Energy Reserve Allocation in the Trade-Off between Migration and Reproduction in Fall Armyworm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Observation of Migratory Behavior
2.3. Tethered Flight Test and Ovarian Dissection
2.4. RNA Sequencing and Transcriptomic Analysis
2.5. Triglyceride and Glycogen Measurement
2.6. Statistical Analyses
3. Results
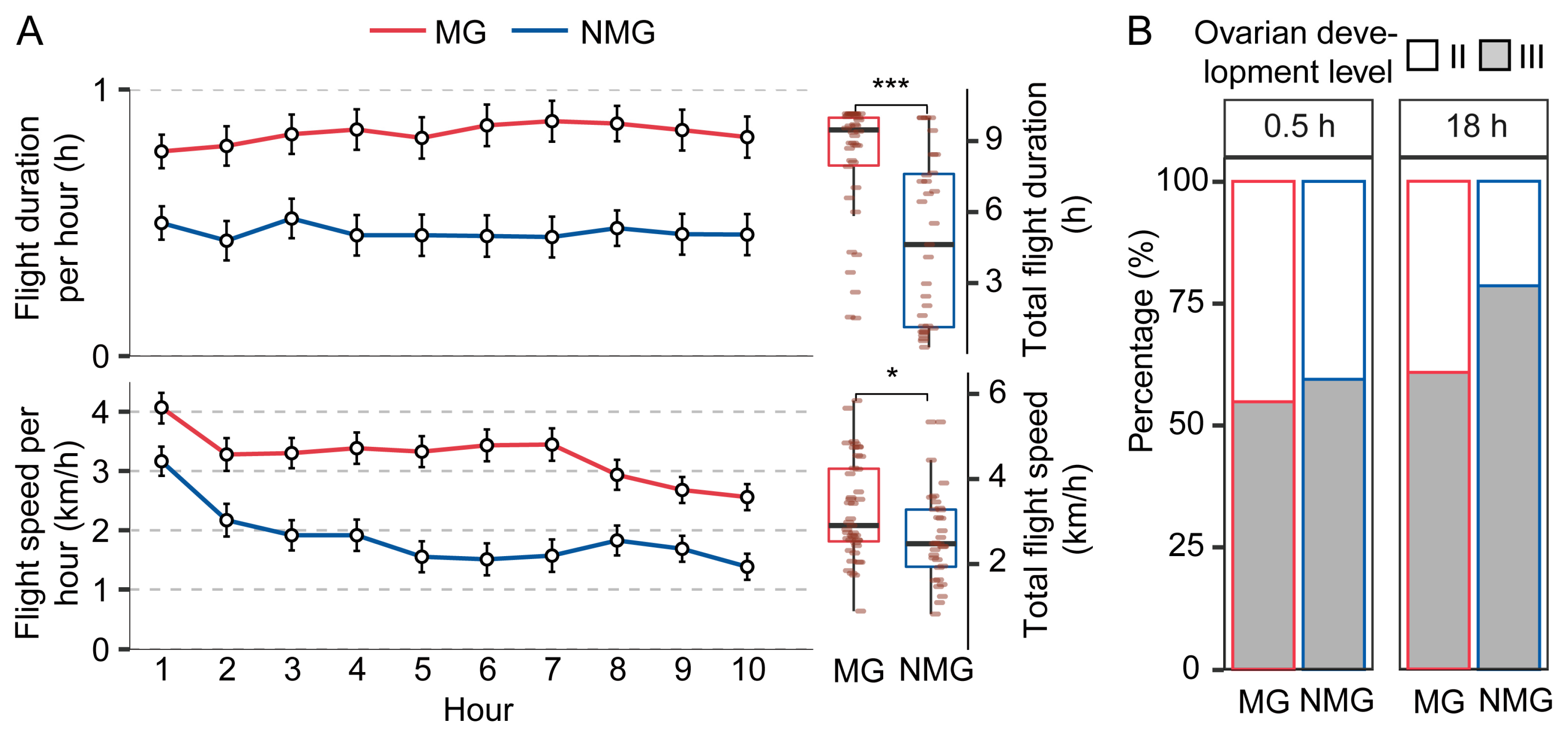
3.1. Migratory Females Display Delayed Ovarian Development but Higher Flight Performance
3.2. Transcriptome Analysis of Migratory and Non-Migratory Females with Different Levels of Ovarian Development
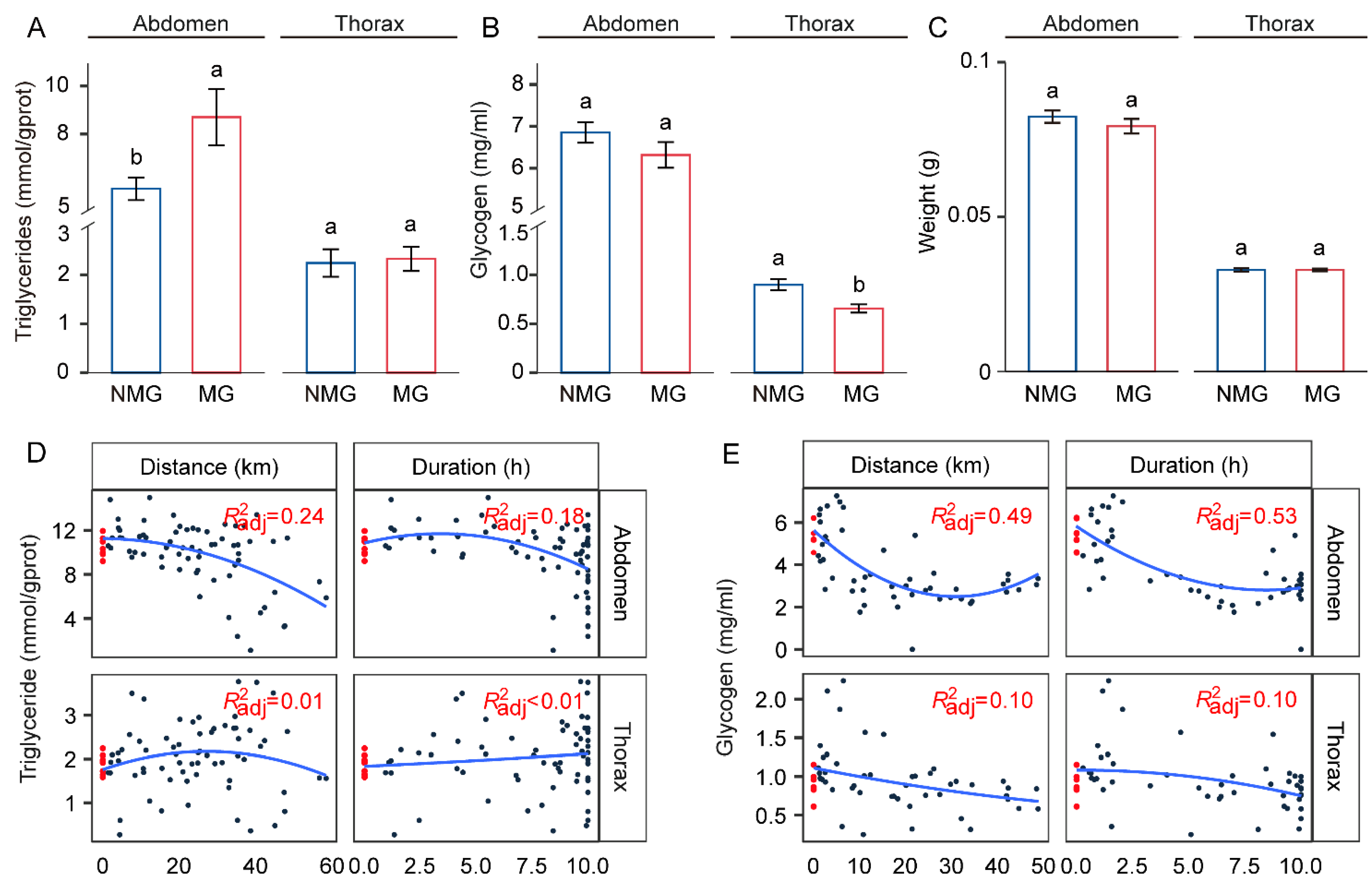
3.3. Triglyceride Levels Are Increased in the Abdomen of Migratory Individuals
3.4. Triglycerides and Glycogen Are Utilized during Flight
4. Discussion
4.1. Down-Regulated Genes Associated with Fatty Acid Synthesis May Be a Key Trait of Migratory Females with Ovaries at Level III
4.2. Lipids Are Accumulated More in Migratory Moths and Stored as Fuel for Later Phase of Migratory Flight
4.3. Glycogen Is Utilized during the Initial Phases of Flight in Migratory Females
4.4. Other Functions Involved in Regulating Individual Migratory Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MG | Migratory |
| NMG | non-migratory |
| DEGs | differentially expressed genes |
| HSD17B12 | hydroxysteroid 17-beta dehydrogenase 12 |
| HK | hexokinase |
| AMY | alpha-Amylase |
| NKA-α | Na+/K+ transport ATPase |
| PLC-β | phosphatidylinositol phospholipase C, beta |
| ACACA | acetyl-CoA carboxylase |
| FAS | fatty acid synthase |
| FACL | fatty acid coenzyme A ligase |
| PPT | palmitoyl-protein thioesterase |
| SCD | stearoyl-CoA desaturase |
| ELOVL4 | very-long-chain fatty acid 4 |
| ELOVL7 | very-long-chain fatty acid 7 |
| VLCAD | very-long-chain acyl-CoA dehydrogenase |
| TSL | torso-like protein |
| GABA | gamma-aminobutyric acid |
| GABAB | gamma-aminobutyric acid type B receptor |
References
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Dingle, H. Migration: The Biology of Life on the Move, 2nd ed.; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Menz, M.H.M.; Reynolds, D.R.; Gao, B.; Hu, G.; Chapman, J.W.; Wotton, K.R. Mechanisms and consequences of partial migration in insects. Front. Ecol. Evol. 2019, 7, 403. [Google Scholar] [CrossRef]
- Dingle, H.; Drake, V.A. What is migration? Bioscience 2007, 57, 113–121. [Google Scholar] [CrossRef]
- Holland, R.A.; Wikelski, M.; Wilcove, D.S. How and why do insects migrate? Science 2006, 313, 794–796. [Google Scholar] [CrossRef]
