Simple Summary
Little is known about the molecular data and phylogenetic relationships of scoliid wasps. In this study, we determined 10 mitochondrial genomes representing eight genera in two tribes of the family Scoliidae distributed in China, compared and analyzed all Chinese genus representatives for general features and rearrangement. Phylogenetic relationships between them were inferred using MrBayes and IQtree based on four data matrices. Gene rearrangement was chosen for ancestral state reconstruction. Our results support most of the relationships between genera and tribes of Scoliidae in former morphological studies except for the Colpa group, which is proved to be closer to Scoliini based on our genome features, phylogenetic analyses and some morphological evidence.
Abstract
Scoliidae, also known as scarab hunters or flower wasps, are important in the biological control of scarabs and for pollination. Mitogenomic and phylogenetic studies are rare for this group. In this study, 10 mitochondrial genomes representing eight genera in two tribes of the family Scoliidae were determined. The general features and rearrangements of the mitochondrial genomes for 15 Scoliidae species representing all genera distributed in China were described and compared and the phylogenetic relationships among them were inferred using MrBayes and IQtree based on four data matrices. Most sequences of Scoliidae have one extra trnM gene. Species belonging to Campsomerini have lower A + T content than all Scoliini species except for Colpa tartara in this study. The AT-skew is positive in 7 out of 15 species. All 15 Scoliidae sequences have similar conserved gene arrangements with the same arrangements of PCGs and rRNA genes, except for Campsomeriella annulata. The tRNA genes have the highest frequency of rearrangement, and C. tartara is always rearranged as in its Scoliini counterparts. Our phylogenetic results support most of the relationships between genera and tribes of Scoliidae in former morphological studies. However, Colpa tartara is proved to be closer to Scoliini according to genome features, phylogenetic analyses and some morphological evidence, which challenges the former attribution of the Colpa group.
1. Introduction
Scoliid wasps (Hymenoptera, Scoliidae), also known as scarab hunters or flower wasps, are pollinators for various plants and larval parasitoids of scarabaeoid beetles [1]. These wasps comprise approximately 560 extant and 19 extinct species worldwide [2,3]. This family traditionally had been grouped with many Tiphiidae, particularly Anthoboscinae in a single superfamily Scolioidea for sharing a general habitus as solitary fossorial aculeate wasps [4]. Brothers [5] (as Königsmann [6]) reevaluated the relationships of the Aculeata and combined the traditional superfamilies Scolioidea, Pompiloidea, Vespoidea and Formicoidea into the superfamily Vespoidea, since these formed a monophyletic clade. The family Scoliidae was also incorporated into Vespoidea later in the phylogenetic analysis of morphological data by Brothers [7]. Phylogenetic relationships among major lineages of the Aculeata have been mainly studied in the 21st century [8,9,10,11]. The Scoliidae was elevated to superfamily level inside the clade Aculeata based on morphological and molecular data by Pilgrim et al. [9]; this was also supported by Branstetter et al. [8], Peters et al. [12] and Blaimer et al. [13], based on transcriptomes and ultraconserved elements (UCE), as well as Zheng et al. [11] and Huang et al. [14], based on mitochondrial genomes.
Scoliid wasps are difficult to determine, as suggested by the description of many subspecies and varieties and shown by their extreme sexual dimorphism. Phylogenetic research on this group has been rare: only a few scattered notes were published during the last century. All scoliid species were classified into Scoliinae and Campsomerinae [15,16] before a new subfamily Proscoliinae was found and the rest were subjected to the subfamily Scoliinae with two tribes, Scoliini and Campsomerini, designated by Rasnitsyn in 1977 [17]. The hierarchical levels were supported by Brothers [7] and adopted by many researchers [1,2,18,19,20,21,22,23,24,25]. Later, Rasnitsyn [26] added an extinct subfamily, Archaeoscoliinae. Argaman [27] divided the family into 143 genera and four subfamilies (Proscoliinae, Colpinae, Scoliinae and Campsomerinae) but his arrangement was not followed by most taxonomists because of insufficient diagnoses without taxonomic revisions. For instance, Osten [2] deliberately ignored it and listed only 43 valid genera. Nevertheless, Argaman discovered some valuable characters for morphological taxonomy and recognised the placement problem of the Colpini or Trielidini (here, we use the latter based on Betrem and Bradley [16], according to the ICZN Code) species. Moreover, the campsomerine species were suggested to be elevated to the subfamily level by Cornelis van Achterberg for their extreme sexual dimorphism and distinct discrepancy in body size of both sexes compared to other groups. No molecular study had attempted to reconstruct a phylogeny of the family before Khouri et al. [28] made the first attempt using UCE data. They confirmed the position of Proscolia as the sister to all other extant scoliids and supported the sister group relationship between Colpa and the Scoliini in their preprint (not formally published), with an emphasis on a dire need for revision of the higher-level taxonomy of the Scoliidae.
In China, only the subfamily Scoliinae (including the Campsomerini) has been reported and it comprises 11 genera with 61 known species currently [24,25,29,30]. The phylogenetic tree [1] based on the COI sequences of 22 morphospecies belonging to nine genera in two tribes of the subfamily Scoliinae from Southern China corroborates the tribal system of Day et al. [4], indicating that mitochondrial sequences are phylogenetically informative for addressing the relationships within Scoliidae. However, the relationships among extant genera of the family are far from clear. For example, Trielidini species were not included in the above study. Recent comparative studies of the mitochondrial genomes of the Aculeata show that both gene arrangements and gene sequences provide insights into the phylogeny of the group [11,14,31,32,33]. Compared to other groups of Hymenoptera, however, there is a serious lack of representative mitochondrial genomes of Scoliidae, which is an obstacle to the study of phylogenetic relationships within Scoliidae.
Here, we successfully collected, sequenced and described 10 mitochondrial genomes of Scoliidae using next-generation sequencing. We also compared genome features of 15 Scoliidae species covering all 11 genera known from China and we analyzed phylogenetic relationships and their influences to illuminate evolutionary patterns across Scoliidae.
2. Material and Methods
2.1. Collection, Identification and DNA Extraction
Specimens were collected by hand netting or Malaise traps (MTs) set up in Guangdong, Yunnan, Hainan and Shaanxi except for one specimen of Campsomeriella annulata (Fabricius, 1793) from Bhutan (Table S1). The collected specimens were stored in 100% ethanol and at −80 °C prior to DNA extraction.
Genomic DNA was extracted from the legs of specimens using an Ezup Column Animal Genomic DNA Purification Kit (Sangon Biotech, Shanghai, China) following the manufacturer’s protocols. Extracted genomic DNA was quantified by NanoPhotometer® (IMPLEN, Westlake Village, CA, USA) and Qubit 3.0 (Invitrogen, Life Technologies, Carlsbad, CA, USA) and a Nanodrop 2000c Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Voucher specimens are deposited in the Laboratory of Hunan University of Arts and Science, Changde, China.
2.2. Genome Sequencing, Assembly, Annotation
The extracted genomic DNA of each specimen was sheared into fragments of approximately 350 bp in length using an Ultrasonic Processor Covaris S220 (Covaris, Inc., Woburn, MS, USA). High-throughput sequencing libraries were constructed using an Illumina TruSeq DNA PCR-Free HT Kit and sequenced using an Illumina Novaseq6000 with the strategy of producing 150 bp paired-ends by the Annoroad Gene Tech. (Beijing, China) Co., Ltd. The quality of raw sequencing reads was checked by a FastQC version 0.11.3 [34], and low-quality reads and sites were filtered by a Trimmomatic version 3.2.57 [35].
The target mitochondrial reads were filtered out using BLAST (BLASTn with an E value: 1 × 10−5) against a reference data set containing Hymenoptera mitochondrial genomes via the FastqExtract script [36]. The mitochondrial genomes were assembled by IDBA_UD version 1.1.3 [37] and SPAdes version 3.15.2 [38] with default parameters.
Annotation of the assembled genomes was performed initially by using MITOS Web Server [39]. Start and stop codons of protein-coding genes (PCGs) were manually adjusted in Geneious Prime v11 by referring to the published mitogenomes of Aculeata. All mitochondrial genomes were submitted to GenBank under the accession numbers PP874258−PP874267.
2.3. Genome Feature and Arrangement Analysis
The base compositions and codon usages (RSCU) in each sequence were calculated according to methods described in Yuan et al. [40]. AT-skew and GC-skew were calculated as AT-skew = (A − T)/(A + T) and GC-skew = (G − C)/(G + C) [41]. Gene arrangements of mitochondrial genomes in Scoliidae were compared using 17 representative mitochondrial genomes (Table 1).

Table 1.
Information on the 17 mitochondrial genomes of Hymenoptera used in this study.
