Prediction and Analysis of the Global Suitable Habitat of the Oryctes rhinoceros (Linnaeus, 1758) (Coleoptera: Scarabaeidae) Based on the MaxEnt Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
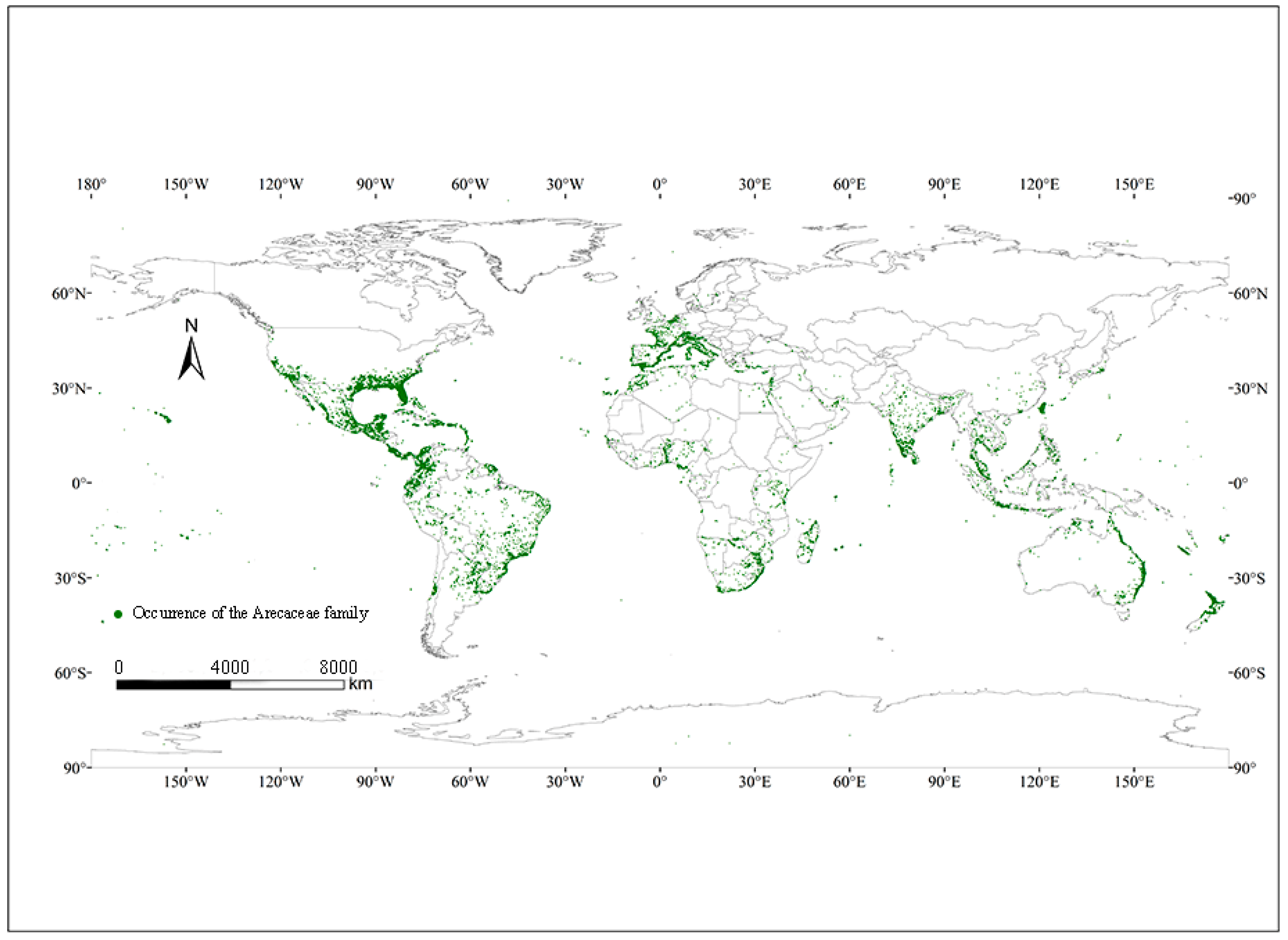
2.1. Species Distribution Data and Processing
2.2. Bioclimatic Factors
2.3. Parameter Settings for MaxEnt Model
2.4. Suitable Area Division and Model Accuracy Evaluation
3. Results
3.1. Verification of Model Accuracy
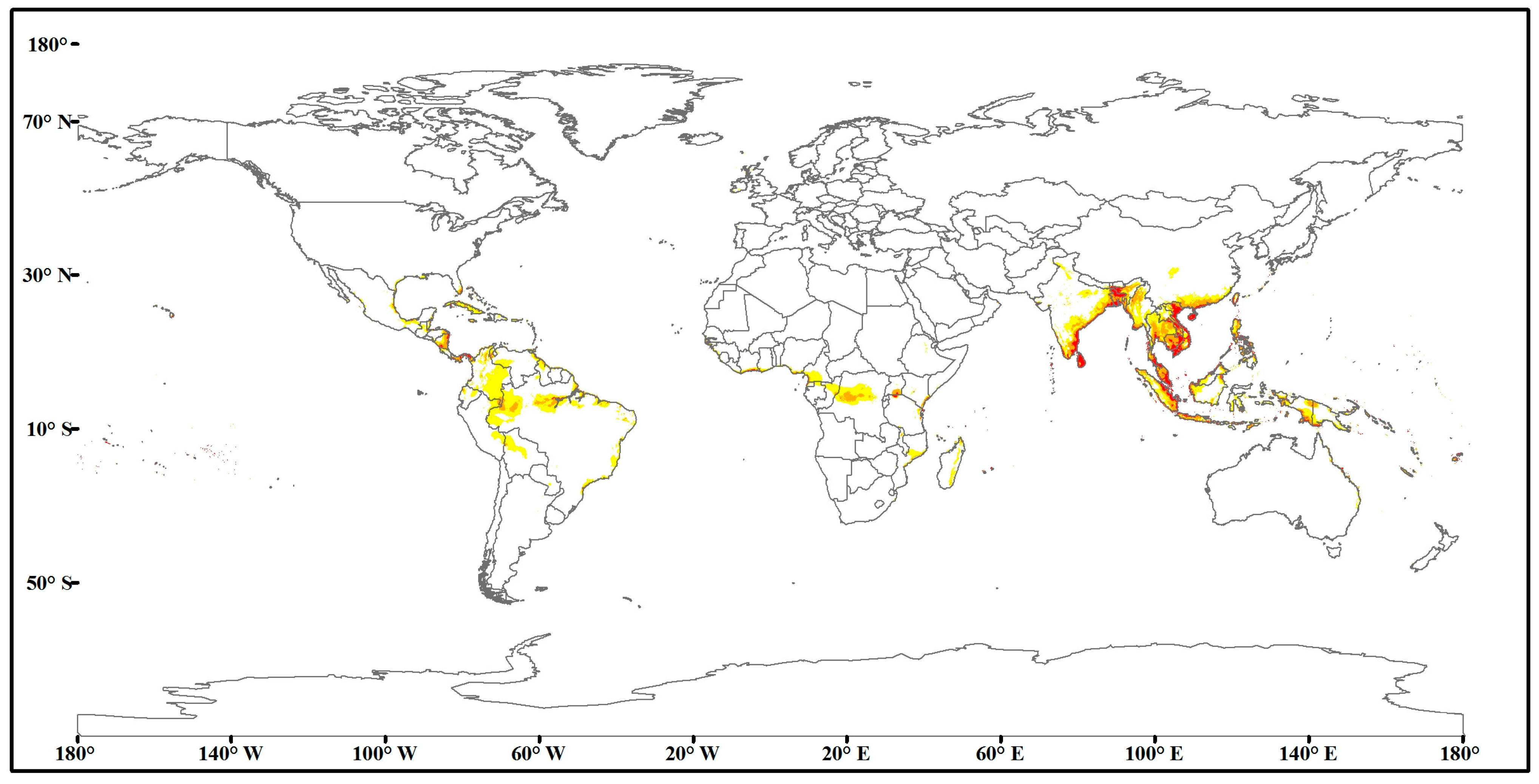
3.2. Current Predicted Distribution Area
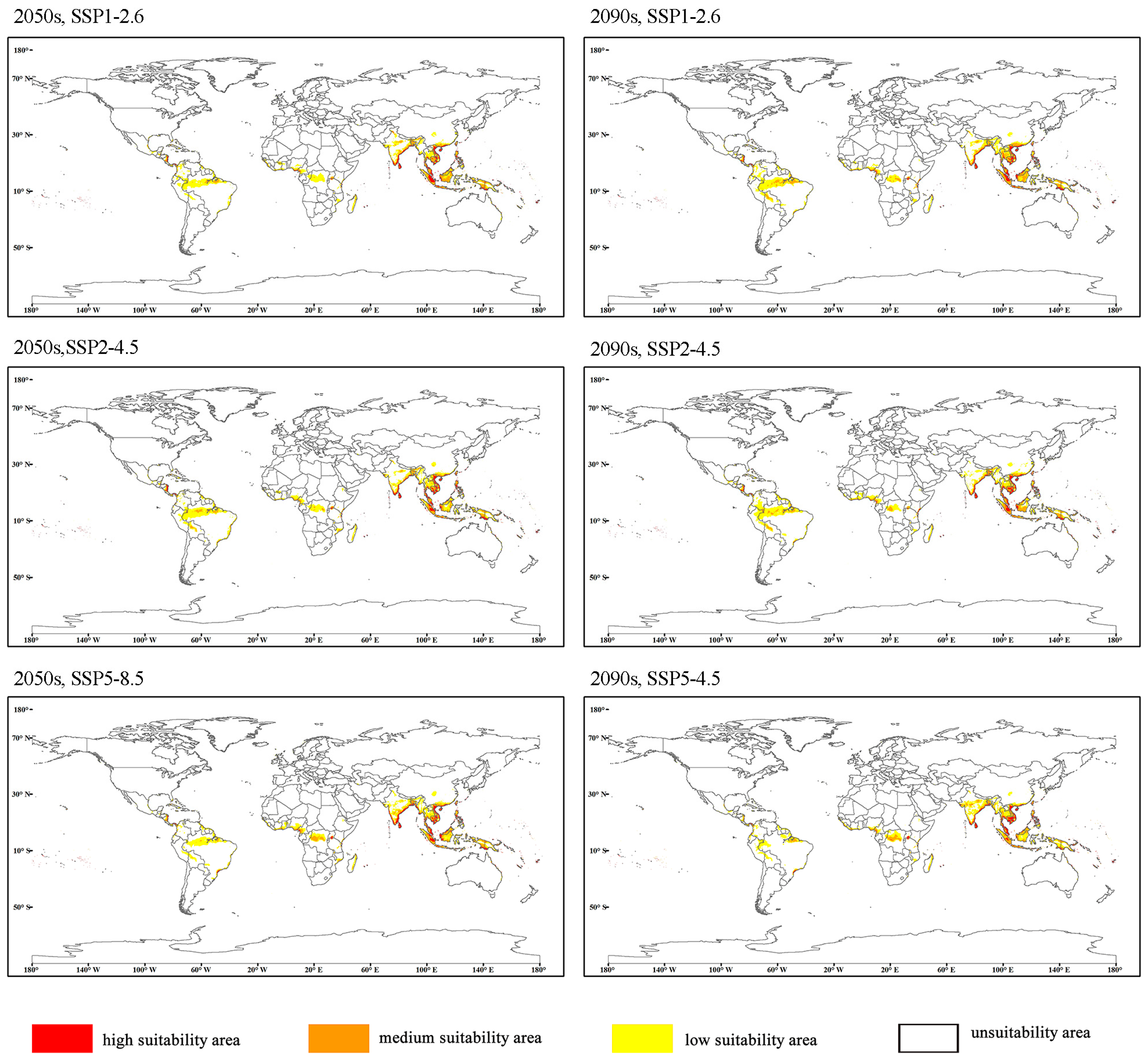
3.3. Potential Future Distribution of O. rhinoceros
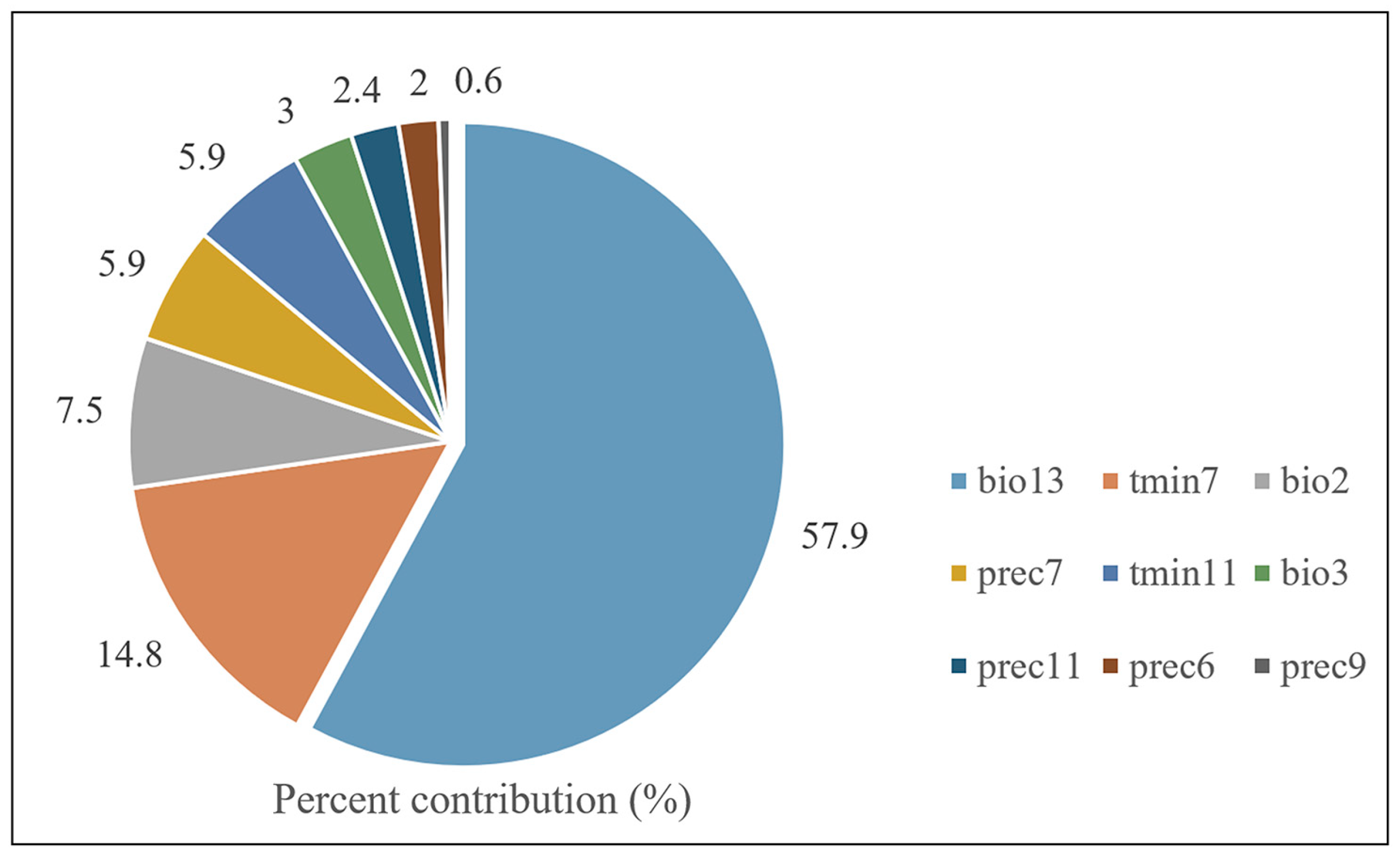
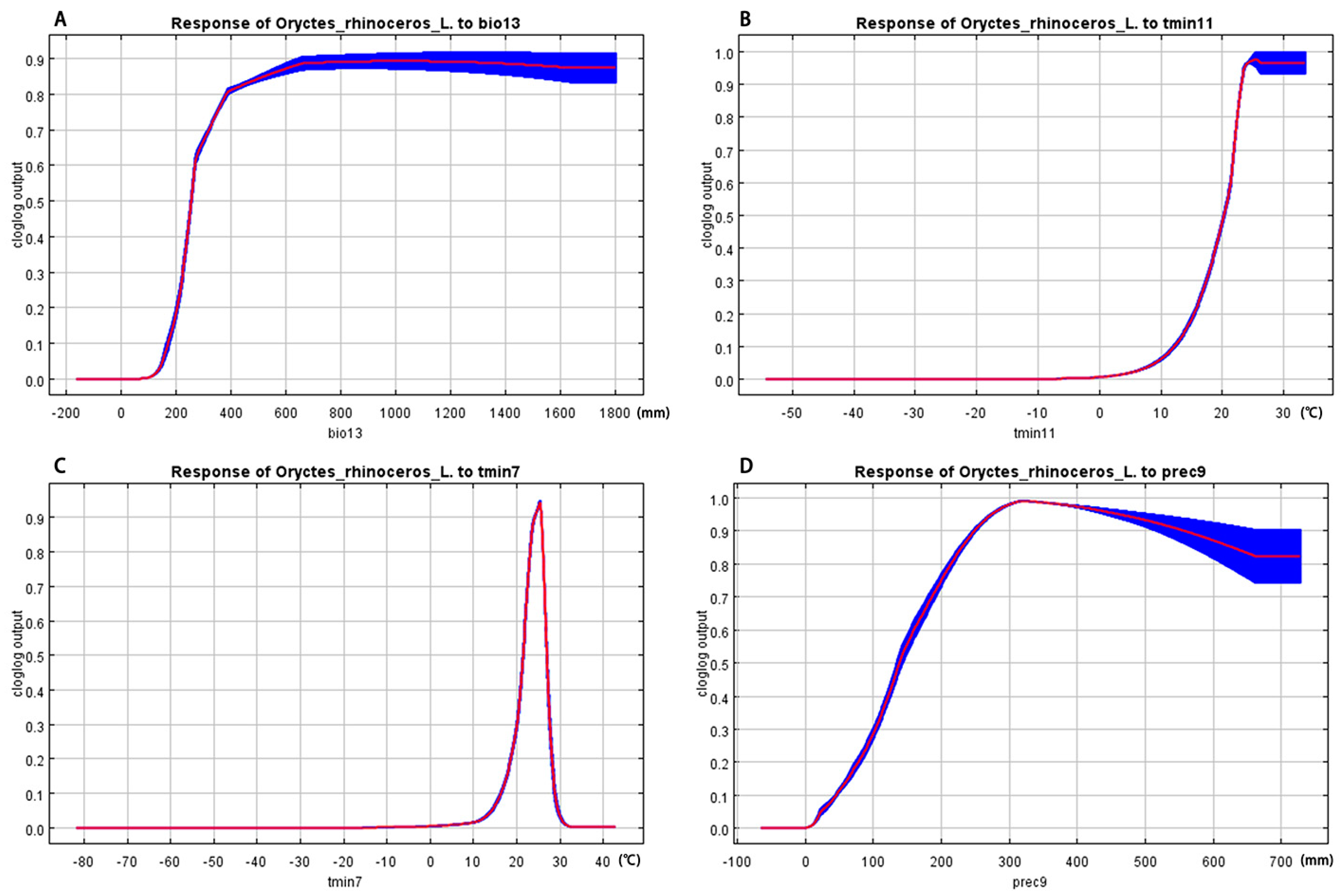
3.4. Environment Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aidoo, O.F.; Corcino Souza, P.G.; Da Silva, R.S.; Santana, P.A., Jr.; Picanco, M.C.; Kyerematen, R.; Setamou, M.; Ekesi, S.; Borgemeister, C. Climate-induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef] [PubMed]
- Bedford, G. Advances in the control of rhinoceros beetle, Oryctes rhinoceros in oil palm. J. Oil Palm Res. 2014, 26, 183–194. [Google Scholar]
- Pradeep Kumar, R.; Dinesh Babu, K.V.; Evans, D.A. Isolation, characterization and mode of action of a larvicidal compound, 22-hydroxyhopane from Adiantum latifolium Lam. against Oryctes rhinoceros Linn. Pestic. Biochem. Phys. 2019, 153, 161–170. [Google Scholar] [CrossRef]
- Hao-jun, C.; Yong-sen, C.; Xiao-dong, S.; Quan-guang, Z.; Chun-tian, W.; He-shuai, L.I. A preliminary survey of Cocos nucifera diseases and insect pests in Guangxi Zhuang Autonomic Region and Guangdong province. Guangdong Agric. Sci. 2011, 38, 73–75. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Sathiamma, B.; Nair, C.P. Control of the coconut pest Oryctes rhinoceros L. using the Oryctes virus. Int. J. Trop Insect Sc. 2001, 21, 93–101. [Google Scholar] [CrossRef]
- Kumara, N.T.; Muniyappa, C.; Kandakoor, S.; Chakravarthy, A. Status and Management of Three Major Insect Pests of Coconut in the Tropics and Subtropics; Springer Link: Berlin, Germany, 2015; pp. 359–381. [Google Scholar] [CrossRef]
