresen, E. Interacción entre primates, semillas y escarabajos coprófagos en bosques húmedos tropicales: Un caso de diplocoria. Univ. Cienc. 2005, 2, 73–84. [Google Scholar]
- Ponce-Santizo, G.; Andresen, E.; Cano, E.; Cuarón, A.D. Dispersión Primaria de Semillas por Primates y Dispersión Secundaria por Escarabajos Coprófagos en Tikal, Guatemala. Biotropica 2006, 38, 390–397. [Google Scholar] [CrossRef]
- Andresen, E. Dung beetle assemblages in primary forest and disturbed habitats in a tropical dry forest landscape in western Mexico. J. Insect Conserv. 2008, 12, 639–650. [Google Scholar] [CrossRef]
- Santos-Heredia, C.; Andresen, E.; Zárate, D.A. Secondary seed dispersal by dung beetles in a Colombian rain forest: Effects of dung type and defecation pattern on seed fate. J. Trop. Ecol. 2010, 26, 355–364. [Google Scholar] [CrossRef]
- Culot, L.; Mann, D.J.; Muñoz Lazo, F.J.; Huynen, M.C.; Heymann, E.W. Tamarins and dung beetles: An efficient diplochorous dispersal system in the Peruvian Amazonia. Biotropica 2011, 43, 84–92. [Google Scholar] [CrossRef]
- Santos-Heredia, C.; Andresen, E.; Stevenson, P. Secondary seed dispersal by dung beetles in an Amazonian forest fragment of Colombia: Influence of dung type and edge effect. Integr. Zool. 2011, 6, 399–408. [Google Scholar] [CrossRef]
- Guevara, S.; Laborde, J. Monitoring seed dispersal at isolated standing trees in tropical pastures: Consequences for local species availability. In Frugivory and Seed Dispersal: Ecological and Evolutionary Aspects. Advances in Vegetation Science; Fleming, T.H., Estrada, A., Eds.; Springer: Dordrecht, The Netherlands, 1993; Volume 15, pp. 84–92. [Google Scholar] [CrossRef]
