Bugs on Drugs: Paracetamol Exposure Reveals Genotype-Specific Generational Effects on Life History Traits in Drosophila melanogaster
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Specimens
2.2. Experiment I: Paracetamol Dose–Response Experiment
2.3. Experiment II: Generational Effects of Paracetamol
2.4. Fecundity
2.5. Longevity
2.6. Spontaneous Locomotor Activity
2.7. Statistics
2.7.1. Experiment I
2.7.2. Experiment II
3. Results
3.1. Experiment I: Paracetamol Dose–Response Experiment
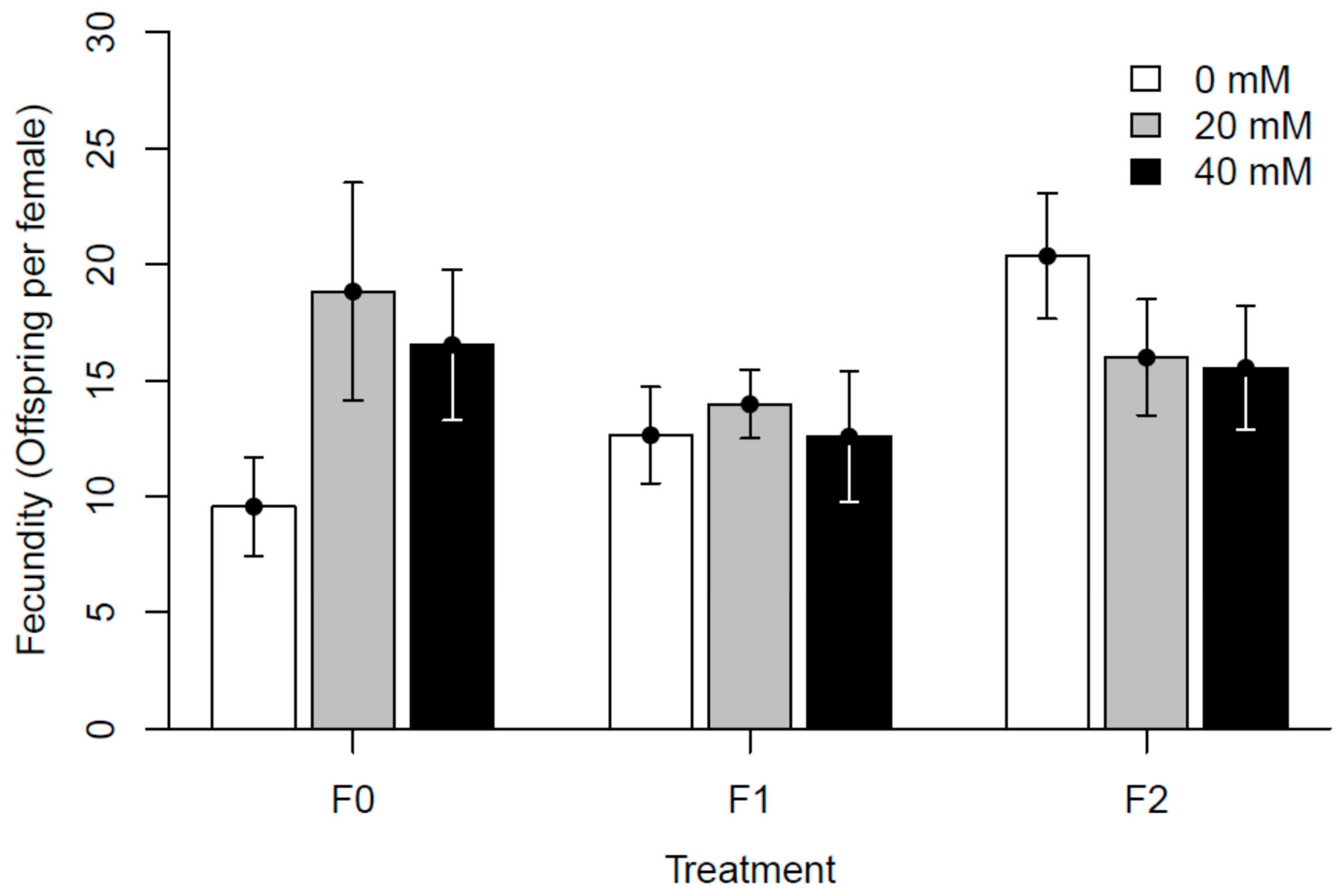
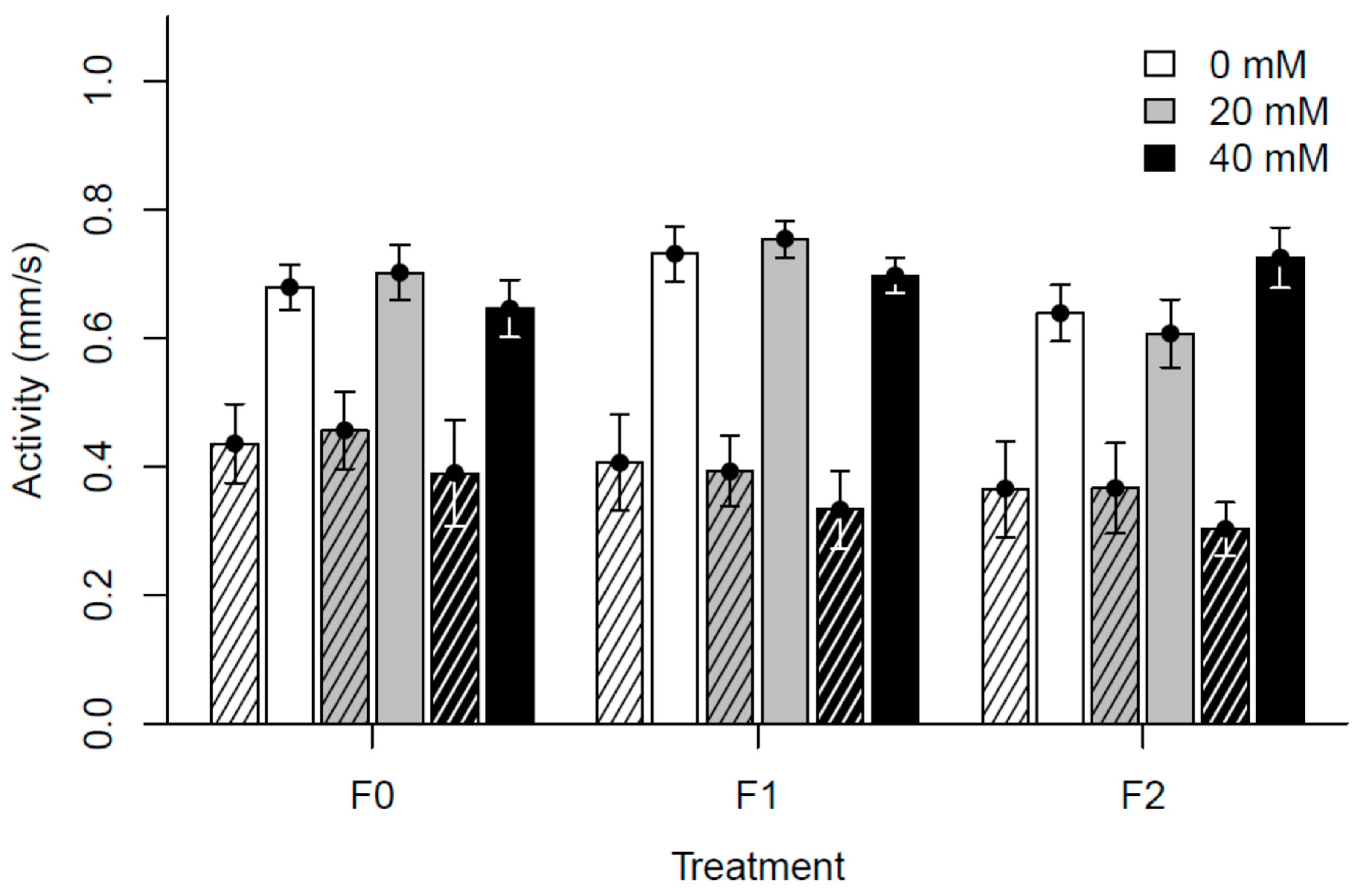
3.2. Experiment II: Generational Effects of Paracetamol
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heard, E.; Martienssen, R.A. Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Barrett, R.D.H. Epigenetics in natural animal populations. J. Evol. Biol. 2017, 30, 1612–1632. [Google Scholar] [CrossRef]
- Brehm, E.; Flaws, J.A. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.R.; Gould, T.J. Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur. J. Neurosci. 2019, 50, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bosagna, C.; Skinner, M. Epigenetic Transgenerational Effects of Endocrine Disruptors on Male Reproduction. Semin. Reprod. Med. 2009, 27, 403–408. [Google Scholar] [CrossRef]
- Lee, M.K.; Blumberg, B. Transgenerational effects of obesogens. Basic. Clin. Pharmacol. Toxicol. 2019, 125, 44–57. [Google Scholar] [CrossRef]
- Raj, A.; Shah, P.; Agrawal, N. Dose-dependent effect of silver nanoparticles (AgNPs) on fertility and survival of Drosophila: An in-vivo study. PLoS ONE 2017, 12, e0178051. [Google Scholar] [CrossRef]
- Gong, Y.; Xue, Y.; Li, X.; Zhang, Z.; Zhou, W.; Marcolongo, P.; Benedetti, A.; Mao, S.; Han, L.; Ding, G.; et al. Inter- and Transgenerational Effects of Paternal Exposure to Inorganic Arsenic. Adv. Sci. 2021, 8, 2002715. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, W.; Chen, M.; Gu, H.; Tang, Q.; Guo, D.; Chen, T.; Chen, Y.; Lu, C.; Song, L.; et al. From the Cover: Metabolomics Reveals a Role of Betaine in Prenatal DBP Exposure-Induced Epigenetic Transgenerational Failure of Spermatogenesis in Rats. Toxicol. Sci. 2017, 158, 356–366. [Google Scholar] [CrossRef]
- Nomura, T. Transgenerational effects from exposure to environmental toxic substances. Mutat. Res. 2008, 659, 185–193. [Google Scholar] [CrossRef]
- Kaati, G.; Bygren, L.O.; Edvinsson, S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur. J. Hum. Genet. 2002, 10, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.M.; Watson, S.-A.; Donelson, J.M.; McCormick, M.I.; Munday, P.L. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2012, 2, 858–861. [Google Scholar] [CrossRef]