- Moore, A.; Jackson, T.; Quitugua, R.; Bassler, P.; Campbell, R. Coconut Rhinoceros Beetles (Coleoptera: Scarabaeidae) Develop in Arboreal Breeding Sites in Guam. Fla Entomol. 2015, 98, 1012–1014. [Google Scholar] [CrossRef]
- Lin, M.; Han, Y.; Li, W.; Liu, F.; Xu, W.; Ao, S.; Wang, X. Monitor and survey of pest insects and deseases of coconut trees in Hainan. Plant Quar. 2010, 24, 21–24. [Google Scholar] [CrossRef]
- Bedford, G. Biology and Management of Palm Dynastid Beetles: Recent Advances. Annu. Rev. Entomol. 2013, 58, 353–372. [Google Scholar] [CrossRef]
- Etebari, K.; Hereward, J.; Sailo, A.; Ahoafi, E.; Tautua, R.; Tsatsia, H.; Jackson, G.; Furlong, M. Genetic structure of the Coconut Rhinoceros Beetle (Oryctes rhinoceros) population and the incidence of its biocontrol agent (Oryctes rhinoceros nudivirus) in the South Pacific Islands. BioRxiv, 2020. [Google Scholar] [CrossRef]
- Etebari, K.; Parry, R.; Beltran, M.J.; Furlong, M. Transcription profile and genomic variation of Oryctes rhinoceros nudivirus (OrNV) in Coconut Rhinoceros Beetle. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Bedford, G. Biology, Ecology, and Control of Palm Rhinoceros Beetles. Annu. Rev. Entomol. 1980, 25, 309–339. [Google Scholar] [CrossRef]
- Dhileepan, K. Incidence and intensity of Rhinoceros beetle infestation in the oil palm plantations in India. J. Plant. Crops 1988, 16, 126–129. [Google Scholar]
- Zhong, B.; Lv, C.; LI, H.; Wang, D.; Qin, W.; Wang, Z. Oviposition selection of Oryctes rhinoceros among different host stems. J. Environ. Entomol. 2013, 35, 13–17. [Google Scholar]
- Gopal, M.; Gupta, A.; Thomas, G.V. Prospects of using Metarhizium anisopliae to check the breeding of insect pest, Oryctes rhinoceros L. in coconut leaf vermicomposting sites. Bioresour. Technol. 2006, 97, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Oehlschlager, C. Optimizing Trapping of Palm Weevils and Beetles. Acta Hort. 2007, 736, 347–368. [Google Scholar] [CrossRef]
- Widihastuty; Tobing, M.C.; Marheni; Kuswardani, R.A.; Fudholi, A. Biological aspects of Myopopone castanea on it’s prey Oryctes rhinoceros larvae. J. Insect. Physiol. 2020, 125, 104089. [Google Scholar] [CrossRef]