- Ferguson, B.G.; Vandermeer, J.; Morales, H.; Griffith, D.M. Post-agricultural succession in El Petén, Guatemala. Conserv. Biol. 2003, 17, 818–828. [Google Scholar] [CrossRef]
- Milotić, T.; Quidé, S.; Van Loo, T.; Hoffmann, M. Linking functional group richness and ecosystem functions of dung beetles: An experimental quantification. Oecologia 2017, 183, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Milotić, T.; Baltzinger, C.; Eichberg, C.; Eycott, A.E.; Heurich, M.; Müller, J.; Noriega, J.A.; Menendez, R.; Stadler, J.; Ádám, R.; et al. Dung beetle assemblages, dung removal and secondary seed dispersal: Data from a large-scale, multi-site experiment in the Western Palaearctic. Front. Biogeogr. 2018, 10, e37829. [Google Scholar] [CrossRef]
- Santos-Heredia, C.; Andresen, E.; Zárate, D.A.; Escobar, F. Dung beetles and their ecological functions in three agroforestry systems in the Lacandona rainforest of Mexico. Biodiv. Conserv. 2018, 27, 2379–2394. [Google Scholar] [CrossRef]
- Ferguson, B.G.; Alemán-Santillán, T.; Jiménez-Ferrer, G.; Nahed-Toral, J.; Carmona-Muñóz, I.; Gómez-Castro, H. Desarrollo regional. In Ganadería, Desarrollo y Ambiente: Una Visión Para Chiapas, 1st ed.; Alemán Santillán, T., Ferguson, B.G., Medina Jonapá, F.J., Eds.; ECOSUR-Fundación Produce Chiapas: Tapachula, Chiapas, Mexico, 2007; pp. 89–98. [Google Scholar]
- Miceli-Méndez, C.L.; Ferguson, B.G.; Ramírez-Marcial, N. Seed dispersal by cattle: Natural history and applications to neotropical forest restoration and agroforestry. In Post-Agricultural Succession Neotrop; Myster, R.W., Ed.; Springer Science Business Media, LCC: New York, NY, USA, 2008; pp. 165–191. [Google Scholar]