- Painter, R.; Osmond, C.; Gluckman, P.; Hanson, M.; Phillips, D.; Roseboom, T. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 2008, 115, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Pembrey, M.E. Male-line transgenerational responses in humans. Hum. Fertil. 2010, 13, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Salinas, S.; Munch, S.B. Thermal legacies: Transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett. 2012, 15, 159–163. [Google Scholar] [CrossRef]
- Veenendaal, M.; Painter, R.; de Rooij, S.R.; Bossuyt, P.; Van Der Post, J.; Gluckman, P.; Hanson, M.; Roseboom, T. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG 2013, 120, 548–554. [Google Scholar] [CrossRef]
- Berger, S.L.; Nakanishi, O.; Haendler, B. The Histone Code and Beyond: New Approaches to Cancer Therapy; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Chuang, J.C.; Jones, P.A. Epigenetics and MicroRNAs. Pediatr. Res. 2007, 61, 24R–29R. [Google Scholar] [CrossRef]
- Pratt, A.J.; MacRae, I.J. The RNA-induced silencing complex: A versatile gene-silencing machine. J. Biol. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Swan, S.H.; Kriebel, D.; Liew, Z.; Taylor, H.S.; Bornehag, C.-G.; Andrade, A.M.; Olsen, J.; Jensen, R.H.; Mitchell, R.T.; et al. Paracetamol use during pregnancy—A call for precautionary action. Nat. Rev. Endocrinol. 2021, 17, 757–766. [Google Scholar] [CrossRef]
- Anderson, B.J. Paracetamol (Acetaminophen): Mechanisms of action. Paediatr. Anaesth. 2008, 18, 915–921. [Google Scholar] [CrossRef]
- Ghanem, C.I.; Pérez, M.J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef]
- Graham, G.G.; Davies, M.J.; Day, R.O.; Mohamudally, A.; Scott, K.F. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013, 21, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, A. Prostaglandin E2 and Pain; An Update. Biol. Pharm. Bull. 2011, 34, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.; Yoshida, K.; Coppari, R.; Bass, C.E.; Mochizuki, T.; Lowell, B.B.; Saper, C.B. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007, 10, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vas. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef]
- Ugur, B.; Chen, K.; Bellen, H. Drosophila tools and assays for the study of human diseases. Dis. Models Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Hunter-Chang, S.; Luo, L.; Li, K.; Liu, K.H.; Robinson, B.S. Oxidative stress mediates end-organ damage in a novel model of acetaminophen-toxicity in Drosophila. Sci. Rep. 2022, 12, 19309. [Google Scholar] [CrossRef]
