Simple Summary
Scaphoideus titanus is the main transmission vector Flavescence doreé (FD) in Europe, causing significant damage to grapevine production. The causative agent of FD is the bacterium ‘Candidatus Phytoplasma vitis’. However, little is known about the interaction of this plant pathogen with the bacterial communities within the insect host. In this study, we characterize the bacterial communities in S. titanus across different European populations and different life stages. We found significant differences in the microbial composition across different populations but did not observe any differences between nymphs and adults of the same population.
Abstract
The Nearctic leafhopper Scaphoideus titanus (Hemiptera: Cicadellidae) is the primary vector of ‘Candidatus Phytoplasma vitis’, the causative agent of Flavescence doreé in Europe. Although microorganisms play an important role in the ecology and behavior of insects, knowledge about the interaction between S. titanus and microbes is limited. In this study, we employed an amplicon metabarcoding approach for profiling the V4 region of the 16S rRNA gene to characterize the bacterial communities of S. titanus across several populations from four European localities. Additionally, we investigated changes in bacterial communities between nymphal and adult stages. In total, we identified 7,472 amplicon sequence variants (ASVs) in adults from the European populations. At the genus level, ‘Candidatus Karelsulcia’ and ‘Candidatus Cardinium’ were the most abundant genera, with both being present in every individual. While we found significant changes in the microbial composition of S. titanus across different European populations, no significant differences were observed between nymphal and adult stages. Our study reveals new insights into the microbial composition of S. titanus and highlights the role of geography in influencing its bacterial community.
1. Introduction
Phytoplasmas are important plant pathogens which are mainly transmitted by sap-feeding hemipterans [1]. In Europe, the Nearctic leafhopper Scaphoideus titanus Ball (Hemiptera: Auchenorrhyncha: Cicadellidae) is the most important vector of ‘Candidatus Phytoplasma vitis’ in grapevines, which is associated with the grapevine yellow Flavescence doreé [2]. The native distribution range of S. titanus in North America covers Midwestern and Eastern United States and Canada [3]. In the 1960s, S. titanus was introduced in Europe, in France [4], and subsequently invaded 18 European countries, from the Russian Caucasus to Portugal [3], including European territories overseas like Madeira [5].
In the order Hemiptera, plant sap feeders are inherently dependent on symbiotic microorganisms, which offset the lack of amino acids in their diet [6]. These microorganisms are known as primary endosymbionts and are widespread in herbivorous hemipteran species [7]. Hemipterans often maintain one or more essential symbiotic bacteria, such as ‘Candidatus Buchnera aphidicola’ in aphids [8], ‘Candidatus Carsonella ruddii’ in psyllids [9], and ‘Candidatus Portiera aleyrodidarum’ in whiteflies [10]. These symbionts are housed in specialized organs of the insect host called bacteriomes [11]. In most Auchenorrhyncha, the suborder of Hemiptera that includes cicadas, leafhoppers, planthoppers, and treehoppers, at least two symbionts co-occur within one host species. The Bacteroidota ’Candidatus Karelsulcia muelleri‘ (hereafter Karelsulcia) is present in most Auchenorrhyncha and has been associated with its host for about 270 million years [12]. However, symbiotic associations are not always stable, and occasional losses and replacements can occur, as shown in Philaenini spittlebugs [13] and various lineages of cicadas [14]. While Karelsulcia provides most of the 10 essential amino acids (EAAs), a co-occurring Betaproteobacteria or Gammaproteobacteria symbiont synthesizes the remaining ones [15,16]. In Deltocephalinae leafhoppers, Karelsulcia co-occurs with ‘Candidatus Nasuia deltocephalinicola’ [17] and occasionally with Sodalis, where triple/quadruple associations, e.g., Karelsulcia–Nasuia–Arsenophonus–Sodalis have been observed [18]. Additionally, some lineages have lost the Betaproteobacteria symbiont and acquired a yeast-like symbiont [19].