- Jones, C.M.; Papanicolaou, A.; Mironidis, G.K.; Vontas, J.; Yang, Y.; Lim, K.S.; Oakeshott, J.G.; Bass, C.; Chapman, J.W. Genomewide transcriptional signatures of migratory flight activity in a globally invasive insect pest. Mol. Ecol. 2015, 24, 4901–4911. [Google Scholar] [CrossRef]
- Rankin, M.A.; Burchsted, J.C.A. The cost of migration in insects. Annu. Rev. Entomol. 1992, 37, 533–559. [Google Scholar] [CrossRef]
- Tanaka, S.; Suzuki, Y. Physiological trade-offs between reproduction, flight capability and longevity in a wing-dimorphic cricket, Modicogryllus confirmatus. J. Insect Physiol. 1998, 44, 121–129. [Google Scholar] [CrossRef]
- Zera, A.J.; Harshman, L.G. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 2001, 32, 95–126. [Google Scholar] [CrossRef]
- Green, D.A.; Kronforst, M.R. Monarch butterflies use an environmentally sensitive, internal timer to control overwintering dynamics. Mol. Ecol. 2019, 28, 3642–3655. [Google Scholar] [CrossRef]
- Guerra, P.A. Evaluating the life-history trade-off between dispersal capability and reproduction in wing dimorphic insects: A meta-analysis. Biol. Rev. 2011, 86, 813–835. [Google Scholar] [CrossRef]
- Liu, P.C.; Diao, Y.H.; Guo, J.W.; Gao, B.Y.; Hu, G. Insect migration behavior and its regulation. Chin. J. Appl. Entomol. 2021, 58, 520–529. [Google Scholar] [CrossRef]
- Johnson, C.G. Physiological factors in insect migration by flight. Nature 1963, 4879, 423–427. [Google Scholar] [CrossRef]
- Johnson, C.G. Migration and Dispersal of Insects by Flight; Methuen: London, UK, 1969. [Google Scholar]
- Zhao, X.C.; Feng, H.Q.; Wu, B.; Wu, X.F.; Liu, Z.F.; Wu, K.M.; McNeil, J.N. Does the onset of sexual maturation terminate the expression of migratory behaviour in moths? A study of the oriental armyworm, Mythimna separata. J. Insect Physiol. 2009, 55, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W. Oogenesis-flight syndrome in crickets: Age-dependent egg production, flight performance, and biochemical composition of the flight muscles in adult female Gryllus bimaculatus. J. Insect Physiol. 2007, 53, 819–832. [Google Scholar] [CrossRef]
- Zera, A.J. Intermediary metabolism and life history trade-offs: Lipid metabolism in lines of the wing-polymorphic cricket, Gryllus firmus, selected for flight capability vs. early age reproduction. Integr. Comp. Biol. 2005, 45, 511–524. [Google Scholar] [CrossRef]
- Beenakkers, A.M.T.; Vanderhorst, D.J.; Vanmarrewijk, W. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985, 24, 19–67. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Nation, J.L. Insect Physiology and Biochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781482247589. [Google Scholar]