- Hochschild, R. Effect of membrane stabilizing drugs on mortality in Drosophila melanogaster. Exp. Gerontol. 1971, 6, 133–151. [Google Scholar] [CrossRef]
- Trappe, T.A.; Carroll, C.C.; Dickinson, J.M.; LeMoine, J.K.; Haus, J.M.; Sullivan, B.E.; Lee, J.D.; Jemiolo, B.; Weinheimer, E.M.; Hollon, C.J. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R655–R662. [Google Scholar] [CrossRef]
- Wu, M.; Desai, D.H.; Kakarla, S.K.; Katta, A.; Paturi, S.; Gutta, A.K.; Rice, K.M.; Walker, E.M., Jr.; Blough, E.R. Acetaminophen prevents aging-associated hyperglycemia in aged rats: Effect of aging-associated hyperactivation of p38-MAPK and ERK1/2. Diabetes Metab. Res. Rev. 2009, 25, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Katta, A.; Gadde, M.K.; Liu, H.; Kakarla, S.K.; Fannin, J.; Paturi, S.; Arvapalli, R.K.; Rice, K.M.; Wang, Y.; et al. Aging-Associated Dysfunction of Akt/Protein Kinase B: S-Nitrosylation and Acetaminophen Intervention. PLoS ONE 2009, 4, e6430. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, H.; Fannin, J.; Katta, A.; Wang, Y.; Arvapalli, R.K.; Paturi, S.; Karkala, S.K.; Rice, K.M.; Blough, E.R. Acetaminophen improves protein translational signaling in aged skeletal muscle. Rejuvenation Res. 2010, 13, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; Grotewiel, M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp. Gerontol. 2011, 46, 320–325. [Google Scholar] [CrossRef]
- Bohler, S.; Liu, X.; Krauskopf, J.; Caiment, F.; Aubrecht, J.; Nicolaes, G.A.F.; Kleinjans, J.C.S.; Briedé, J.J. Acetaminophen Overdose as a Potential Risk Factor for Parkinson’s Disease. Clin. Transl. Sci. 2019, 12, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Alakilli, S.Y.M. Genotoxicity of paracetamol on the germ cells of Drosophilla melanogaster. Egypt. J. Hosp. Med. 2007, 29, 486–491. [Google Scholar] [CrossRef]
- Rannug, U.; Holme, J.A.; Hongslo, J.K.; Srám, R. An evaluation of the genetic toxicity of paracetamol. Mutat. Res. 1995, 327, 179–200. [Google Scholar] [CrossRef]
- Rehfeld, A.; Frederiksen, H.; Rasmussen, R.H.; David, A.; Chaker, J.; Nielsen, B.S.; Nielsen, J.E.; Juul, A.; Skakkebaek, N.E.; Kristensen, D.M. Human sperm cells can form paracetamol metabolite AM404 that directly interferes with sperm calcium signalling and function through a CatSper-dependent mechanism. Hum. Reprod. 2022, 37, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Rohde, P.D.; Jensen, I.R.; Sarup, P.M.; Orsted, M.; Demontis, D.; Sorensen, P.; Kristensen, T.N. Genetic Signatures of Drug Response Variability in Drosophila melanogaster. Genetics 2019, 213, 633–650. [Google Scholar] [CrossRef]