2.4. Phylogenetic Inference
A total of 15 species covering all 11 genera traditionally or morphologically accepted Chinese scoliids were used in phylogenetic analyses. Two species representing two families of Ichneumonoidae were chosen as outgroups (Table 1). The nucleotide sequences of 13 protein-coding genes (PCGs) were aligned using G-INS-i algorithms implemented in MAFFT v7.407 [44].
The ambiguously aligned positions were identified and removed by the combination of Aliscore version 2.2 and Alicut version 3.2 to reduce noise [45]. The best partition schemes of the substitution models for the matrix were searched in PartitionFinder v2.1.12 with model selection = BIC and Branch lengths = unlinked between different subsets [46].
Four data matrices for phylogenetic inferences, i.e., DNA sequences of all 1st, 2nd and 3rd positions of codons in protein-coding genes (PCG123), all 1st and 2nd protein-coding genes (PCG12), DNA sequences of all protein-coding and RNA genes (PCG + RNA) and amino acid sequences of all protein-coding genes (AA).
Bayesian inference (BI) analyses in MrBayes v3.2.7a and Maximum Likelihood (ML) analyses in IQtree v1.6.12 [47,48] were used to reconstruct the phylogenetic relationship. In MrBayes analysis, substitution models were applied for each partition chosen by PartitionFinder. Four independent Markov chains were run for 10 million generations, with tree sampling occurring every 1000 generations and a burn-in of 25% of trees. The stationarity of the run was checked by the program Tracer version 1.4.0 (effective sample sizes > 200). In ML analysis, 200 runs were conducted to find the highest-likelihood tree, followed by analysis of 1000 bootstrap replicates.
2.5. Phylogenetic Hypothesis Tests
We examined three conflicting nodes by four-clusters likelihood mapping analysis in TREE-PUZZLE version 5.2 [49]. All four matrices were used for the hypothesis testing. The TVM + F + I + G4 model was applied for PCG123 and PCG12, the GTR + F + I + G4 was for PCG + RNA and the mtZOA + F + R4 was for AA.
Gene rearrangement clusters (1 and 2 with two modes each) were chosen for ancestral state reconstruction. The data about rearrangement of clusters in Scoliidae mitogenomes were mapped on the phylogenetic tree (PCG123 + RNA) using Mesquite v3.81 [50]. The lack of data about the clusters was coded as missing data. We applied maximum parsimony and maximum likelihood reconstruction methods. Depending on the AIC criterion, we used the better fit model for the data: either the Mk1 model (Markov k-state 1parameter model) or the AsymmMk model (asymmetrical Markov k-state 2 parameter model).
3. Results
3.1. General Feature of the Mitochondrial Genomes in Scoliidae
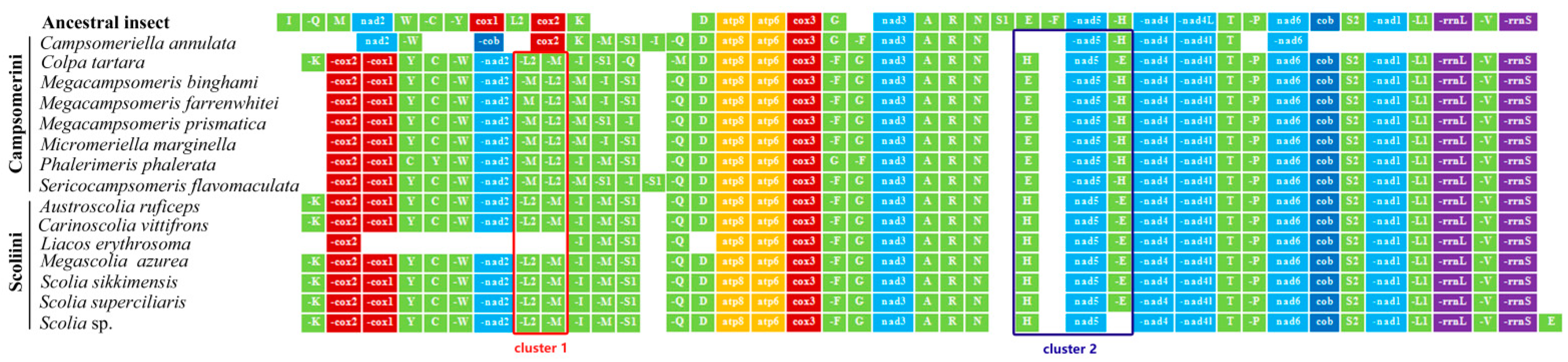