Apart from the primary symbionts, hemipterans harbor a variety of other secondary symbionts that are not essential for host survival [20]. In leafhoppers, common facultative symbionts are the Alphaproteobacteria Wolbachia [21] and Rickettsia [22], as well as the Bacteroidota Cardinium [23]. The effect of these symbionts on the insect host are broad and can range from negative effects such as feminization of genetic males [24] and cytoplasmatic incompatibility between female and males [25] to positive effects such as increasing the lifespan of the host [26] and the defense from natural enemies [27]. The associations of insects with secondary symbionts are much more dynamic than the intimate relationship with primary symbionts [28]. While facultative symbionts have evolved ways to propagate within a host population, affecting the reproduction of its host [29], they can also be transmitted horizontally among species when occupying the same ecological niche [30], or they can be acquired from the diet, via plant sap feeding [31]. Moreover, the composition of the microbiome can change during the host different developmental stages [32].
In S. titanus, Cardinium was reported in female reproductive tissues and embryos [33]. Although it is generally assumed that Cardinium is not a nutritional symbiont, a biotin pathway was described in the genome of Cardinium in Encarsia pergandiella (Hymenoptera: Aphelinidae) [34], suggesting a possible contribution to the nutrition of the host. The distribution pattern of Cardinium reveals a wide range of invertebrate hosts, including arthropods [35,36] and nematodes [37]. Cardinium can be transmitted vertically and induce cytoplasmatic incompatibility in crosses where males are infected with the symbiont and females are not, thus promoting its own spread [38]. Molecular studies have reported that S. titanus harbors other symbionts, like Asaia [39] and various other bacteria that are considered grapevine endophytes, such as Agrobacterium, Pseudomonas, Erwinia, Paracoccus, and Lysobacter [40].
Despite the understanding of the microorganisms associated with S. titanus has increased in recent years [40,41], comprehensive knowledge of the taxonomic composition across populations is limited. Moreover, knowledge of the diversity of the bacterial communities associated with S. titanus across different life stages is lacking. Therefore, we characterize the bacterial community composition of S. titanus across four European countries and compare the bacterial composition and diversity among different life stages.
2. Materials and Methods
2.1. Sample Collection and Identification
In total, we analyzed 45 individuals of Scaphoideus titanus: 40 adults and five nymphs (Table 1). To investigate the bacterial community across different geographic regions, we analyzed eight adults from Bordeaux, France (Fr); ten from Breganze, Italy (It); eight from Brno, Czech Republic (Cz); seven from Malca, Serbia (Sr1) and seven from Belgrade, Serbia (Sr2). To compare the microbial composition among different life stages, we characterized the microbial community of five nymphs (fifth stage) and compared them with adults from the same locality (Fr1). All samples were collected between August 2020 and May 2023. Individuals of S. titanus from Italy, Czech Republic, and Serbia were collected by using sticky traps and sweeping nets in vineyards, placed immediately in absolute ethanol and stored at −20 °C. Samples from France were obtained by collecting logs containing S. titanus eggs during winter, which were stored at 4–8 °C in a cold chamber. Egg hatchings were obtained by placing the logs in a climate chamber at 22 °C, 16:8 (L:D) photoperiod, and 65–70% relative humidity, as described in [42]. Nymphs were reared on broad beans, and after completing development, young adults were collected, placed in absolute ethanol, and stored at −20 °C.

Table 1.
List of all sampling locations of Scaphoideus titanus, including population code, coordinates, number of analyzed individuals, life stage, and date of collection. * Individuals from France were obtained from a rearing laboratory as described in [42].
2.2. DNA Extraction and Sequencing
DNA was extracted by using DNeasy Blood & Tissue Extraction Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol without surface sterilization of the insect. DNA quality and quantity were measured with the fluorometer DS-11 FX+ (Denovix Inc., Wilmington, DE, USA) and the Qubit 1X dsDNA High Sensitivity (HS) kit (Invitrogen, Waltham, WI, USA).
Taxonomic identification was verified by performing molecular barcoding targeting the cytochrome oxidase II (COXII) gene. PCR was performed by using the primers mtd13 and mtd18 [43]. PCR reactions were performed in a total volume of 25 µL containing 2 µL of DNA, 0.5 µL of each primer (10 µM), 12.5 µL of 2X DreamTaq Master Mix (ThermoFisher, Waltham, USA), and 9.5 µL of sterile water under the following thermal conditions: 3 min at 95 °C for initial denaturation; 35 cycles of 30 s at 95 °C; 40 s at 50 °C, 1 min at 72 °C, and 10 min at 72 °C. PCR products were Sanger-sequenced at Eurofins Genomics (Ebersberg, Germany) and then analyzed by BLAST search [44] by using the NCBI database.