- Stefanicka-Wojtas, D.; Kurpas, D. Personalised Medicine-Implementation to the Healthcare System in Europe (Focus Group Discussions). J. Pers. Med. 2023, 13, 380. [Google Scholar] [CrossRef]
- Schou, M.F.; Loeschcke, V.; Kristensen, T.N. Inbreeding depression across a nutritional stress continuum. Heredity 2015, 115, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Holland-Letz, T.; Kopp-Schneider, A. Optimal experimental designs for dose–response studies with continuous endpoints. Arch. Toxicol. 2015, 89, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Juhl, D.W.; Tosner, Z.; Vosegaard, T. Versatile NMR simulations using SIMPSON. In Annual Reports on NMR Spectroscopy; Academic Press: Cambridge, MA, USA, 2020; Volume 100, pp. 1–59. [Google Scholar] [CrossRef]
- Leech, T.; Sait, S.M.; Bretman, A. Sex-specific effects of social isolation on ageing in. J. Insect Physiol. 2017, 102, 12–17. [Google Scholar] [CrossRef] [PubMed]
- MacLean, H.J.; Hansen, J.H.; Sorensen, J.G. Validating the automation of different measures of high temperature tolerance of small terrestrial insects. J. Insect Physiol. 2022, 137, 104362. [Google Scholar] [CrossRef] [PubMed]
- Noldus, L.P.J.J.; Spink, A.J.; Tegelenbosch, R.A.J. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behav. Res. Methods Instrum. Comput. 2001, 33, 398–414. [Google Scholar] [CrossRef]
- Dell, A.I.; Bender, J.A.; Branson, K.; Couzin, I.D.; De Polavieja, G.G.; Noldus, L.P.J.J.; Pérez-Escudero, A.; Perona, P.; Straw, A.D.; Wikelski, M.; et al. Automated image-based tracking and its application in ecology. Trends Ecol. Evol. 2014, 29, 417–428. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Kassambara, A. Pipe-Friendly Framework for Basic Statistical Tests • Rstatix. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 20 August 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Huang, W.; Campbell, T.; Carbone, M.A.; Jones, W.E.; Unselt, D.; Anholt, R.R.H.; Mackay, T.F.C. Context-dependent genetic architecture of Drosophila life span. PLoS Biol. 2020, 18, e3000645. [Google Scholar] [CrossRef]
- Huang, W.; Carbone, M.A.; Lyman, R.F.; Anholt, R.R.H.; Mackay, T.F.C. Genotype by environment interaction for gene expression in Drosophila melanogaster. Nat. Commun. 2020, 11, 5451. [Google Scholar] [CrossRef] [PubMed]