We sequenced mitochondrial genomes of 10 species from Scoliidae, representing eight genera in two tribes from more than 3 Gb of data for each specimen, covering 73% of the scoliid wasp genera distributed in China. Mitochondrial genomes were first reported for seven genera here, viz., Austroscolia, Campsomeriella, Carinoscolia, Liacos, Micromeriella, Phalerimeris and Sericocampsomeris. Ten nearly complete mitogenomes ranged in size from 15,183 bp in Sericocampsomeris flavomaculata to 16,863 bp in Austroscolia ruficeps, except for the shortly assembled Campsomeriella annulata and Liacos erythrosoma with 11,035 bp and 12,273 bp length, respectively. All protein-coding and rRNA genes were obtained except for cox1 and nad1 in Campsomeriella annulata, cox1, cox2 and nad2 in Liacos erythrosoma (Figure 1). Unlike most animal mitochondrial genomes, however, they all (except for the shortly assembled C. annulata and L. erythrosoma) have one extra trnM gene, just like the three sequences of Megacampsomeris published in Huang et al. [14].

Figure 1.
Gene rearrangement in 15 mitochondrial genomes of Scoliidae. Green stands for trnA genes, red stands for Cyclooxygenase genes, light blue stands for NADH dehydrogenase subunits genes, dark blue stands for cytochrome b gene, orange stands for ATP Synthase Membrane Subunits, and purple stands for ribosomal RNA genes. Two clusters (red box for cluster 1 and blue box for cluster 2) are selected for gene rearrangement analysis.
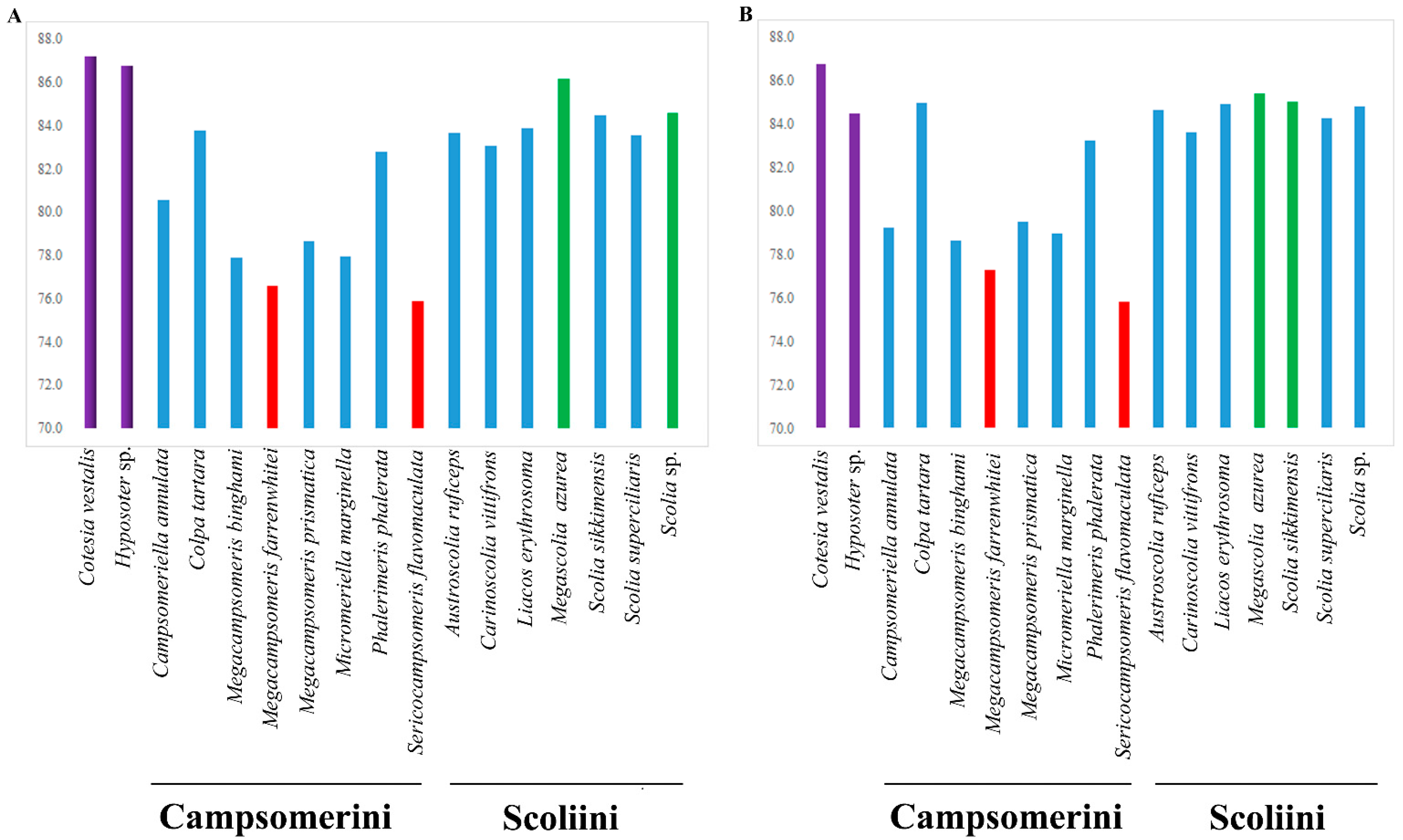
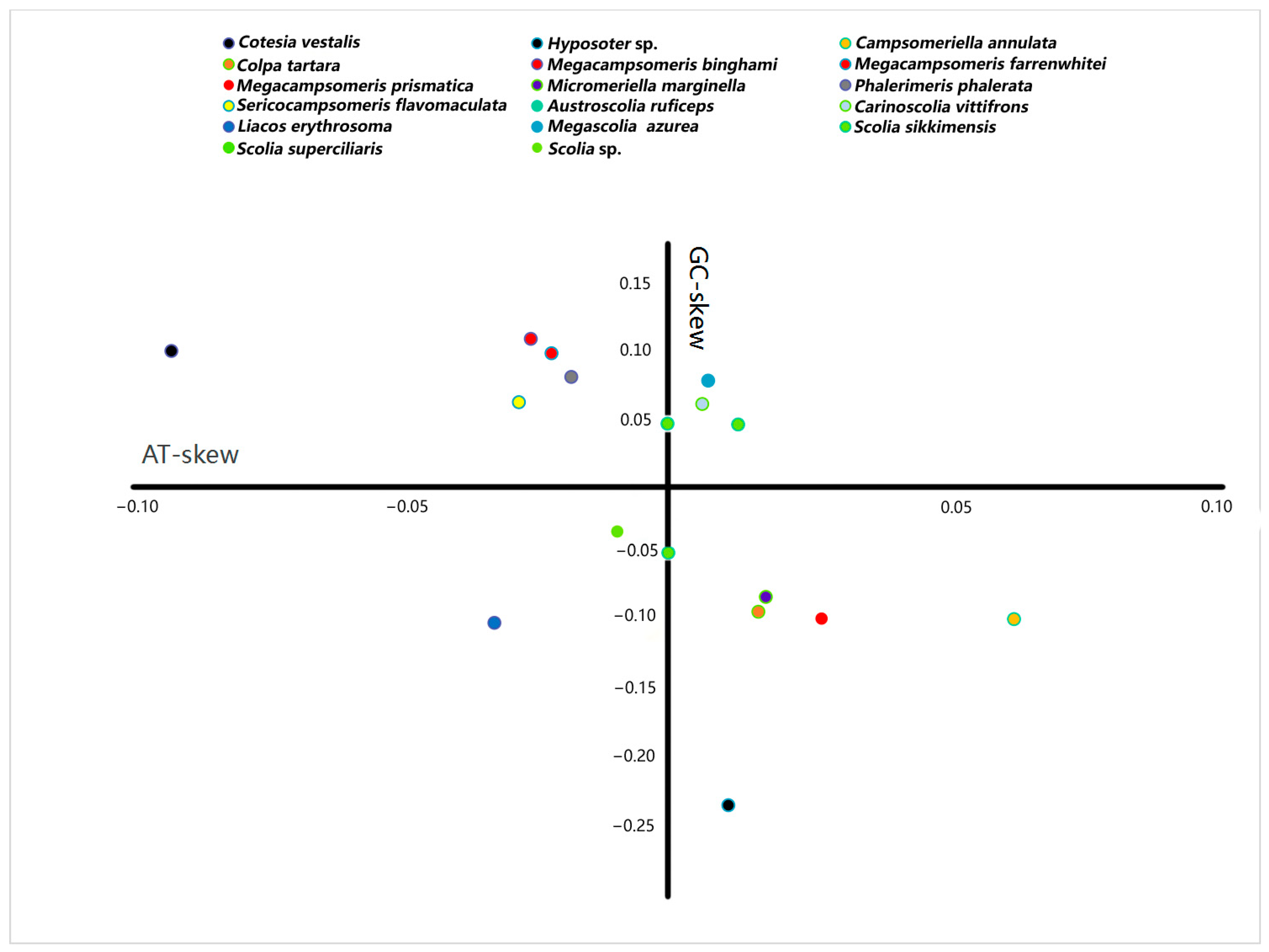
The A + T content for the sequenced region of the mitochondrial genomes in Scoliidae ranged from 75.9% (Sericocampsomeris flavomaculata) to 86.2% (Megascolia azurea). The lowest A + T content was found in the Campsomerini species, while the highest A + T content was found in the Scoliini species. Moreover, when compared with other available mitochondrial genomes from NCBI, all Campsomerini species had a lower A + T content than all Scoliini species in this study except for Colpa tartara, the only representative of Trielidini in China, with a relatively higher value (83.8%) than other Campsomerini representatives (Figure 2A). The A + T content was lower in the Scoliini (81.6%) than in the two outgroup species (87%) from the “Parasitica” group, which corresponded with the other Aculeata species in Zheng et al. [11]. The results were similar to 13 protein-coding genes (Figure 2B). In the Scoliidae, the AT-skew is positive in 7 out of 15 species while the GC-skew is negative in 7 out of 15 species (Figure 3), which is common in the Aculeata (see Zheng et al. [11]). However, AT-skew is positive and the GC-skew is negative in most species of insects [51].
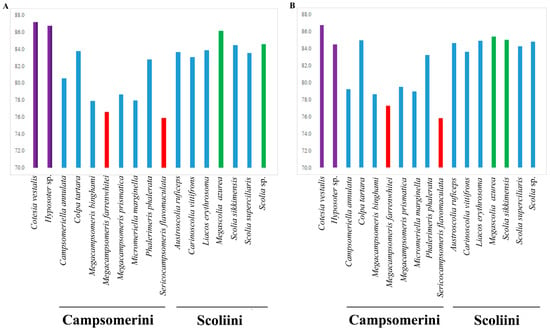
Figure 2.
Base composition of the mitochondrial genomes of the Scoliidae and their hymenopteran outgroups. (A), A + T content for the sequenced region of the mitochondrial genomes in 17 species; (B), A + T content for protein-coding genes in 17 species. Red indicates the two lowest A + T content species. Green marks the two highest A + T content species (except the outgroups). Purple stands for outgroups. Blue is for other species.
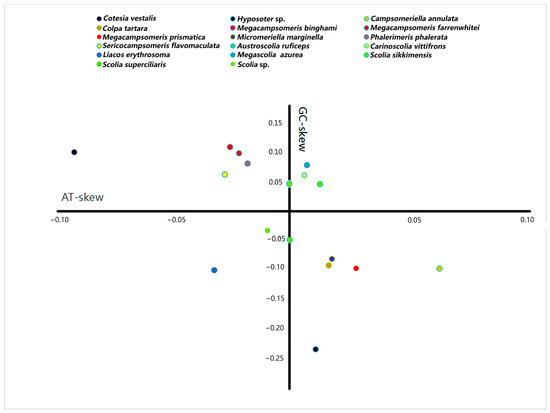
Figure 3.
Strand asymmetry of the Scoliidae and the outgroups calculated from the majority strand of the mitochondrial genomes. Each mitochondrial genome is indicated by a filled circle. Different colors represent different genera of the Scoliidae and outgroups.