- Finch, D.M.; Butler, J.L.; Runyon, J.B.; Fettig, C.J.; Kilkenny, F.F.; Jose, S.; Frankel, S.J.; Cushman, S.A.; Cobb, R.C.; Dukes, J.S.; et al. Effects of Climate Change on Invasive Species. In Invasive Species in Forests and Rangelands of the United States: A Comprehensive Science Synthesis for the United States Forest Sector; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 57–83. [Google Scholar]
- Peterson, A.; Soberón, J.; Pearson, R.; Anderson, R.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011; Volume 49. [Google Scholar] [CrossRef]
- Zhu, G.; Guoqing, L.; Bu, W.; Yubao, G. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Booth, T.; Nix, H.; Busby, J.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Stockwell, D.; Peters, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G.F. A computerised system for matching climates in ecology. Agric. Ecosyst. Environ. 1985, 13, 281–299. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Yunsheng, W. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 15, 365. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.; Anderson, R.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.; Huettmann, F.; Leathwick, J.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Lv, C.; Zhong, B.; Qin, W.; Yan, W. Risk analysis and control strategy of Oryctes rhinoceros on Elaeis guineensis and Cocos nucifera. J. Biosaf. 2020, 29, 39–43. [Google Scholar]
- Tomie, T.; Ishibashi, J.; Furukawa, S.; Kobayashi, S.; Sawahata, R.; Asaoka, A.; Tagawa, M.; Yamakawa, M. Scarabaecin, a novel cysteine-containing antifungal peptide from the rhinoceros beetle, Oryctes rhinoceros. Biochem. Bioph. Res. Co. 2003, 307, 261–266. [Google Scholar] [CrossRef]
- Xu, D.; Zhuo, Z.; Li, X.; Wang, R. Distribution and invasion risk assessment of Oryctes rhinoceros (L.) in China under changing climate. J. Appl. Entomol. 2022, 146, 385–395. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Wu, D.; Zhu, C. Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e856. [Google Scholar] [CrossRef]