- Murata, M.; Tojo, S. Utilization of lipid for flight and reproduction in Spodoptera litura (Lepidoptera: Noctuidae). Eur. J. Entomol. 2002, 99, 221–224. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Chen, H.; Wu, M.F.; Liu, J.; Chen, A.D.; Jiang, Y.Y.; Hu, G. Migratory routes and occurrence divisions of the fall armyworm Spodoptera frugiperda in China. J. Plant Prot. 2020, 47, 747–757. [Google Scholar] [CrossRef]
- Li, X.J.; Wu, M.F.; Ma, J.; Gao, B.Y.; Wu, Q.L.; Chen, A.D.; Liu, J.; Jian, Y.Y.; Zhai, B.P.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2020, 76, 454–463. [Google Scholar] [CrossRef]
- Ge, S.; He, W.; He, L.; Yan, R.; Zhang, H.W.; Wu, K.M. Flight activity promotes reproductive processes in the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 727–735. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhu, Q.Z.; Tan, Y.T.; Ma, Q.L.; Wang, R.F.; Zhang, M.F.; Xu, H.H.; Zhang, Z.X. Artificial diets and rearing technique of Spodoptera frugiperda (J.E. Smith) in laboratory. J. Environ. Entomol. 2019, 41, 742–747. [Google Scholar]
- Guo, J.; Yang, F.; Li, P.; Liu, X.D.; Wu, Q.L.; Hu, G.; Zhai, B.P. Female bias in an immigratory population of Cnaphalocrocis medinalis moths based on field surveys and laboratory tests. Sci. Rep. 2019, 9, 18388. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Y.; Huang, L.; Xu, C.F.; Li, J.H.; Wang, F.Y.; Cheng, W.; Gao, B.Y.; Chapman, J.W.; Hu, G. Flight capability and the low temperature threshold of a chinese field population of the fall armyworm Spodoptera frugiperda. Insects 2022, 13, 422. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Yang, X.M.; He, W.; Zhang, H.W.; Jiang, Y.Y.; Wu, K.M. Ovarian development gradation and reproduction potential prediction in Spodoptera frugiperda. Plant Prot. 2019, 45, 28–34. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Berkopec, A. HyperQuick algorithm for discrete hypergeometric distribution. J. Discret. Algorithms 2007, 5, 341–347. [Google Scholar] [CrossRef]
- Liu, L.; Davis, R.L.; Roman, G. Exploratory activity in Drosophila requires the kurtz nonvisual arrestin. Genetics 2007, 175, 1197–1212. [Google Scholar] [CrossRef]
- Mustard, J.A.; Jones, L.; Wright, G.A. GABA signaling affects motor function in the honey bee. J. Insect Physiol. 2020, 120, 103989. [Google Scholar] [CrossRef] [PubMed]