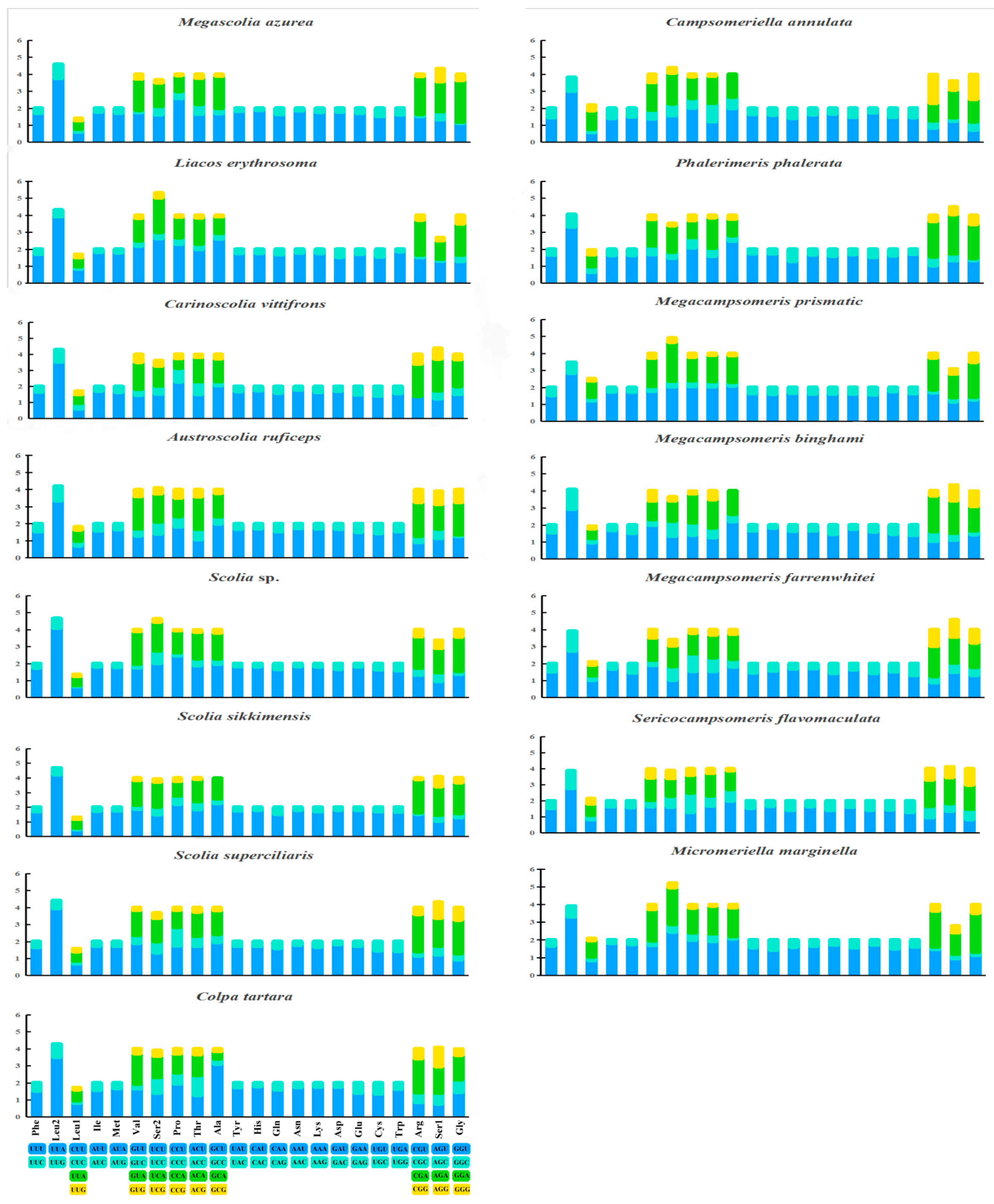
The relative synonymous codon usages (RSCUs) are shown in Figure 4. The PCGs, except for the stop codons, consist of 3412 codons in Campsomeriella annulata to 5241 codons in Micromeriella marginella. Seven AT-rich codons: AUU Ile (8.7%), AAU Asn (8.2%), AAA Lys (8.0%), UUU Phe (8.0%), UUA Leu (7.9%), AUA Met (7.1%), and UAU Tyr (6.8%), are the most frequent codons, which corresponds to the higher AT content in mitogenomes.
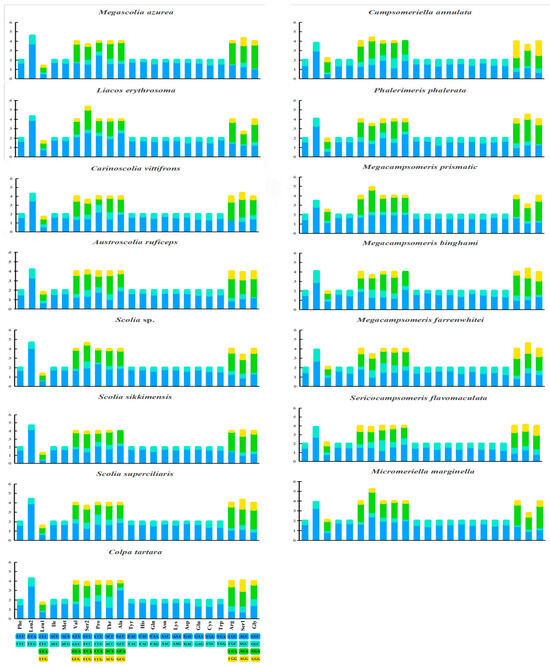
Figure 4.
Relative synonymous codon usage (RSCU) of protein coding genes (PCGs) in 15 (10 new sequenced and 5 from NCBI) mitochondrial genomes of Scoliidae.
3.2. Gene Rearrangement in Scoliidae
Generally, in Scoliidae gene rearrangements were evident when compared with the ancestral type of the Drosophila melanogaster (Figure 1). Obviously, shuffled clusters between trnI to trnK and inverse transpositions in trnF, trnS1, and sometimes trnE occurred in all 15 Scoliidae sequences.
Overall, all 15 Scoliidae sequences had similar conserved gene arrangements with the same arrangements of PCGs and rRNA genes, except for the shortly assembled C. annulata which had a distinct inverse transposition of cob and shuffled cluster between cox2 and nad2.
However, the tRNA genes had the highest frequency of rearrangement followed by PCGs in Scoliidae. The tRNA genes located in the tRNA clusters had a relatively higher frequency of rearrangement, such as the cluster between nad2 and trnD. The trnE, trnH and trnF genes showed the most frequent transposition or inverse transpositions elsewhere. Other tRNA (trnD, trnA, trnR, trnN, trnT, trnP, trnS2, trnL1 and trnV) genes without rearrangement were the most conservative.
Two obvious clusters can be observed to be different when comparing the two tribes: cluster 1 (red box in Figure 1) and cluster 2 (blue box in Figure 1). Scoliini members rearranged as “-trnL2-(-trnM)” (mode 1) for cluster 1 and “trnH-nad5-(-trnE)” (mode 3) for cluster 2, while Campsomerini mostly displayed as “-trnM-(-trnL2)” (mode 2) for cluster 1 and “trnE-(-nad5)-(-trnH)” (mode 4) for cluster 2. Surprisingly, C. tartara was always rearranged as the Scoliini referring above two clusters (mode 1 & 3). Within Scoliini, however, it exhibited fewer changes, with only one transposition of trnE in the Scolia sp. sequence. In Campsomerini, conversely, it still showed more changes though excluding shortly assembled C. annulata and unusual C. tartara referring tRNA cluster “trnM-trnQ”. Phalerimeris phalerata, exclusively, showed transpositions of trnC-trnY and trnG-trnF in Campsomerini or even in all Scoliidae. It coincided with the morphological appearance between these two tribes. Campsomerini, in general, display a more distinct sexual dimorphism and the genera are distinctive.