- Santos-Heredia, C.; Andresen, E. Upward movement of buried seeds: Another ecological role of dung beetles promoting seedling establishment. J. Trop. Ecol. 2014, 30, 409–417. [Google Scholar] [CrossRef]
- Huerta, C.; Anduaga, S.; López-Portillo, J.; Halffter, G. Use of food and space by tunneler dung beetles (Coleoptera; Scarabaeinae) during reproduction. Environ. Entomol. 2010, 39, 1165–1169. [Google Scholar] [CrossRef]
- Castillo, A.; Godínez, C.; Schroeder, N.; Galicia, C.; Pujadas-Botey, A.; Martínez Hernández, L. El bosque tropical seco en riesgo: Conflictos entre uso agropecuario, desarrollo turístico y provisión de servicios ecosistémicos en la costa de Jalisco, México. Interciencia 2009, 34, 844–850. [Google Scholar]
| Species | O | F1 (0) | F2 (0) | F3 (0) | P1 (4) | P2 (10) | P3 (10) | P4 (14) | P5 (18) | P6 (25) | P7 (26) | P8 (30) | P9 (35) | P10 (39) | P11 (40) | T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ateuchus rodriguezi (Borre, 1886) | N | 2 | 0 | 0 | 0 | 1 | 3 | 0 | 7 | 2 | 1 | 5 | 0 | 0 | 0 | 21 |
| Canthon indigaceus Le Conte 1859 | N | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 22 | 1 | 0 | 32 |
| Copris lugubris Boheman, 1868 | N | 5 | 3 | 4 | 8 | 30 | 0 | 7 | 23 | 0 | 9 | 56 | 4 | 0 | 6 | 155 |
| Dichotomius amplicollis (Harold, 1869) | N | 139 | 131 | 100 | 42 | 24 | 1 | 24 | 27 | 1 | 24 | 9 | 2 | 0 | 1 | 525 |
| Dichotomius colonicus (Say, 1835) | N | 40 | 33 | 51 | 23 | 15 | 8 | 21 | 3 | 2 | 21 | 7 | 35 | 3 | 13 | 275 |
| Digitonthophagus gazella (Fabricius, 1787) | E | 0 | 0 | 0 | 27 | 16 | 96 | 11 | 8 | 36 | 16 | 29 | 82 | 99 | 182 | 542 |