- Strambi, C.; Cayre, M.; Sattelle, D.B.; Augier, R.; Charpin, P.; Strambi, A. Immunocytochemical mapping of an RDL-like GABA receptor subunit and of GABA in brain structures related to learning and memory in the cricket Acheta domesticus. Learn. Mem. 1998, 5, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, Y.; Hu, Y.; Shi, J.; Li, Q.; Wang, Y.; Xia, Q.; Guo, P. Structural characterization and functional analysis of mevalonate kinase from Tribolium castaneum (red flour beetle). Int. J. Mol. Sci. 2024, 25, 2552. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Chokki, T.; Nishioka, T.; Morimoto, K.; Nakayama, A.; Nakae, H.; Ogasawara, M.; Terasaki, A.G. The emergence of nebulin repeats and evolution of lasp family proteins. Cytoskeleton 2021, 78, 419–435. [Google Scholar] [CrossRef]
- Henstridge, M.A.; Aulsebrook, L.; Koyama, T.; Johnson, T.K.; Whisstock, J.C.; Tiganis, T.; Mirth, C.K.; Warr, C.G. Torso-like is a component of the hemolymph and regulates the insulin signaling pathway in Drosophila. Genetics 2018, 208, 1523–1533. [Google Scholar] [CrossRef]
- Cho, J.; Hur, J.H.; Graniel, J.; Benzer, S.; Walker, D.W. Expression of yeast NDI1 rescues a Drosophila complex I assembly defect. PLoS ONE 2012, 7, e50644. [Google Scholar] [CrossRef]
- French, C.M.; Bertola, L.D.; Carnaval, A.C.; Economo, E.P.; Kass, J.M.; Lohman, D.J.; Marske, K.A.; Meier, R.; Overcast, I.; Rominger, A.J.; et al. Global determinants of insect mitochondrial genetic diversity. Nat. Commun. 2023, 14, 5276. [Google Scholar] [CrossRef]
- Dixit, S.; Thakur, N.; Shukla, A.; Upadhyay, S.K.; Verma, P.C. Molecular characterization of N-methyl-d-aspartate receptor from Bemisia tabaci. Insect Mol. Biol. 2021, 30, 231–240. [Google Scholar] [CrossRef]
- Sajjadian, S.M.; Ahmed, S.; Al Baki, M.A.; Kim, Y. Prostaglandin D2 synthase and its functional association with immune and reproductive processes in a lepidopteran insect, Spodoptera exigua. Gen. Comp. Endocr. 2020, 287, 113352. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Klett, E.L.; De Paula, I.F.; Ramos, I.B.; Coleman, R.A.; Gondim, K.C. Long-chain acyl-CoA synthetase 2 knockdown leads to decreased fatty acid oxidation in fat body and reduced reproductive capacity in the insect Rhodnius prolixus. BBA Mol. Cell Biol. Lipids 2016, 1861, 650–662. [Google Scholar] [CrossRef]
- Luo, J.; Liang, S.; Li, J.; Xu, Z.; Li, L.; Zhu, B.; Li, Z.; Lei, C.L.; Lindsey, K.; Chen, L.Z.; et al. A transgenic strategy for controlling plant bugs (Adelphocoris suturalis) through expression of double-stranded RNA homologous to fatty acyl-coenzyme A reductase in cotton. New Phytol. 2017, 215, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Anand, A.N. Changes in the biochemical composition of fat body stores during adult development of female crickets, Gryllus bimaculatus. Arch. Insect Biochem. Physiol. 2004, 56, 110–119. [Google Scholar] [CrossRef]