- Rohde, P.D.; Bocker, A.; Jensen, C.A.B.; Bergstrom, A.L.; Madsen, M.I.J.; Christensen, S.L.; Villadsen, S.B.; Kristensen, T.N. Genotype and Trait Specific Responses to Rapamycin Intake in Drosophila melanogaster. Insects 2021, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Ryan, D.H. Evidence-based weight loss interventions: Individualized treatment options to maximize patient outcomes. Diabetes Obes. Metab. 2021, 23, 50–62. [Google Scholar] [CrossRef]
- Penzias, A.S. Improving results with assisted reproductive technologies: Individualized patient-tailored strategies for ovulation induction. Reprod. Biomed. Online 2011, 22 (Suppl. 1), S83–S86. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Holmstrup, M.; Sarup, P.; Loeschcke, V. Evolutionary Theory and Studies of Model Organisms Predict a Cautiously Positive Perspective on the Therapeutic Use of Hormesis for Healthy Aging in Humans. Dose-Response 2010, 8, 53–57. [Google Scholar]
- Wiger, R.; Hongslo, J.K.; Evenson, D.P.; De Angelis, P.; Schwarze, P.E.; Holme, J.A. Effects of acetaminophen and hydroxyurea on spermatogenesis and sperm chromatin structure in laboratory mice. Reprod. Toxicol. 1995, 9, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Flatt, T. Survival costs of reproduction in Drosophila. Exp. Gerontol. 2011, 46, 369–375. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Prowse, N.; Partridge, L. The effects of reproduction on longevity and fertility in male Drosophila melanogaster. J. Insect Physiol. 1997, 43, 501–512. [Google Scholar] [CrossRef]
- Hansen, L.S.; Laursen, S.F.; Bahrndorff, S.; Kargo, M.; Sørensen, J.G.; Sahana, G.; Nielsen, H.M.; Kristensen, T.N. Estimation of genetic parameters for the implementation of selective breeding in commercial insect production. Genet. Sel. Evol. 2024, 56, 21. [Google Scholar] [CrossRef]
- Kim, P.; Choi, C.S.; Park, J.H.; Joo, S.H.; Kim, S.Y.; Ko, H.M.; Kim, K.C.; Jeon, S.J.; Park, S.H.; Han, S.H.; et al. Chronic exposure to ethanol of male mice before mating produces attention deficit hyperactivity disorder-like phenotype along with epigenetic dysregulation of dopamine transporter expression in mouse offspring. J. Neurosci. Res. 2014, 92, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Rohde, P.D.; Madsen, L.S.; Neumann Arvidson, S.M.; Loeschcke, V.; Demontis, D.; Kristensen, T.N. Testing candidate genes for attention-deficit/hyperactivity disorder in fruit flies using a high throughput assay for complex behavior. Fly 2016, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Rai-Bhogal, R.; Wong, C.; Kissoondoyal, A.; Davidson, J.; Li, H.; Crawford, D.A. Maternal exposure to prostaglandin E2 modifies expression of Wnt genes in mouse brain—An autism connection. Biochem. Biophys. Rep. 2018, 14, 43–53. [Google Scholar] [CrossRef]