- Zhu, G.; Qiang, L.; Yubao, G. Improving ecological niche model transferability to predict the potential distribution of invasive exotic species. Biodivers. Sci. 2014, 22, 223. [Google Scholar] [CrossRef]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Xu, D.; Zhuo, Z.; Wang, R.; Ye, M.; Pu, B. Modeling the distribution of Zanthoxylum armatum in China with MaxEnt modeling. Glob. Ecol. Conserv. 2019, 19, e691. [Google Scholar] [CrossRef]
- Walden-Schreiner, C.; Leung, Y.; Kuhn, T.; Newburger, T.; Tsai, W. Environmental and managerial factors associated with pack stock distribution in high elevation meadows: Case study from Yosemite National Park. J. Environ. Manag. 2017, 193, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Castex, V.; Beniston, M.; Calanca, P.; Fleury, D.; Moreau, J. Pest management under climate change: The importance of understanding tritrophic relations. Sci. Total Environ. 2018, 616–617, 397–407. [Google Scholar] [CrossRef]
- Booth, T.H. Species distribution modelling tools and databases to assist managing forests under climate change. For. Ecol. Manag. 2018, 430, 196–203. [Google Scholar] [CrossRef]
- Laeseke, P.; Martinez, B.; Mansilla, A.; Bischof, K. Correction to: Future range dynamics of the red alga Capreolia implexa in native and invaded regions: Contrasting predictions from species distribution models versus physiological knowledge. Biol. Invasions 2021, 23, 3279–3280. [Google Scholar] [CrossRef]
- Carlson, C.J. embarcadero: Species distribution modelling with Bayesian additive regression trees inr. Methods Ecol. Evol. 2020, 11, 850–858. [Google Scholar] [CrossRef]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, X.; Yang, Z.; Wieprecht, S.; Zhang, S.; Wu, Y. Evaluating the ecological influence of hydraulic projects: A review of aquatic habitat suitability models. Renew. Sustain. Energy Rev. 2017, 68, 748–762. [Google Scholar] [CrossRef]
- Cao, X.; Qian, G.; Hu, B.; Liu, F. [Prediction of potential suitable distribution area of Flaveria bidentis in China based on niche models]. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol./Zhongguo Sheng Tai Xue Xue Hui Zhongguo Ke Xue Yuan Shenyang Ying Yong Sheng Tai Yan Jiu Suo Zhu Ban 2010, 21, 3063–3069. [Google Scholar]