- Du, B.; Ding, D.; Ma, C.; Guo, W.; Kang, L. Locust density shapes energy metabolism and oxidative stress resulting in divergence of flight traits. Proc. Natl. Acad. Sci. USA 2022, 119, e2115753118. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Wu, K. Biological characteristics and energy metabolism of migrating insects. Metabolites 2023, 13, 439. [Google Scholar] [CrossRef]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Saraiva, F.B.; Alves-Bezerra, M.; Majerowicz, D.; Paes-Vieira, L.; Braz, V.; Almeida, M.G.; Meyer-Fernandes, J.R.; Gondim, K.C. Blood meal drives de novo lipogenesis in the fat body of Rhodnius prolixus. Insect Biochem. Mol. 2021, 133, 103511. [Google Scholar] [CrossRef]
- Verkruyse, L.A.; Hofmann, S.L. Lysosomal targeting of palmitoyl-protein thioesterase. J. Biol. Chem. 1996, 271, 15831–15836. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, J.; Xu, B.; Ge, L.Q.; Yang, G.Q.; Wu, J.C. Long chain fatty acid coenzyme A ligase (FACL) regulates triazophos-induced stimulation of reproduction in the small brown planthopper (SBPH), Laodelphax striatellus (Fallen). Pestic. Biochem. Phys. 2018, 148, 81–86. [Google Scholar] [CrossRef]
- Jakobsson, A.; Westerberg, R.; Jacobsson, A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog. Lipid Res. 2006, 45, 237–249. [Google Scholar] [CrossRef]
- Nagasaki, S.; Suzuki, T.; Miki, Y.; Akahira, J.I.; Kitada, K.; Ishida, T.; Handa, H.; Ohuchi, N.; Sasano, H. 17β-Hydroxysteroid dehydrogenase type 12 in human breast carcinoma: A prognostic factor via potential regulation of fatty acid synthesis. Cancer Res. 2009, 69, 1392–1399. [Google Scholar] [CrossRef]
- Bakhache, W.; Neyret, A.; McKellar, J.; Clop, C.; Bernard, E.; Weger-Lucarelli, J.; Briant, L. Fatty acid synthase and stearoyl-CoA desaturase-1 are conserved druggable cofactors of Old World Alphavirus genome replication. Antivir. Res. 2019, 172, 104642. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Denlinger, D.L. Transcription profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipiens. Physiol. Genom. 2009, 39, 202–209. [Google Scholar] [CrossRef]
- Moraes, B.; Braz, V.; Santos-Araujo, S.; Oliveira, I.A.; Bomfim, L.; Ramos, I.; Gondim, K.C. Deficiency of acetyl-CoA carboxylase impairs digestion, lipid synthesis, and reproduction in the kissing bug Rhodnius prolixus. Front. Physiol. 2022, 13, 934667. [Google Scholar] [CrossRef]