| Euoniticellus intermedius (Reiche) | E | 0 | 0 | 0 | 96 | 8 | 556 | 51 | 43 | 299 | 64 | 176 | 337 | 158 | 412 | 1788 |
| Onthophagus igualensis Bates, 1887 | N | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| Onthophagus landolti Harold, 1880 | N | 0 | 0 | 0 | 10 | 0 | 0 | 1 | 3 | 3 | 0 | 0 | 4 | 0 | 0 | 21 |
| Phanaeus obliquans Bates, 1887 | N | 58 | 91 | 56 | 45 | 20 | 2 | 47 | 28 | 42 | 17 | 18 | 10 | 0 | 4 | 438 |
| Phanaeus tridens Castelnau, 1840 | N | 3 | 2 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 13 |
| Abundance | 247 | 260 | 212 | 251 | 122 | 668 | 162 | 144 | 387 | 153 | 303 | 497 | 261 | 618 | ||
| Zero-order diversity (species richness: q0) | 6 | 5 | 5 | 7 | 9 | 7 | 7 | 9 | 8 | 8 | 9 | 9 | 4 | 6 | ||
| First-order diversity (q1) | 3.09 | 2.89 | 3.15 | 5.32 | 6.81 | 2.34 | 5.05 | 6.13 | 2.21 | 5.15 | 3.78 | 2.93 | 2.1 | 2.22 | ||
| Second-order diversity (q2) | 2.5 | 2.54 | 2.85 | 4.36 | 6.04 | 1.73 | 4.37 | 5.15 | 1.61 | 4.07 | 2.59 | 2.02 | 1.95 | 1.88 |
| Linear | Exponential | ||||||
|---|---|---|---|---|---|---|---|
| Variable ~~Duration of Livestock Grazing | R2 | p-Value | R2 | p-Value | Relationship | ||
| Species richness (q0) | 0.08 | 0.39 | 0.08 | 0.70 | - | ||
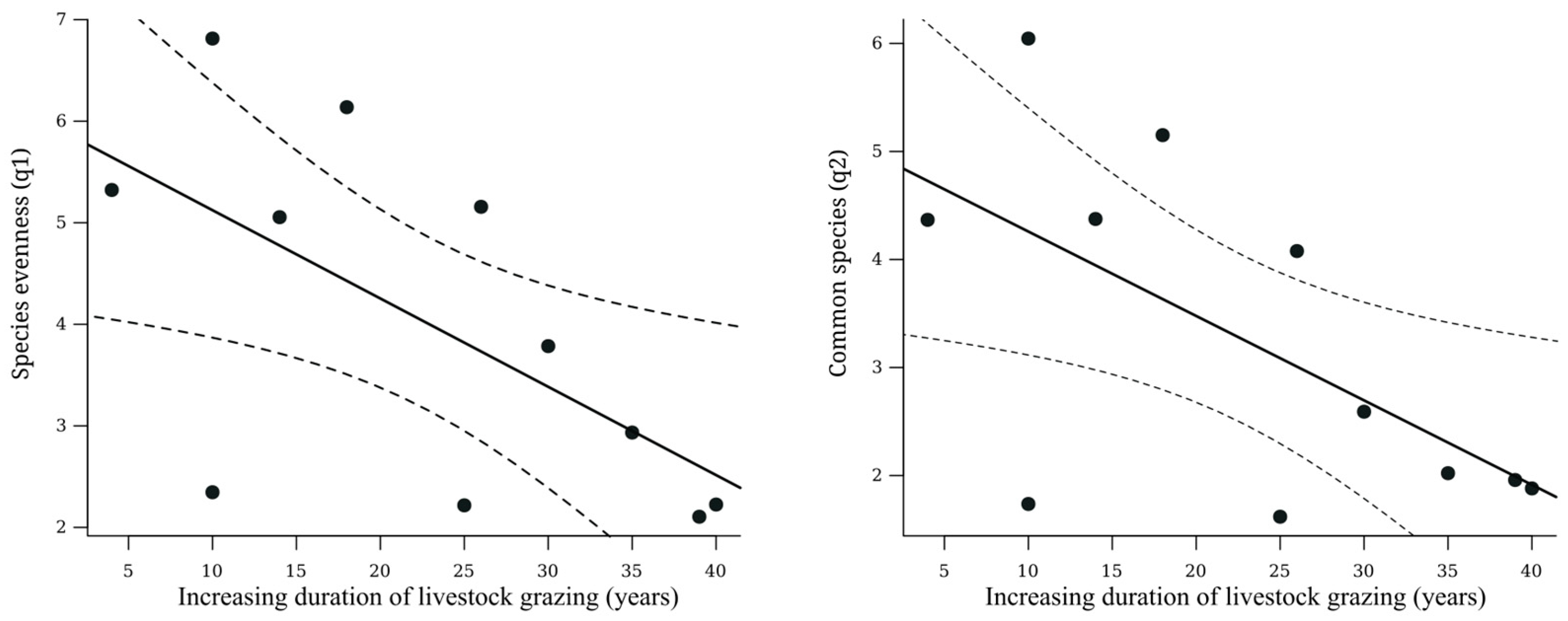
| Shannon diversity (q1) | 0.39 | 0.04 | * | 0.39 | 0.14 | Negative | |
| Simpson diversity (q2) | 0.38 | 0.04 | * | 0.38 | 0.15 | Negative | |
| Total abundance | 0.09 | 0.36 | 0.18 | 0.46 | - | ||
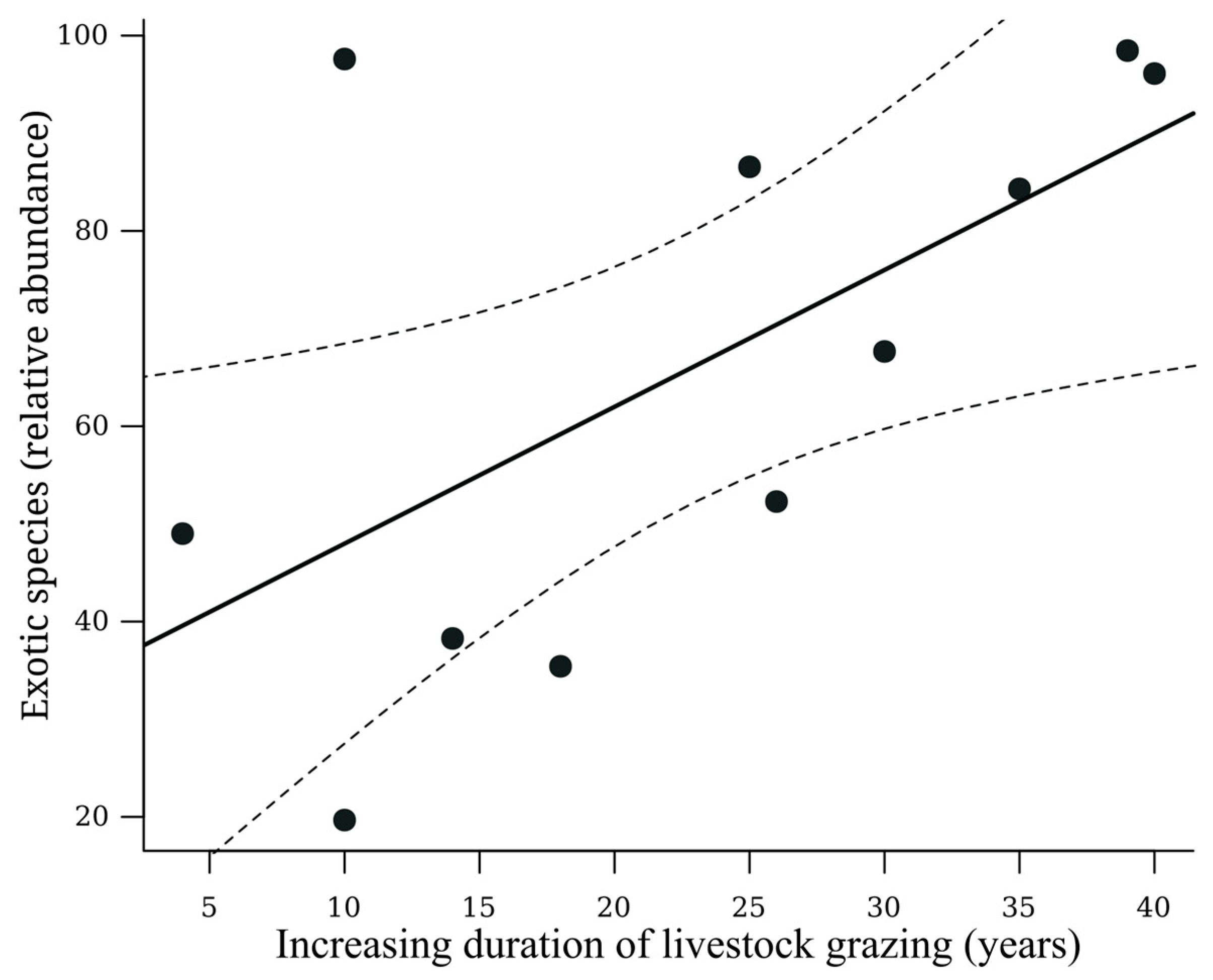
| Exotic species (+) | 0.38 | 0.04 | * | 0.44 | 0.10 | Positive | |
| A. rodriguezi (+) | 0.02 | 0.69 | 0.00 | 1.00 | - | ||
| C. indigaceus (+) | 0.05 | 0.52 | 0.00 | 1.00 | - | ||