- Li, B.; Wei, W.; Ma, J.; Zhang, R. Maximum entropy niche-based modeling (Maxent) of potential geographical distributions of fruit flies Dacus bivittatus, D. ciliatus and D. vertebrates (Diptera: Tephritidae). Acta Entomol. Sin. 2009, 52, 1122–1131. [Google Scholar]
- Zhang, H.; Luo, D.; Xidong, M.; Xu, M.; Wei, H.; Luo, J.; Zhang, J.; Hu, Y. Predicting the potential suitable distribution area of the apple snail Pomacea canaliculata in China based on multiple ecological niche models. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. / Zhongguo Sheng Tai Xue Xue Hui Zhongguo Ke Xue Yuan Shenyang Ying Yong Sheng Tai Yan Jiu Suo Zhu Ban 2016, 27, 1277–1284. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Chen, D.; Guo, X.; Li, Q.; Wang, M. Analysis of the potential distribution of the Asian citrus psyllid, Diaphorina citri Kuwayama in Southwest China using the MaxEnt model. Plant Prot. 2021, 47, 84–90. [Google Scholar]
- Mohammadi, S.; Ebrahimi, E.; Shahriari Moghadam, M.; Bosso, L. Modelling current and future potential distributions of two desert jerboas under climate change in Iran. Ecol. Inf. 2019, 52, 7–13. [Google Scholar] [CrossRef]
- Heya, H.; Khamis, F.; Onyambu, G.; Akutse, K.; Mohamed, S.; Kimathi, E.; Ombura, F.; Ekesi, S.; Dubois, T.; Subramanian, S.; et al. Characterization and risk assessment of the invasive papaya mealybug, Paracoccus marginatus, in Kenya under changing climate. J. Appl. Entomol. 2020, 144, 442–458. [Google Scholar] [CrossRef]
- Sunday, C.E.; Olowu, R.A.; Moronkola, B.A.; Tovide, O.O.; Denloye, A.A.; Awokoya, K.N.; Sunday, C.E.; Olujimi, O.O. Assessment of Proximate and Mineral Status of Rhinoceros Beetle Larva, Oryctes Rhinoceros Linnaeus (1758) (Coleoptera: Scarabaeidae) from Itokin, Lagos State, Nigeria. Res. J. Environ. Sci. 2012, 6, 118–124. [Google Scholar] [CrossRef]
- Hao, M.; Aidoo, O.F.; Qian, Y.; Wang, D.; Ding, F.; Ma, T.; Tettey, E.; Ninsin, K.D.; Osabutey, A.F.; Borgemeister, C. Global potential distribution of Oryctes rhinoceros, as predicted by Boosted Regression Tree model. Glob. Ecol. Conserv. 2022, 37, e2175. [Google Scholar] [CrossRef]