3.3. Phylogenetic Analyses
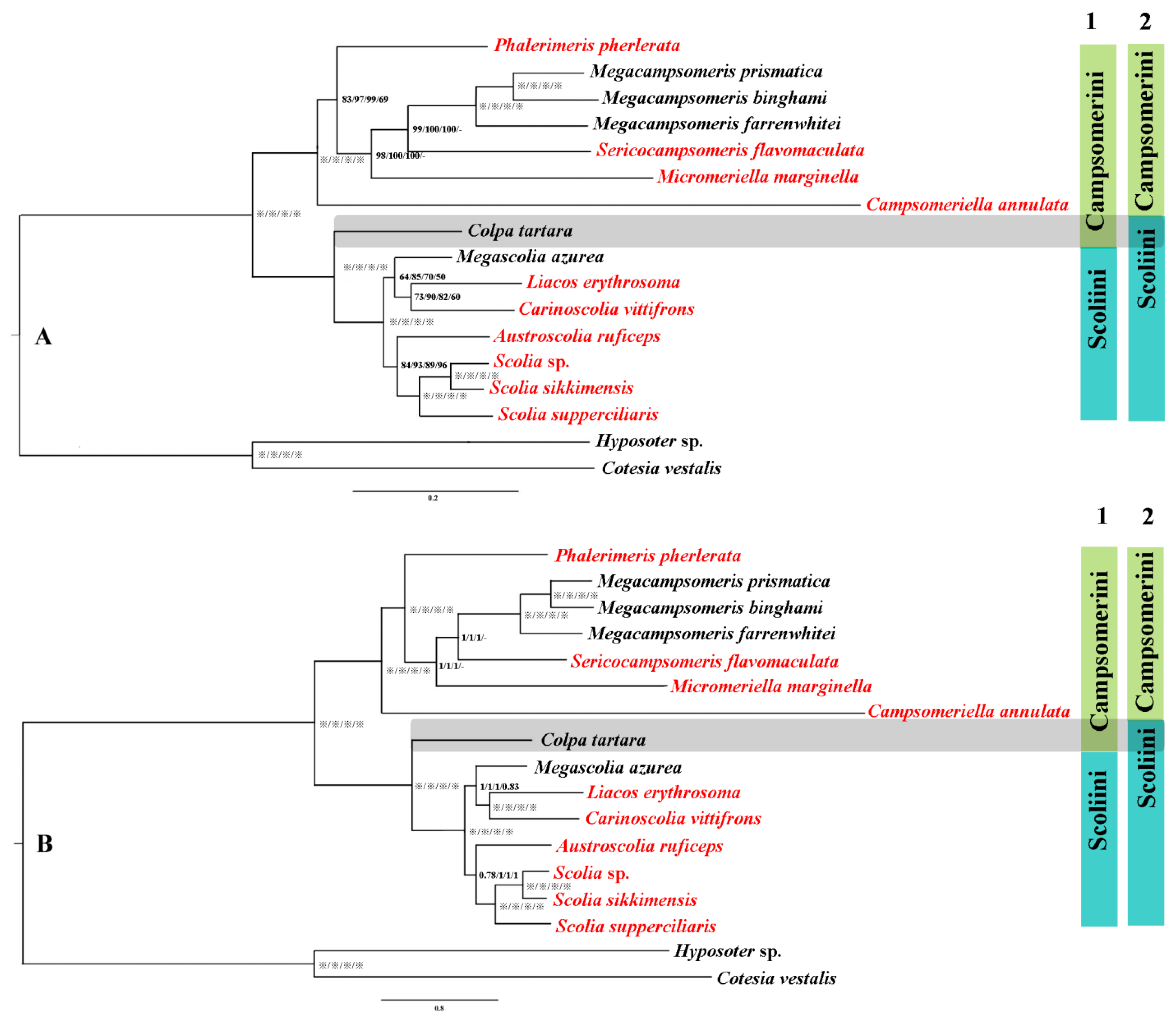
Phylogenetic relationships were inferred using MrBayes and IQtree based on four data matrices (PCG123, PCG123 + RNA, PCG12 and AA), which result in eight highly congruent tree topologies (Figure 5) with a high support value. Optimal partitioning strategy and models were selected using PartitionFinder v2.1.12 (Table S2). Generally, the support values of the corresponding nodes in the phylogenetic tree were relatively higher in BI than MI analysis in this study, which testified that MrBayes performs more reliably than ML methods in mitochondrial phylogenomic analysis of this group, corresponding with other Hymenoptera [14,52,53]. In all analyses, two clusters of the tribes Campsomerini and Scoliini were always recovered, which is consistent with former morphological [17,20] and COI analyses [1]. Within Campsomerini, three species of Megacampsomeris clustered together and they together were sister to Sericocampsomeris in BI and ML trees based on PCG123, PCG123 + RNA, PCG12 or (Sericocampsomeris + Micromeriella) based on AA matrixes (Figure 5). Phalerimeris together with the former genera was sister to Campsomeriella in all analyses. Colpa tartara, however, was always included as a sister group to other Scoliini members rather than clustered together with members of Campsomerini. Similar results were extracted when Yao et al. [33] used the same sequence to obtain the phylogenetic relationship of Scoliidae with other stinging wasps. Three Scolia species together with Austroscolia were sister to the cluster of Megascolia + (Liacos + Carinoscolia) in Scoliini in all analyses.
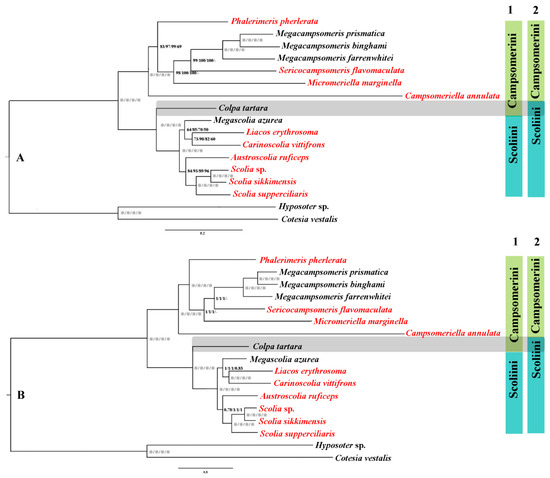
Figure 5.
Phylogenetic tree of 15 representative lineages in Scoliidae based on matrices of PCG123/PCG123 + RNA/PCG12/AA separately. (A), the phylogenetic tree using maximum likelihood (ML) methods; (B), the phylogenetic tree using Bayesian inference (BI) methods. Note: “※” indicates bootstrap percentage = 100 or Bayesian posterior probability = 1; “-” means not applicable; 1 refers to former morphological attribution; 2 refers to new molecular attribution; red font refers to newly sequenced Scoliidae.
3.4. Distribution of Mitogenomic Rearrangements in Phylogenetic Trees of Scoliidae
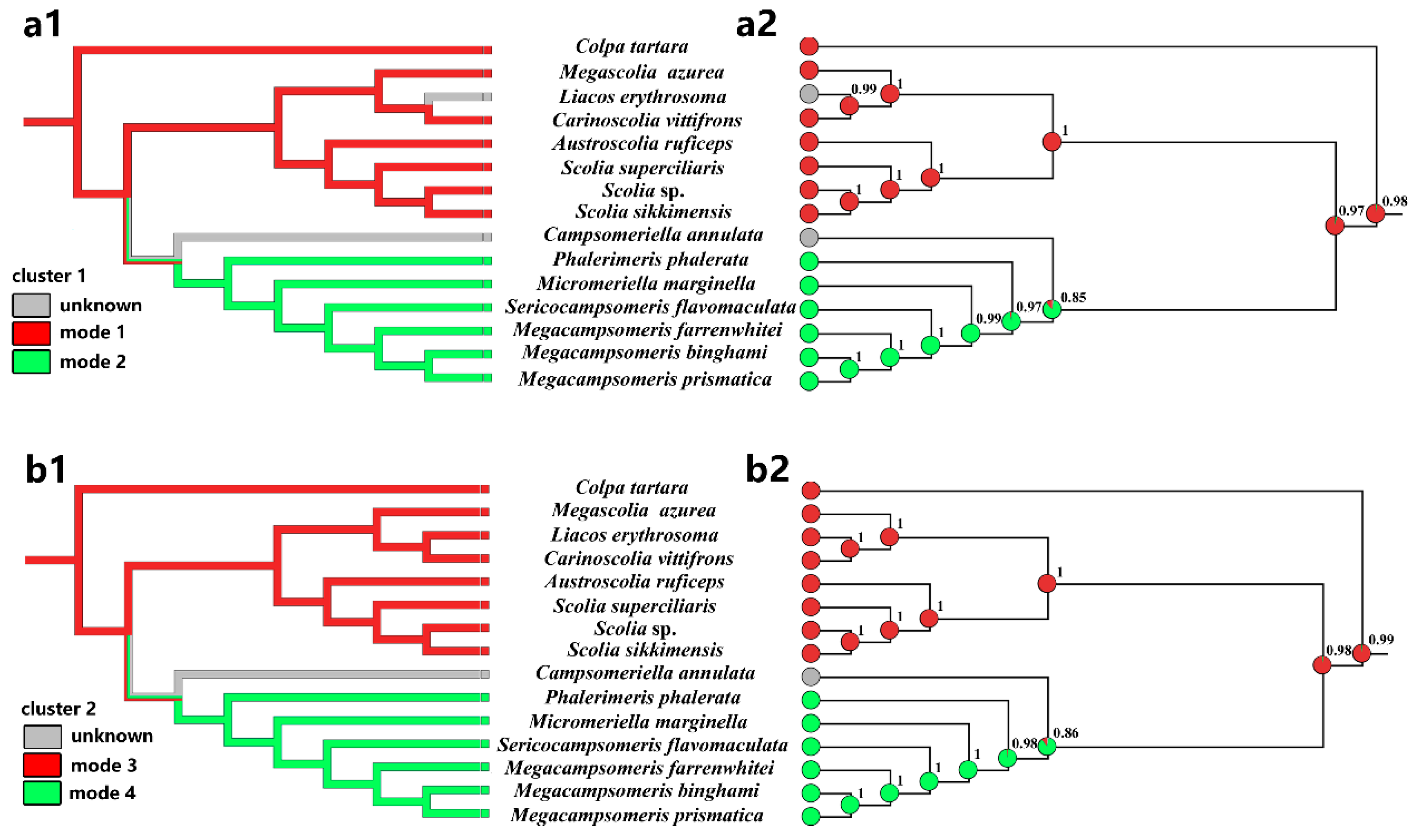
We considered PCG123 + RNA topology to analyze the clusters 1 and cluster 2 rearrangements in the phylogenetic context. Using these relationships, we mapped the mitogenomic features onto the topology and inferred ancestral states for the individual lineages using maximum parsimony (MP) and maximum likelihood (ML) methods (Figure 6).