| C. lugubris (+) | 0.08 | 0.39 | 0.08 | 0.71 | - | ||
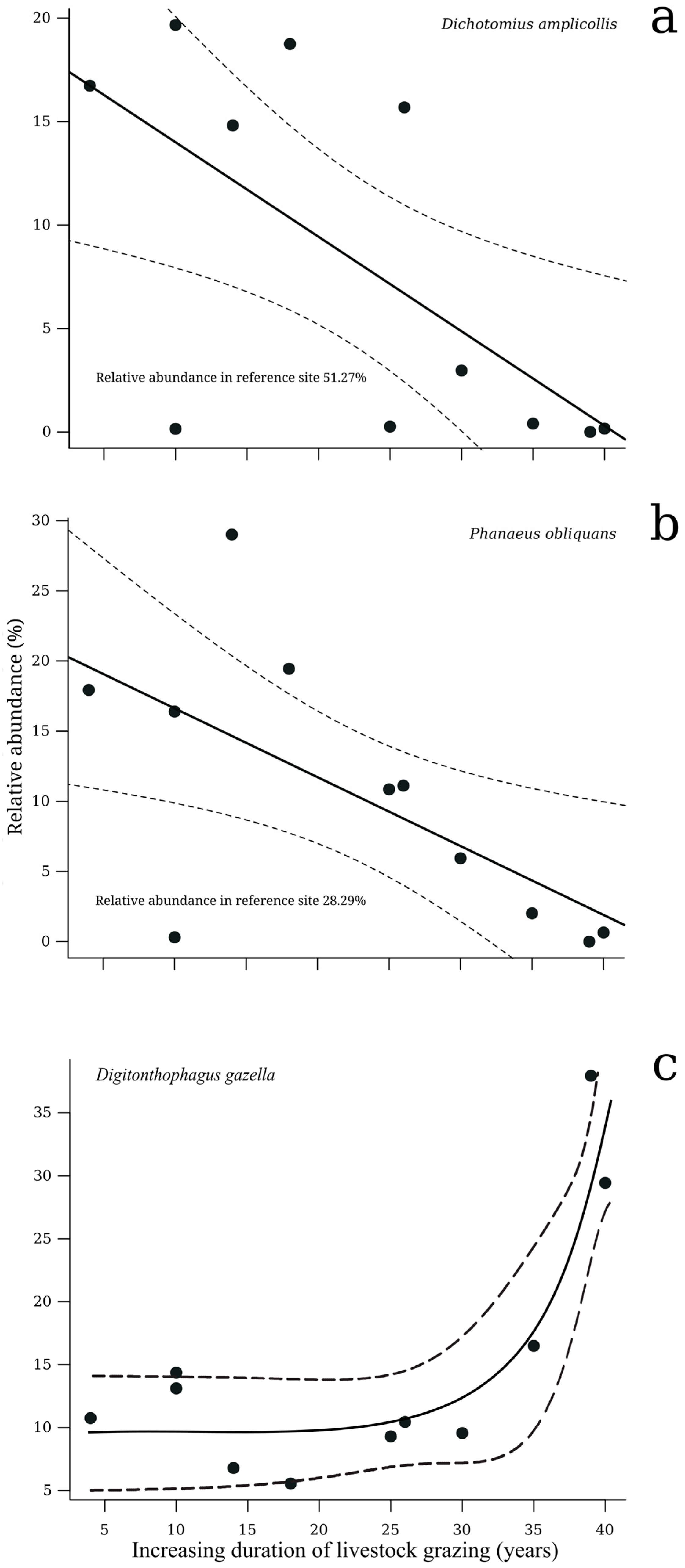
| D. amplicollis (+) | 0.43 | 0.03 | * | 0.43 | 0.11 | Positive | |
| D. colonicus (+) | 0.17 | 0.22 | 0.17 | 0.49 | - | ||
| D. gazella (+) | 0.41 | 0.03 | * | 0.83 | 0.01 | ** | Positive |
| E. intermedius (+) | 0.22 | 0.14 | 0.23 | 0.36 | - | ||
| O. igualensis (+) | 0.00 | 0.87 | 0.00 | 1.00 | - | ||
| O. landolti (+) | 0.26 | 0.11 | 0.00 | 1.00 | - | ||
| P. obliquans (+) | 0.41 | 0.03 | * | 0.41 | 0.12 | Positive | |
| P. tridens (+) | 0.10 | 0.34 | 0.10 | 0.65 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Trejo, J.J.; Dáttilo, W.; Zurita, G.; Arellano, L. Duration of Cattle Ranching Affects Dung Beetle Diversity and Secondary Seed Removal in Tropical Dry Forest Landscapes. Insects 2024, 15, 749. https://doi.org/10.3390/insects15100749
Morales-Trejo JJ, Dáttilo W, Zurita G, Arellano L. Duration of Cattle Ranching Affects Dung Beetle Diversity and Secondary Seed Removal in Tropical Dry Forest Landscapes. Insects. 2024; 15(10):749. https://doi.org/10.3390/insects15100749
Chicago/Turabian StyleMorales-Trejo, Juan J., Wesley Dáttilo, Gustavo Zurita, and Lucrecia Arellano. 2024. "Duration of Cattle Ranching Affects Dung Beetle Diversity and Secondary Seed Removal in Tropical Dry Forest Landscapes" Insects 15, no. 10: 749. https://doi.org/10.3390/insects15100749
APA StyleMorales-Trejo, J. J., Dáttilo, W., Zurita, G., & Arellano, L. (2024). Duration of Cattle Ranching Affects Dung Beetle Diversity and Secondary Seed Removal in Tropical Dry Forest Landscapes. Insects, 15(10), 749. https://doi.org/10.3390/insects15100749