- Monteith, J.L. Agricultural Meteorology: Evolution and application. Agric. For. Meteorol. 2000, 103, 5–9. [Google Scholar] [CrossRef]
- Qin, J.; Yang, X.; Yang, Z.; Luo, J.; Lei, X. New technology for using meteorological information in forest insect pest forecast and warning systems. Pest Manag. Sci. 2017, 73, 2509–2518. [Google Scholar] [CrossRef]
- Chang, X.N.; Gao, H.J.; Chen, F.J.; Zhai, B.P. Effects of environmental moisture and precipitation on insects: A review. Chin. J. Ecol. 2008, 27, 619–625. [Google Scholar]
- Wang, L. Study on the Insect Diversity in Different Environment in Xiaowutai Mountain Natural Reserve Areas. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2013. [Google Scholar]
- Xu, D.; Li, X.; Jin, Y.; Zhuo, Z.; Yang, H.; Hu, J.; Wang, R. Influence of climatic factors on the potential distribution of pest Heortia vitessoides Moore in China. Glob. Ecol. Conserv. 2020, 23, e1107. [Google Scholar] [CrossRef]
- Jacob, T.K.; Bhumannavar, B.S. The coconut rhinoceros beetle Oryctes rhinoceros L.–its incidence and extent of palm damage in the Andaman and Nicobar Islands (India). Trop. Pest Manag. 1991, 37, 80–84. [Google Scholar] [CrossRef]
- Kamarudin, N. Immigration and activity of Oryctes rhinoceros within a small oil palm replanting area. J. Oil Palm Res. 2004, 16, 64–77. [Google Scholar]
- Young, E.C. The rhinoceros beetle project: History and review of the research programme. Agric. Ecosyst. Environ. 1986, 15, 149–166. [Google Scholar] [CrossRef]
- Jalaeian, M.; Golizadeh, A.; Sarafrazi, A.; Naimi, B. Inferring climatic controls of rice stem borers’ spatial distributions using maximum entropy modelling. J. Appl. Entomol. 2018, 142, 388–396. [Google Scholar] [CrossRef]



| Code | Environmental Variables |
|---|---|
| bio1 | Annual Mean Temperature |
| bio2 | Mean Diurnal Range (Mean of monthly (max temp–min temp)) |
| bio3 | Isothermality (bio 2/bio 7) (×100) |
| bio4 | Temperature Seasonality (standard deviation × 100) |
| bio5 | Max Temperature of Warmest Month |
| bio6 | Min Temperature of Coldest Month |
| bio7 | Temperature Annual Range (bio5-bio6) |