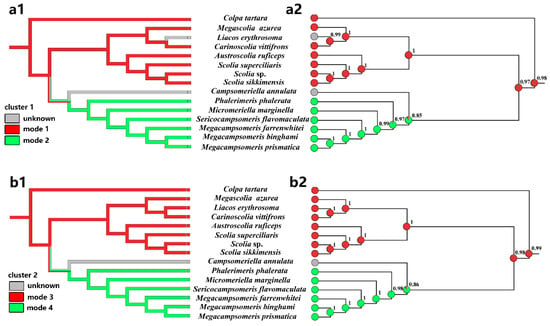
Figure 6.
Reconstruction of ancestral states and mapping of mitogenomic clusters of different modes onto the Chinese Scoliidae tree (PCG123 + RNA). Two methods, maximum parsimony (a1,b1) and maximum likelihood (a2,b2) were applied for two clusters: cluster 1 (a1,a2) and cluster 2 (b1,b2). The area of colors at nodes in (a2,b2) corresponds to the probability of the given state, modes 1/2 and modes 3/4. The probability value for a more likely state was also given at these nodes. Mk1 model was applied for ML approach.
Two methods applied to the topology for four modes in two clusters clearly indicated that the last common ancestor of Scoliidae contained a rearrangement of cluster 1 and 2 as the modes of Scoliini (modes 1 and 3) (Figure 6). This state was inherited also by the Colpa in its mitogenome, while Campsomerini excluding Colpa and unknown Campsomeriella rearranged a different mode referring cluster 1 and 2 (modes 2 and 4). The ML method provided the probability p = 0.977 (cluster 1)/0.987 (cluster 2) of this state for the last common ancestors of all Scoliidae. The last common ancestor of the Campsomerini groups could also rearrange as modes 1 (p = 0.849) & 3 (p = 0.860) as Scoliidae and so do the unknown modes of cluster 1 and 2 in Campsomeriella annulata. However, it will not happen in the last common ancestor of groups excluding Campsomeriella in Campsomerini (p = 0.966 (mode 2)/0.980 (mode 4)). In turn, an unknown rearrangement of cluster 1 in Liacos erythrosoma could be as Scoliini, with its last common ancestor rearranged as mode 1 with p = 0.988.
4. Discussion
4.1. Informative Mitochondrial Sequences and Phylogenetic Results
This is the first comprehensive study to use mitochondrial genomes to reconstruct the scoliid wasp phylogeny. In this study, 10 mitochondrial genomes representing eight genera of Scoliidae were newly sequenced. Comparative analysis including all Chinese Scoliidae genera provides valuable information for understanding the phylogenetic relationships within Scoliidae wasps. As members of the Hymenoptera, they showed high A + T compared to other orders in previous studies [40,53], while exhibiting lower content when compared to their “Parasitica” counterparts, which correspond to other Aculeata groups in Zheng et al. [11]. The results were similar to 13 protein-coding genes. However, we observed higher A + T for Scoliini than Campsomerini species except for Colpa tartara, which may suggest it is closer to Scoliini.
Gene rearrangements can help to define a variety of taxonomic scales below the ordinal level though no gene rearrangements are shared between insect orders [54,55]. In the Aculeata, gene rearrangement occurred in all species [11]. Synapomorphic rearrangement of rRNA genes and protein-coding genes were found in Scoliidae. The tRNA genes, however, had the highest frequency of rearrangement and were in sharp contrast between two tribes with the exception of C. tartara falling into the same mode of Scoliini in major rearrangements, which also suggested a closer relationship between Colpa and members of Scoliini rather than its traditional placement near the Campsomerini. We also found that Campsomeriella annulata, with the varied pattern gene rearrangement, was always inferred as being an outlier of Campsomerini and the longest branch in Scoliidae in later phylogenetic analysis. These indicated that an association between gene arrangement and sequence evolution may exist [11,56].
Phylogenetic relationships were inferred for all representative Chinese Scoliidae genera using MrBayes and IQtree based on four data matrices (PCG123, PCG123 + RNA, PCG12 and AA). The results in eight tree topologies showed high congruence with high support values excluding a slightly unstable position for (Sericocampsomeris + Micromeriella) in AA data matrices which may be the reason we lack sufficient representatives of these two groups. In general, MrBayes performs more reliably than ML methods in mitochondrial phylogenomic analysis of this group, corresponding with other Hymenoptera [14,52,53]. Our phylogenetic inferences support most of the relationships between genera and tribes of Scoliidae as in former morphological studies. Colpa tartara, however, was always included as a sister group to members of Scoliini, which is contrary to former morphological studies but consistent with recent studies of Khouri et al. [28] and Yao et al. [33].
We mapped the mitogenomic features onto the topology and inferred ancestral states for the individual lineages. Four mapped trees clearly indicated that the last common ancestor of Scoliidae contained a rearrangement of cluster 1 and 2 as the modes of Scoliini. This state was inherited also by the Colpa in its mitogenome, while other Campsomerini members rearranged more likely in a different mode, which is consistent with our other results for supporting Colpa as one of Scoliini.
4.2. Implications for Classification
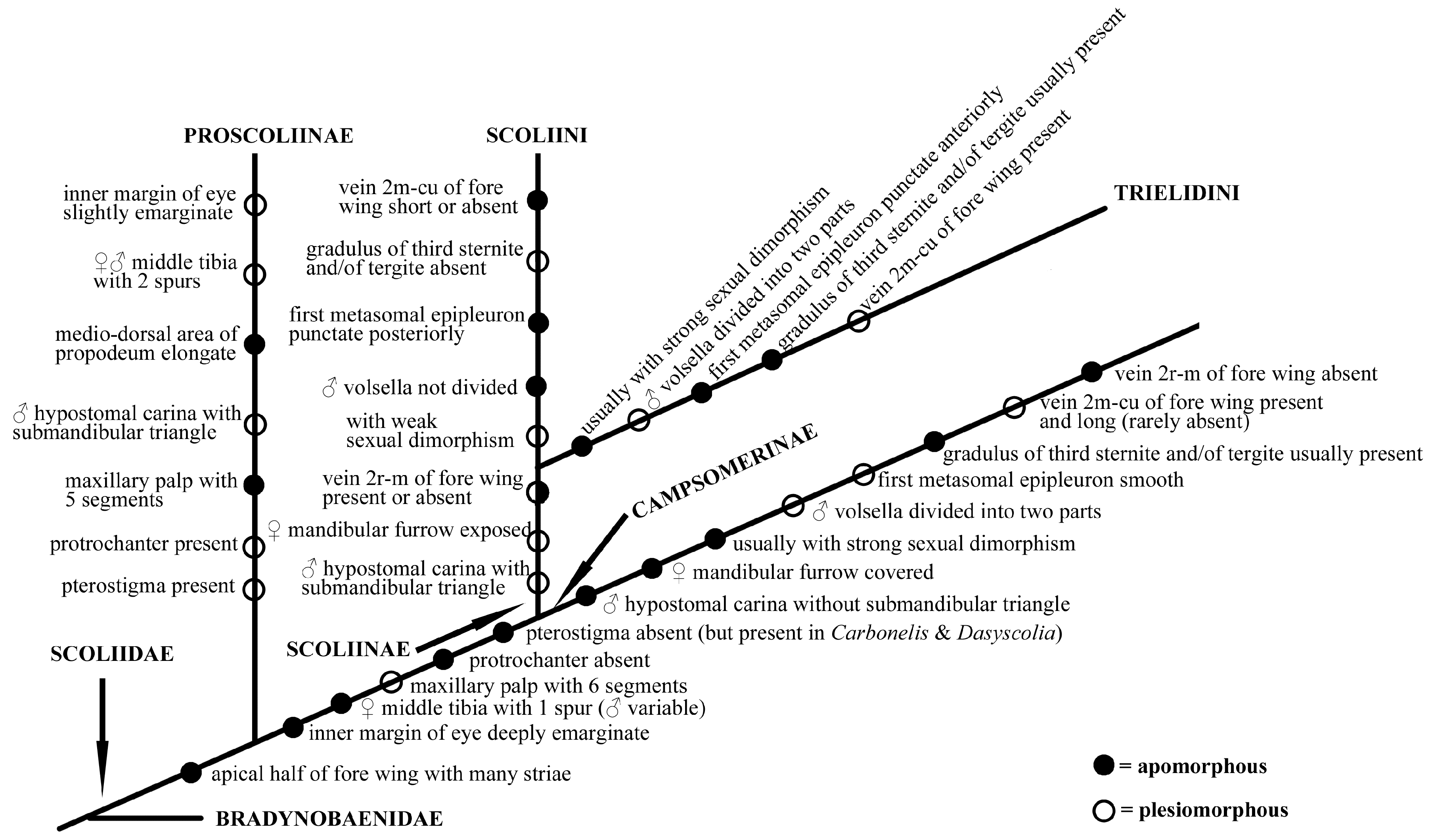
In Scoliidae, the two Proscolia species distributed in Greece and Romania were believed to be the sister group to the remaining extant Scoliidae ever since they were described. And this is strongly supported by later authors [8,28,57]. For most of the remaining groups, on the contrary, the taxonomy has been historically unstable and confusing (see Elliott, 2011 [58]).