- Perveen, S.; Kumari, S.; Raj, H.; Yasmin, S. Effects of sodium fluoride and Ocimum sanctum extract on the lifespan and climbing ability of Drosophila melanogaster. J. Basic. Appl. Zool. 2021, 82, 32. [Google Scholar] [CrossRef]
- Devineni, A.V.; Scaplen, K.M. Neural Circuits Underlying Behavioral Flexibility: Insights From Drosophila. Front. Behav. Neurosci. 2021, 15, 821680. [Google Scholar] [CrossRef]
- Nitta, Y.; Sugie, A. Studies of neurodegenerative diseases using Drosophila and the development of novel approaches for their analysis. Fly 2022, 16, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Glémin, S. How are deleterious mutations purged? Drift versus nonrandom mating. Evolution 2003, 57, 2678–2687. [Google Scholar] [CrossRef]
- Bewick, A.J.; Vogel, K.J.; Moore, A.J.; Schmitz, R.J. Evolution of DNA Methylation across Insects. Mol. Biol. Evol. 2016, 34, 654–665. [Google Scholar] [CrossRef]
- Bird, A.P.; Taggart, M.H. Variable patterns of total DNA and rDNA methylation in animals. Nucleic Acids Res. 1980, 8, 1485–1497. [Google Scholar] [CrossRef]
- Ratel, D.; Ravanat, J.-L.; Berger, F.; Wion, D. N6-methyladenine: The other methylated base of DNA. Bioessays 2006, 28, 309–315. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.W. Understanding investigative clinical trials. J. Vasc. Surg. 1989, 9, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. FDA Approval and Regulation of Pharmaceuticals, 1983-2018. JAMA 2020, 323, 164. [Google Scholar] [CrossRef] [PubMed]
- Metz, B.; Van Den Dobbelsteen, G.; Van Els, C.; Van Der Gun, J.; Levels, L.; Van Der Pol, L.; Rots, N.; Kersten, G. Quality-control issues and approaches in vaccine development. Expert. Rev. Vaccines 2009, 8, 227–238. [Google Scholar] [CrossRef]




| Fecundity | SqrSumOffspring | ||||
|---|---|---|---|---|---|
| Predictors | Df | Sum Sq | Mean Sq | F Value | p-Value |
| Dose | 2 | 33 | 16.67 | 5.734 | 0.003 |
| Gen | 2 | 132 | 65.79 | 22.628 | <0.001 |
| Line | 6 | 694 | 115.66 | 39.782 | <0.001 |
| Dose:Gen | 4 | 236 | 58.88 | 20.252 | <0.001 |
| Dose:Line | 12 | 221 | 18.38 | 6.323 | <0.001 |
| Gen:Line | 12 | 812 | 67.66 | 23.271 | <0.001 |
| Dose:Gen:Line | 24 | 330 | 13.76 | 4.733 | <0.001 |
| Observations | 2445 | ||||
| R2/R2 adjusted | 0.262/0.243 | ||||
| Longevity | SqrDays | ||||
|---|---|---|---|---|---|
| Predictors | Df | Sum Sq | Mean Sq | F Value | p-Value |
| Dose | 2 | 2.1 | 1.07 | 1.576 | 0.207 |
| Gen | 2 | 72.5 | 36.27 | 53.642 | <0.001 |
| Line | 6 | 745.4 | 124.24 | 183.744 | <0.001 |
| Dose:Gen | 4 | 9.6 | 2.4 | 3.552 | 0.007 |