- Suarez, R.K.; Darveau, C.A.; Welch, K.C., Jr.; O’Brien, D.M.; Roubik, D.W.; Hochachka, P.W. Energy metabolism in orchid bee flight muscles: Carbohydrate fuels all. J. Exp. Biol. 2005, 208, 3573–3579. [Google Scholar] [CrossRef]
- Wang, B.; Huang, D.; Cao, C.; Gong, Y. Insect α-amylases and their application in pest management. Molecules 2023, 28, 7888. [Google Scholar] [CrossRef]
- Lin, X.; Xu, W. Hexokinase is a key regulator of energy metabolism and ROS activity in insect lifespan extension. Aging 2016, 8, 245–259. [Google Scholar] [CrossRef]
- Hwang, H.J.; Jang, H.J.; Cocco, L.; Suh, P.G. The regulation of insulin secretion via phosphoinositide-specific phospholipase Cβ signaling. Adv. Biol. Regul. 2019, 71, 10–18. [Google Scholar] [CrossRef]
- Sun, H.J.; Cao, L.; Zhu, M.Y.; Wu, Z.Y.; Shen, C.Y.; Nie, X.W.; Bian, J.S. DR-region of Na+/K+-ATPase is a target to ameliorate hepatic insulin resistance in obese diabetic mice. Theranostics 2020, 10, 6149. [Google Scholar] [CrossRef]
- Heinze, S. Unraveling the neural basis of insect navigation. Curr. Opin. Insect Sci. 2017, 24, 58–67. [Google Scholar] [CrossRef]
- Shichida, Y.; Matsuyama, T. Evolution of opsins and phototransduction. Phil. Trans. R. Soc. B 2009, 364, 2881–2895. [Google Scholar] [CrossRef]
- Landry, C.R.; Castillo-Davis, C.I.; Ogura, A.; Liu, J.S.; Hartl, D.L. Systems-level analysis and evolution of the phototransduction network in Drosophila. Proc. Natl. Acad. Sci. USA 2007, 104, 3283–3288. [Google Scholar] [CrossRef]
- Bähner, M.; Frechter, S.; Da Silva, N.; Minke, B.; Paulsen, R.; Huber, A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron 2002, 34, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, Z.; Cai, L.; Shen, Z.; Michaud, J.P.; Zhu, L.; Yan, S.; Ros, V.I.D.; Hoover, K.; Li, Z.; et al. Baculoviruses hijack the visual perception of their caterpillar hosts to induce climbing behaviour thus promoting virus dispersal. Mol. Ecol. 2022, 31, 2752–2765. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Chen, J.; Si, H.; Yan, L.; Chu, T.; Wu, C.; Chen, J.; Yan, C. Study of the migrations of brown planthopper Nilaparvata lugens Stål. Acta Entomol. Sin. 1979, 22, 1–21. [Google Scholar]
- Yang, H.; Dong, J.; Hu, Z.; Li, D.; Fang, F.; Zhai, B. Temporary inhibition of positive phototaxis in emigratory population of Nilaparvata lugens by mark-release-recapture. PLoS ONE 2019, 14, e0222214. [Google Scholar] [CrossRef]
- Lin, X.; Yao, Y.; Wang, B.; Emlen, D.J.; Lavine, L.C. Ecological trade-offs between migration and reproduction are mediated by the nutrition-sensitive insulin-signaling pathway. Int. J. Biol. Sci. 2016, 12, 607. [Google Scholar] [CrossRef]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef]