Colpa, containing about 29 species worldwide [2], is often mingled with other genera, especially Trielis and Campsoscolia (both synonymized later with Colpa) and its taxonomic treatment has historically varied significantly. Bradley [59] argued for a “basal” placement for Campsoscolia. Betrem [60] erected the tribe Trielini (emended by Betrem and Bradley [16] to Trielidini) within the Campsomerinae to contain the genera Trielis (treated as a subgenus of Colpa, except for Guigliana). For most of the following works, it was included as part of Campsomerinae or Campsomerini after being demoted by Rasnitsyn [17], except for Argaman [27] who erected a subfamily Colpinae for related species and placed it as a sister to his Scoliinae. Recently, more molecular evidence has been applied for supporting Colpa within the Scoliini clade [28,33] though lacking morphological discussion.
Colpa was historically grouped in Campsomerini morphologically mainly for the presence of a vein 2m-cu of the fore wing which connected to the second submarginal cell and volsella of males divided into two parts [25]. Nevertheless, some differences can be observed between them, such as male hypostomal carina with submandibular triangle in the Colpa while being absent in Campsomerini, the female mandibular furrow being exposed while being concealed in Campsomerini, and the first metasomal epipleuron being punctate anteriorly but smooth in Campsomerini. Furthermore, the features mentioned above to distinguish Colpa from Campsomerini are all found in members of the tribe Scoliini, which justifies the inclusion of Colpa in the Scoliini considering the phylogenetic results and the similar AT content and gene rearrangements in our study; all analyses also strongly support Colpa as a member of Scoliini rather than Campsomerini. Hence, we propose a phylogram (Figure 7) hypothesis based on the present study, the suggestion by van Achterberg to treat the Campsomerini as subfamily and former research to explain the possible evolutionary directions of the family Scoliidae here. Nevertheless, more materials from other regions and related genera are needed to better understand the relationships between them.
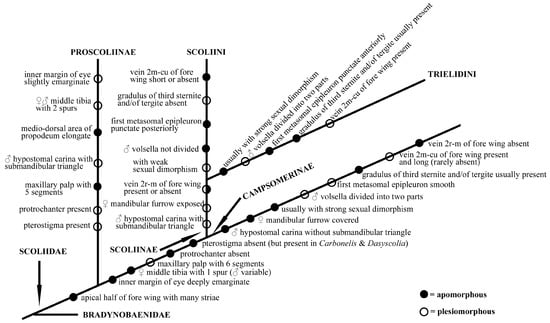
Figure 7.
Hypothesis phylogram based on present study and former research on family Scoliidae.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15100758/s1, Table S1: Detailed collecting data of the sequenced taxa.; Table S2: Opitimal partitioning schemes for mitogenome dataset determined by PartitionFinder.
Author Contributions
Conceptualization, methodology, validation, formal analysis, investigation, data curation, Z.L., C.v.A. and H.C.; writing—original draft preparation, Z.L.; writing—review and editing, Z.L., C.v.A. and H.C.; project administration, Z.L. and H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was provided the Scientific Research Fund of the Hunan Provincial Education Department (23B0654) and the Hunan Provincial Natural Science Foundation of China (2023JJ30434).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data of the research were deposited in Hunan University of Arts and Science.
Acknowledgments
We thank Andrew Polaszek, Natural History Museum, London, for his help during the course of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Z.; Yang, S.-J.; Wang, Y.-Y.; Peng, Y.-Q.; Chen, H.-Y.; Luo, S.-X. Tackling the Taxonomic Challenges in the Family Scoliidae (Insecta, Hymenoptera) Using an Integrative Approach: A Case Study from Southern China. Insects 2021, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Osten, T. Checkliste der Dolchwespen der Welt (Insecta: Hymenoptera, Scoliidae). Naturf. Ges. 2005, 220, 1–62. [Google Scholar]
- Zhang, Q.; Zhang, H.; Rasnitsyn, A.P.; Jarzembowski, E.A. A new genus of Scoliidae (Insecta: Hymenoptera) from the Lower Cretaceous of northeast China. Cretac. Res. 2015, 52, 579–584. [Google Scholar] [CrossRef]
- Day, M.C.; Else, G.R.; Morgan, D. The most primitive Scoliidae (Hymenoptera). J. Nat. Hist. 1981, 15, 671–684. [Google Scholar] [CrossRef]
- Brothers, D.J. Phylogeny and Classification of the Aculeate Hymenoptera, with Special Reference to Mutillidae. Univ. Kans. Sci. Bull. 1975, 50, 483–648. [Google Scholar]
- Königsmann, E. Das phylogenetische System der Hymenoptera Teil 4: Aculeata (Unterordnung Apocrita). Dtsch. Entomol. Z. 1978, 25, 365–435. [Google Scholar] [CrossRef]
- Brothers, D.J. Phylogeny of Aculeata: Chrysidoidea and Vespoidea (Hymenoptera). J. Hymenopt. Res. 1993, 2, 227–304. [Google Scholar]
- Branstetter, M.G.; Danforth, B.N.; Pitts, J.P.; Faircloth, B.C.; Ward, P.S.; Buffington, M.L.; Gates, M.W.; Kula, R.R.; Brady, S.G. Phylogenomic Insights into the Evolution of Stinging Wasps and the Origins of Ants and Bees. Curr. Biol. 2017, 27, 1019–1025. [Google Scholar] [CrossRef]
- Pilgrim, E.M.; Von Dohlen, C.D.; Pitts, J.P. Molecular phylogenetics of Vespoidea indicate paraphyly of the superfamily and novel relationships of its component families and subfamilies. Zool. Scr. 2008, 37, 539–560. [Google Scholar] [CrossRef]
- Wilson, J.S.; von Dohlen, C.D.; Forister, M.L.; Pitts, J.P. Family-Level Divergences in the Stinging Wasps (Hymenoptera: Aculeata), with Correlations to Angiosperm Diversification. Evol. Biol. 2013, 40, 101–107. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Cao, L.-J.; Chen, P.-Y.; Chen, X.-X.; van Achterberg, K.; Hoffmann, A.A.; Liu, J.-X.; Wei, S.-J. Comparative mitogenomics and phylogenetics of the stinging wasps (Hymenoptera: Aculeata). Mol. Phylogenet. Evol. 2021, 159, 107119. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Kozlov, A.; Podsiadlowski, L.; Petersen, M.; Lanfear, R.; et al. Evolutionary History of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Blaimer, B.B.; Santos, B.F.; Cruaud, A.; Gates, M.W.; Kula, R.R.; Mikó, I.; Rasplus, J.-Y.; Smith, D.R.; Talamas, E.J.; Brady, S.G.; et al. Key innovations and the diversification of Hymenoptera. Nat. Commun. 2023, 14, 1212. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-S.; Liu, Z.; Chen, H.-Y.; Chen, X.-X. Two mitochondrial genomes of genus Megacampsomeris Betrem, 1928 (Hymenoptera: Scoliidae) with their phylogenetic implications. J. Asia-Pac. Entomol. 2023, 26, 102067. [Google Scholar] [CrossRef]
- Bradley, J.C. Further notes on the American taxa of Campsomeris (Hymenoptera: Scoliidae). Entomol. News. 1964, 25, 101–108. [Google Scholar]
- Betrem, J.G.; Bradley, J.C. The African Campsomerinae (Hym., Scoliidae). Monogr. NEV 1972, 6, 1–326. [Google Scholar]
- Rasnitsyn, A.P. New subfamily of scoliid wasps (Hymenoptera, Scoliidae, Proscoliinae). Zool. Zhurnal. 1977, 66, 522–599. [Google Scholar]
- Osten, T. Zweiter Beitrag zur Kenntnis der Scoliidenfauna von Zypern (Hymenoptera, Scoliidae). Entomofauna. Z. Für Entomol. 1994, 15, 501–508. [Google Scholar]