Bacterial communities were characterized by performing the amplicon sequencing of the V4 hypervariable region of the 16S rRNA gene by using the barcoded primers 515f and 806r [45]. Sequencing was carried out on an Illumina MiSeq with 250 bp paired-end reads at StarSEQ GmbH (Mainz, Germany). Additionally, a no-template control was included as the negative control.
2.3. Data Analysis
Raw reads were processed by using the QIIME2 pipeline (version 2023.9); [46]. Due to the poor quality of the reverse reads, only the forward reads were used in this study. However, because of the short size of the V4 region of the 16S rRNA gene, the forward reads covered almost the complete gene. The trimming of the forward reads was performed at 230 bp by using the DADA2 plugin in q2-dada2. DADA2 was also used for the clustering and denoising of the raw reads into amplicon sequence variants (ASVs) [47]. Taxonomic classification was performed by using the SILVA database, version 138 [48]. Subsequently, ASVs belonging to archaea, mitochondria, eukaryotes, and chloroplast, as well as rare ASVs (i.e., singletons), were removed. Additionally, ASVs that were present in the no-template control were excluded from the analyses. Unclassified ASVs at the genus level were further investigated by using BLAST search [44] and the NCBI database. The two datasets were analyzed independently; therefore, two ASV tables were generated: one for the microbiome characterization of adults from different European populations (Table S1) and a second ASV table for the comparison of two life stages of one locality in France (Table S2). Raw reads were submitted to NCBI under BioProject PRJNA1155196.
2.4. Statistical Analyses
Statistical analyses were performed in R (Version 4.3.2). The packages ‘phyloseq’ [49] and ‘vegan’ [50] were used for assessing bacterial diversity. For alpha diversity, the number of observed ASVs and the Chao1, Evenness, and Shannon indices were calculated. To test the effect of the sampling site on the Chao1, Evenness, and Shannon indices, we used the one-factor analysis of variance (ANOVA) test. Tukey’s honestly significant difference test was implemented as a post hoc test to show the differences among the populations of different localities. In addition, rarefaction was performed to standardize the sequencing depth across samples, ensuring comparability by subsampling each sample to the same number of reads. To visualize the relative abundance of the observed taxa, we used the ‘ggplot’ package in R [51]. A heatmap was generated by using the ‘pheatmap’ package in R [52] to visualize the relative abundance across the 30 most abundant taxa by using log 2 transformation.
To calculate beta diversity and relative abundance of the data, reads were normalized by dividing the reads of each ASV by the total count within each sample. Beta diversity was assessed by using Bray–Curtis distances matrices. The ordination of the Bray–Curtis matrices was visualized by using principal coordinates analysis (PCoA) performed with the ‘ordinate’ function from ‘phyloseq’ [49]. PCoA plots were generated to visualize the clustering of individuals based on locality and life stage. To statistically test for differences in bacterial composition among groups, a PERMANOVA (Permutational Multivariate Analysis of Variance) was conducted by using the ‘adonis2’ function from the ‘vegan’ package. The analysis was based on the Bray–Curtis distance matrix, with 9999 permutations testing for significant composition across different life stages and populations.
3. Results
3.1. Bacterial Communities of S. titanus from Different European Populations
The molecular barcoding of all individuals confirmed that all the specimens used in this study were correctly identified as S. titanus. A total of 40 adults of S. titanus from different populations (Fr, It, Cz, Sr1, and Sr2) produced a total of 2,880,698 reads with an average of 72,017 ± 16,416 reads per individual, ranging from 30,279 to 102,053 reads per individual. After filtering and trimming, the remaining reads were assigned to 52 bacterial phyla (Table S1).