| Dose:Line | 12 | 97.6 | 8.14 | 12.035 | <0.001 |
| Gen:Line | 12 | 250.6 | 20.88 | 30.883 | <0.001 |
| Dose:Gen:Line | 23 | 110.5 | 4.8 | 7.103 | <0.001 |
| Observations | 3473 | ||||
| R2/R2 adjusted | 0.358/0.347 | ||||
| Spontaneous Locomotor Activity | Distance | ||||
|---|---|---|---|---|---|
| Predictors | Df | Sum Sq | Mean Sq | F Value | p-Value |
| Dose | 2 | 0.152 | 0.076 | 4.55 | 0.011 |
| Gen | 2 | 0.3354 | 0.1677 | 10.04 | <0.001 |
| Line | 6 | 15.2625 | 2.5438 | 152.31 | <0.001 |
| Age | 1 | 23.9584 | 23.9584 | 1434.56 | <0.001 |
| Dose:Gen | 4 | 0.3763 | 0.0941 | 5.63 | <0.001 |
| Dose:Line | 12 | 0.6415 | 0.0535 | 3.2 | <0.001 |
| Gen:Line | 12 | 1.1514 | 0.096 | 5.75 | <0.001 |
| Dose:Age | 2 | 0.189 | 0.0945 | 5.66 | 0.004 |
| Gen:Age | 2 | 0.6102 | 0.3051 | 18.27 | <0.001 |
| Line:Age | 6 | 4.5025 | 0.7504 | 44.93 | <0.001 |
| Dose:Gen:Line | 24 | 1.4453 | 0.0602 | 3.61 | <0.001 |
| Dose:Gen:Age | 4 | 0.2706 | 0.0677 | 4.05 | 0.003 |
| Dose:Line:Age | 12 | 1.0281 | 0.0857 | 5.13 | <0.001 |
| Gen:Line:Age | 12 | 0.938 | 0.0782 | 4.68 | <0.001 |
| Dose:Gen:Line:Age | 21 | 2.2027 | 0.1049 | 6.28 | <0.001 |
| Random Effects | |||||
| ICC | 0.77 | ||||
| N ID | 6626 | ||||
| Observations | 171628 | ||||
| Marginal R2/Conditional R2 | 0.329/0.848 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hundebøl, B.N.R.G.; Rohde, P.D.; Kristensen, T.N.; Jensen, R.W.M.; Vosegaard, T.; Sørensen, J.G. Bugs on Drugs: Paracetamol Exposure Reveals Genotype-Specific Generational Effects on Life History Traits in Drosophila melanogaster. Insects 2024, 15, 763. https://doi.org/10.3390/insects15100763
Hundebøl BNRG, Rohde PD, Kristensen TN, Jensen RWM, Vosegaard T, Sørensen JG. Bugs on Drugs: Paracetamol Exposure Reveals Genotype-Specific Generational Effects on Life History Traits in Drosophila melanogaster. Insects. 2024; 15(10):763. https://doi.org/10.3390/insects15100763
Chicago/Turabian StyleHundebøl, Birk Nete Randlev Gleerup, Palle Duun Rohde, Torsten Nygaard Kristensen, Rune Wittendorff Mønster Jensen, Thomas Vosegaard, and Jesper Givskov Sørensen. 2024. "Bugs on Drugs: Paracetamol Exposure Reveals Genotype-Specific Generational Effects on Life History Traits in Drosophila melanogaster" Insects 15, no. 10: 763. https://doi.org/10.3390/insects15100763
APA StyleHundebøl, B. N. R. G., Rohde, P. D., Kristensen, T. N., Jensen, R. W. M., Vosegaard, T., & Sørensen, J. G. (2024). Bugs on Drugs: Paracetamol Exposure Reveals Genotype-Specific Generational Effects on Life History Traits in Drosophila melanogaster. Insects, 15(10), 763. https://doi.org/10.3390/insects15100763