- Matsuo, R.; Takatori, Y.; Hamada, S.; Koyanagi, M.; Matsuo, Y. Expression and light-dependent translocation of β-arrestin in the visual system of the terrestrial slug Limax valentianus. J. Exp. Biol. 2017, 220, 3301–3314. [Google Scholar] [CrossRef]
- Osborne, R.H. Insect neurotransmission: Neurotransmitters and their receptors. Pharmacol. Ther. 1996, 69, 117–142. [Google Scholar] [CrossRef]

| Gene ID | Annotation | log2 FC | Function | |
|---|---|---|---|---|
| MG-II vs. NMG-II | MG-III vs. NMG-III | |||
| LOC118262805 | beta-arrestin | 1.25 | / | Locomotion [33] |
| XLOC_008866 | gamma-aminobutyric acid type B receptor | −1.77 | / | Locomotion [34] |
| LOC118263634 | gamma-aminobutyric acid receptor subunit beta | / | −2.02 | Learning and memory [35] |
| LOC118265756 | mevalonate kinase | 1.07 | / | Juvenile hormone synthesis [36] |
| LOC118270612 | actin-binding LIM protein | −1.22 | / | Muscle contraction [37] |
| LOC118271205 | torso-like protein | / | 2.01 | Insulin signaling [38] |
| LOC118272993 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex assembly factor 1 | / | 1.11 | Mitochondrion metabolism [39] |
| LOC126913043 | cytochrome c oxidase subunit 1 | / | −29.06 | Mitochondrion metabolism [40] |
| LOC118278287 | glutamate receptor ionotropic, NMDA 1 | / | −2.08 | Reproduction [41] |
| XLOC_002619 | prostaglandin-H2 D-isomerase/glutathione transferase | −1.54 | −1.71 | Reproduction [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.-F.; Liu, P.-C.; Chapman, J.W.; Wotton, K.R.; Qi, G.-J.; Wang, Y.-M.; Hu, G. Energy Reserve Allocation in the Trade-Off between Migration and Reproduction in Fall Armyworm. Insects 2024, 15, 809. https://doi.org/10.3390/insects15100809
Xu C-F, Liu P-C, Chapman JW, Wotton KR, Qi G-J, Wang Y-M, Hu G. Energy Reserve Allocation in the Trade-Off between Migration and Reproduction in Fall Armyworm. Insects. 2024; 15(10):809. https://doi.org/10.3390/insects15100809
Chicago/Turabian StyleXu, Chuan-Feng, Peng-Cheng Liu, Jason W. Chapman, Karl R. Wotton, Guo-Jun Qi, Yu-Meng Wang, and Gao Hu. 2024. "Energy Reserve Allocation in the Trade-Off between Migration and Reproduction in Fall Armyworm" Insects 15, no. 10: 809. https://doi.org/10.3390/insects15100809
APA StyleXu, C.-F., Liu, P.-C., Chapman, J. W., Wotton, K. R., Qi, G.-J., Wang, Y.-M., & Hu, G. (2024). Energy Reserve Allocation in the Trade-Off between Migration and Reproduction in Fall Armyworm. Insects, 15(10), 809. https://doi.org/10.3390/insects15100809