| bio8 | Mean Temperature of Wettest Quarter |
| bio9 | Mean Temperature of Driest Quarter |
| bio10 | Mean Temperature of Warmest Quarter |
| bio11 | Mean Temperature of Coldest Quarter |
| bio12 | Annual Precipitation |
| bio13 | Precipitation of Wettest Month |
| bio14 | Precipitation of Driest Month |
| bio15 | Precipitation Seasonality (Coefficient of Variation) 1 |
| bio16 | Precipitation of Wettest Quarter |
| bio17 | Precipitation of Driest Quarter |
| bio18 | Precipitation of Warmest Quarter |
| bio19 | Precipitation of Coldest Quarter |
| Tmin | Minimum Temperature of Each Month |
| Tmax | Maximum Temperature of Each Month |
| Tmean | Mean Temperature of Each Month |
| Prec | Precipitation of Each Month |
| bio2 | bio3 | bio13 | prec6 | prec7 | prec9 | prec11 | tmin7 | |
|---|---|---|---|---|---|---|---|---|
| bio3 | 0.140 | |||||||
| bio13 | −0.181 * | 0.039 | ||||||
| prec6 | −0.233 ** | −0.227 ** | 0.782 ** | |||||
| prec7 | −0.110 | −0.279 ** | 0.782 ** | 0.776 ** | ||||
| prec9 | −0.210 ** | −0.126 | 0.480 ** | 0.640 ** | 0.700 ** | |||
| prec11 | −0.184 * | 0.638 ** | 0.030 | −0.279 ** | −0.374 ** | −0.130 | ||
| tmin7 | −0.324 ** | −0.231 ** | 0.068 | 0.160 * | 0.173 * | 0.343 ** | 0.002 | |
| tmin11 | −0.418 ** | 0.557 ** | 0.084 | −0.008 | −0.079 | 0.093 | 0.490 ** | 0.457 ** |
| Continent | Low Suitability | Medium Suitability | High Suitability | Total Suitability Area |
|---|---|---|---|---|
| Africa | 122.57 | 46.75 | 14.41 | 183.73 |
| Asia | 118.38 | 118.51 | 69.79 | 306.68 |
| Europe | 2.37 | 0.10 | 0.04 | 2.51 |
| North America | 29.66 | 16.27 | 2.70 | 48.63 |
| Oceania | 12.63 | 8.06 | 1.98 | 22.67 |
| South America | 179.63 | 26.78 | 0.98 | 207.39 |
| Total | 465.24 | 216.47 | 89.90 | 771.61 |
| Decade | Scenarios | Predicted Suitable Areas (×104 km2) | Comparison with Current (%) | ||||
|---|---|---|---|---|---|---|---|
| Low Suitability | Medium Suitability | High Suitability | Low Suitability | Medium Suitability | High Suitability | ||
| Current | 465.24 | 216.47 | 89.90 | ||||
| 2050s | SSP1-2.6 | 494.59 | 243.70 | 94.28 | 6.31% | 12.58% | 4.87% |
| SSP2-4.5 | 518.62 | 242.64 | 95.49 | 11.47% | 12.09% | 6.22% | |