- Osten, T. Die Scoliiden des Mittelmeer-Gebietes und angrenzender Regionen (Hymenoptera). Ein Bestimmungsschlüssel. Linz. Biol. Beiträge 2000, 32, 537–593. [Google Scholar]
- Gupta, S.K.; Jonathan, J.K. Fauna of India and the Adjacent Countries, Hymenoptera: Scoliidae; Zoological Survey of India: Kolkata, India, 2003; pp. 1–277. [Google Scholar]
- Bhattacharjee, S.; Saha, S.; Raychaudhuri, D. Scoliid wasps (Hymenoptera: Vespoidea) of Jaldapara Wildlife Sanctuary, West Bengal, India. Mun. Ent. Zool. 2010, 5, 661–669. [Google Scholar]
- Kim, J.-K. Taxonomic Review of the Tribe Campsomerini (Scoliinae, Scoliidae, Hymenoptera) in Korea. Anim. Syst. Evol. Divers. 2009, 25, 99–106. [Google Scholar] [CrossRef][Green Version]
- Schulten, G.G.M.; Feijen, H.R.; Feijen, C. The genus Bellimeris Betrem (Hymenoptera: Scoliidae, Campsomerinae). Zool. Meded. 2011, 85, 887–903. [Google Scholar]
- Liu, Z.; van Achterberg, C.; He, J.-H.; Chen, X.-X. A checklist of Scoliidae (Insecta: Hymenoptera) from China. Zootaxa 2021, 4966, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; van Achterberg, C.; He, J.H.; Chen, X.-X.; Chen, H.-Y. Illustrated keys to Scoliidae (Insecta, Hymenoptera, Scolioidea) from China. ZooKeys 2021, 1025, 139–175. [Google Scholar] [CrossRef] [PubMed]
- Rasnitsyn, A.P. Archaeoscoliinae, an extinct subfamily of scoliid wasps (Insecta: Vespida = Hymenoptera: Scoliidae). J. Hymenopt. Res. 1993, 2, 85–96. [Google Scholar]
- Argaman, Q. Generic synopsis of Scoliidae (Hymenoptera, Scolioidea). Ann. Hist.-Nat. Mus. Natl. Hung. 1996, 88, 171–222. [Google Scholar]
- Khouri, Z.; Gillung, J.P.; Kimsey, L.S. The evolutionary history of mammoth wasps (Hymenoptera: Scoliidae). bioRxiv 2022. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Liu, Z.; Wang, Z.; Yang, S.-J.; Luo, S.-X. Review of the genus Sericocampsomeris Betrem, 1941 (Hymenoptera, Scoliidae) from China. Berl. Èntomol. Z. 2022, 69, 125–138. [Google Scholar] [CrossRef]
- Taylor, C.; Barthélémy, C. A review of the digger wasps (Insecta: Hymenoptera: Scoliidae) of Hong Kong, with description of one new species and a key to known species. Eur. J. Taxon. 2021, 786, 1–92. [Google Scholar] [CrossRef]
- Zheng, B.-Y.; Cao, L.-J.; Tang, P.; van Achterberg, K.; Hoffmann, A.A.; Chen, H.-Y.; Chen, X.-X.; Wei, S.-J. Gene arrangement and sequence of mitochondrial genomes yield insights into the phylogeny and evolution of bees and sphecid wasps (Hymenoptera: Apoidea). Mol. Phylogenet. Evol. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, C.; Chen, S.Q. The mitochondrial genome of Megascolia azurea (Christ) (Hymenoptera: Scoliidae) with its phylogenetic implications. Entomotaxonomia 2023, 45, 140–150. [Google Scholar] [CrossRef]
- Yao, Y.J.; Chen, B.; Li, T.J. Sequencing and analysis of the mitochondrial genome of Scoliidae. Acta Entomol. Sin. 2023, 66, 99–107. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 26 April 2010).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Crampton-Platt, A.; Timmermans, M.J.; Gimmel, M.L.; Kutty, S.N.; Cockerill, T.D.; Khen, C.V.; Vogler, A.P. Soup to Tree: The Phylogeny of Beetles Inferred by Mitochondrial Metagenomics of a Bornean Rainforest Sample. Mol. Biol. Evol. 2015, 32, 2302–2316. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Leung, H.C.M.; Yiu, S.M.; Chin, F.Y.L. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. Comput. Biol. J. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Yuan, R.Z.; Zhou, J.J.; Shu, X.H.; Ye, X.-Q.; Tang, P.; Chen, X.-X. The mitochondrial genome of Chelonus formosanus (Hymenoptera: Braconidae) with novel gene orders and phylogenetic implications. Arch Insect Biochem. 2022, 111, e21870. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Patterns of nueleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Wei, S.-J.; Shi, M.; Sharkey, M.J.; van Achterberg, C.; Chen, X.-X. Comparative mitogenomics of Braconidae (Insecta: Hymenoptera) and the phylogenetic utility of mitochondrial genomes with special reference to Holometabolous insects. BMC Genom. 2010, 11, 371. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, J.-C.; Zheng, B.-Y.; Wei, S.-J.; Sharkey, M.; Chen, X.-X.; Vogler, A.P. Mitochondrial phylogenomics of the Hymenoptera. Mol. Phylogenet. Evol. 2018, 131, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kück, P. ALICUT: A Perl Script Which Cuts ALISCORE Identified RSS; Department of Bioinformatics, Zoologisches Forschungs Museum A. Koenig (ZFMK): Bonn, Germany, 2009. [Google Scholar]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Schmidt, H.A.; Strimmer, K.; Vingron, M.; von Haeseler, A. TREE-PUZZLE: Maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 2002, 18, 502–504. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 3.81 ed.; 2023; Available online: http://www.mesquiteproject.org (accessed on 1 April 2023).
- Gilbert, M.T.P.; Wei, S.-J.; Shi, M.; Chen, X.-X.; Sharkey, M.J.; van Achterberg, C.; Ye, G.-Y.; He, J.-H. New views on strand asymmetry in insect mitochondrial genomes. PLoS ONE 2010, 5, e12708. [Google Scholar]
- Dowton, M.; Cameron, S.L.; Austin, A.D.; Whiting, M.F. Phylogenetic approaches for the analysis of mitochondrial genome sequence data in the Hymenoptera—A lineage with both rapidly and slowly evolving mitochondrial genomes. Mol. Phylogenet. Evol. 2009, 52, 512–519. [Google Scholar] [CrossRef]
- Wei, S.-J.; Li, Q.; van Achterberg, K.; Chen, X.-X. Two mitochondrial genomes from the families Bethylidae and Mutillidae: Independent rearrangement of protein-coding genes and higher-level phylogeny of the Hymenoptera. Mol. Phylogenet. Evol. 2014, 77, 1–10. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect Mitochondrial Genomics: Implications for Evolution and Phylogeny. Annu. Rev. Èntomol. 2014, 59, 95–117. [Google Scholar] [CrossRef]
- Song, F.; Li, H.; Liu, G.-H.; Wang, W.; James, P.; Colwell, D.D.; Tran, A.; Gong, S.; Cai, W.; Shao, R. Mitochondrial Genome Fragmentation Unites the Parasitic Lice of Eutherian Mammals. Syst. Biol. 2018, 68, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Dowton, M.; Murrell, A.; Barker, S.C. Rates of Gene Rearrangement and Nucleotide Substitution Are Correlated in the Mitochondrial Genomes of Insects. Mol. Biol. Evol. 2003, 20, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Debevec, A.H.; Cardinal, S.; Danforth, B.N. Identifying the sister group to the bees: A molecular phylogeny of Aculeata with an emphasis on the superfamily Apoidea. Zool. Scr. 2012, 41, 527–535. [Google Scholar] [CrossRef]
- Elliott, M. Annotated catalogue of the Australian Scoliidae (Hymenoptera). Tech. Rep. Aust. Mus. Online 2011, 22, 1–17. [Google Scholar] [CrossRef]
- Bradley, J.C. The most primitive Scoliidae. EOS 1950, 1, 427–438. [Google Scholar]
- Betrem, J.G. The African Scoliids and their Affinities. In Proceedings of the XIIth International Congress of Entomology, London, UK, 8–16 July 1964; Ince: London, UK, 1965; p. 120. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).