| SSP5-8.5 | 486.06 | 248.21 | 112.39 | 4.48% | 14.66% | 25.02% | |
| 2090s | SSP1-2.6 | 508.73 | 287.20 | 102.97 | 8.55% | 32.67% | 14.54% |
| SSP2-4.5 | 519.56 | 261.56 | 95.02 | 11.68% | 20.83% | 5.70% | |
| SSP5-8.5 | 431.53 | 244.96 | 94.68 | −7.25% | 13.16% | 5.32% | |
| Continent | Suitable Area (×104 km2) | ||||||
|---|---|---|---|---|---|---|---|
| Current | 2050s | 2090s | |||||
| SSP1-2.6 | SSP2-4.5 | SSP5-8.5 | SSP1-2.6 | SSP2-4.5 | SSP5-8.5 | ||
| Africa | 183.73 | 235.00 | 218.59 | 262.49 | 221.23 | 207.78 | 250.10 |
| Asia | 306.68 | 327.49 | 335.19 | 342.04 | 359.25 | 333.12 | 328.15 |
| Europe | 2.51 | 1.85 | 1.56 | 3.60 | 0.78 | 3.28 | 1.87 |
| North America | 48.63 | 32.75 | 32.22 | 25.43 | 28.32 | 34.20 | 20.06 |
| Oceania | 22.67 | 34.04 | 30.44 | 32.74 | 31.89 | 31.32 | 31.45 |
| South America | 207.39 | 193.98 | 231.29 | 173.15 | 249.97 | 259.00 | 132.22 |
| Total | 771.61 | 825.11 | 849.29 | 839.45 | 891.44 | 868.70 | 763.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Qian, Q.; Deng, X.; Zhuo, Z.; Xu, D. Prediction and Analysis of the Global Suitable Habitat of the Oryctes rhinoceros (Linnaeus, 1758) (Coleoptera: Scarabaeidae) Based on the MaxEnt Model. Insects 2024, 15, 774. https://doi.org/10.3390/insects15100774
Fu C, Qian Q, Deng X, Zhuo Z, Xu D. Prediction and Analysis of the Global Suitable Habitat of the Oryctes rhinoceros (Linnaeus, 1758) (Coleoptera: Scarabaeidae) Based on the MaxEnt Model. Insects. 2024; 15(10):774. https://doi.org/10.3390/insects15100774
Chicago/Turabian StyleFu, Chun, Qianqian Qian, Xinqi Deng, Zhihang Zhuo, and Danping Xu. 2024. "Prediction and Analysis of the Global Suitable Habitat of the Oryctes rhinoceros (Linnaeus, 1758) (Coleoptera: Scarabaeidae) Based on the MaxEnt Model" Insects 15, no. 10: 774. https://doi.org/10.3390/insects15100774
APA StyleFu, C., Qian, Q., Deng, X., Zhuo, Z., & Xu, D. (2024). Prediction and Analysis of the Global Suitable Habitat of the Oryctes rhinoceros (Linnaeus, 1758) (Coleoptera: Scarabaeidae) Based on the MaxEnt Model. Insects, 15(10), 774. https://doi.org/10.3390/insects15100774