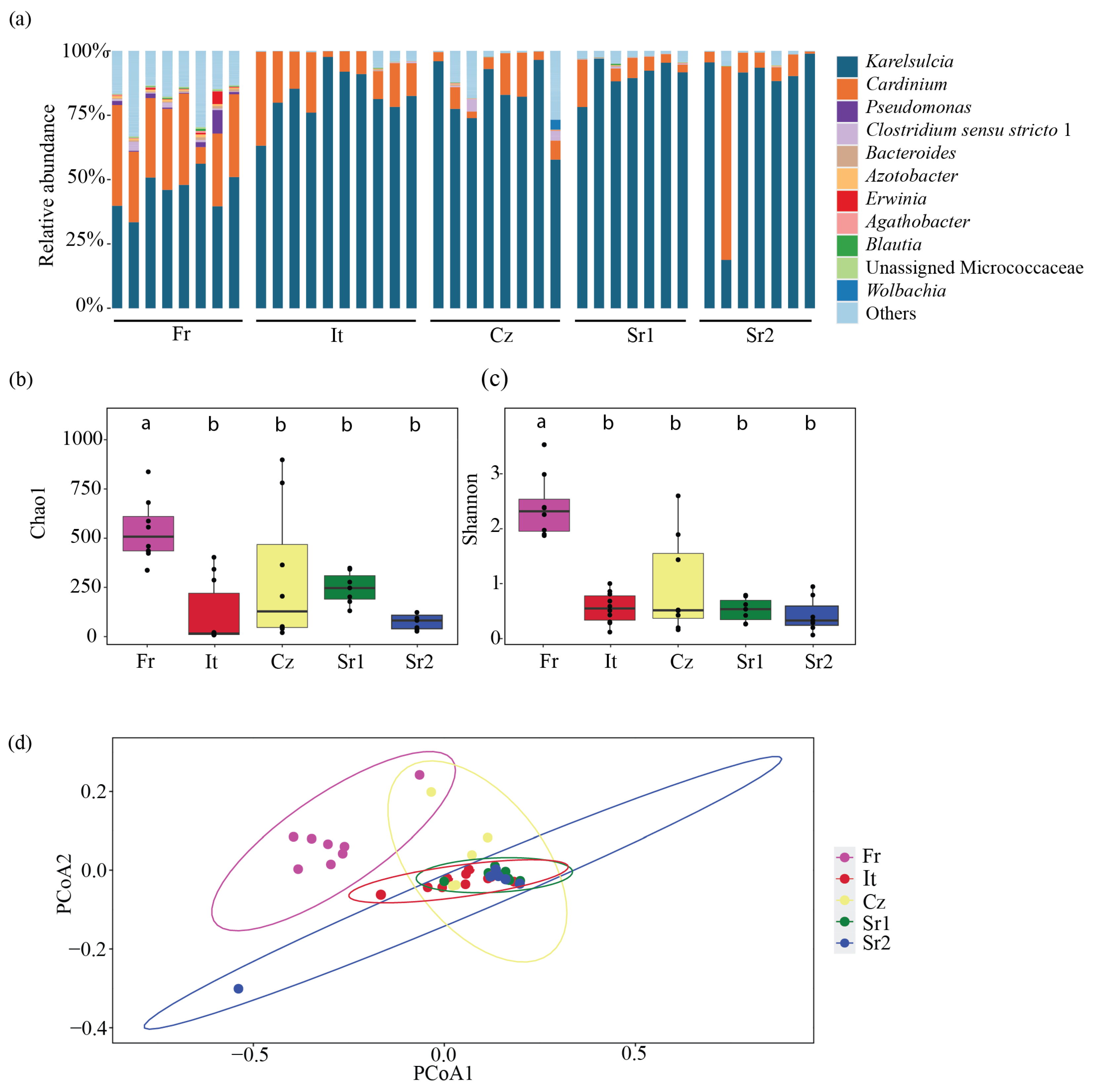
At the genus level, 11 bacterial taxa were predominantly found in adults from different European populations: Karelsulcia, Cardinium, Pseudomonas, Clostridium sensu stricto 1, Bacteroides, Azotobacter, Erwinia, Agathobacter, Blautia, Unassigned Microccocaceae, and Wolbachia (Figure 1a). Karelsulcia was detected in all samples, representing 78.3% of all reads. The second most abundant ASV was assigned to Cardinium, representing 13.5% of all reads. The highest relative abundance of Cardinium was detected in one individual from Serbia (Sr2) with a relative abundance of 75.21%, while individuals from Czech Republic (Cz) and Serbia (Sr1 and Sr2) and one individual from Italy (It) had low relative abundance, ranging from 0.07 to 2.43%.
Figure 1.
(Bacterial community composition and diversity in adults of Scaphoideus titanus. (a) Relative abundance at genus level. (b) Comparison of bacterial species richness based on Chao1 index of adults in S. titanus. (c) Comparison of bacterial diversity based on Shannon index of adults in S. titanus. (d) Principal coordinates analysis based on Bray–Curtis dissimilarity matrix showing differences among individuals across Europe. Different letters represent statistically significant differences.
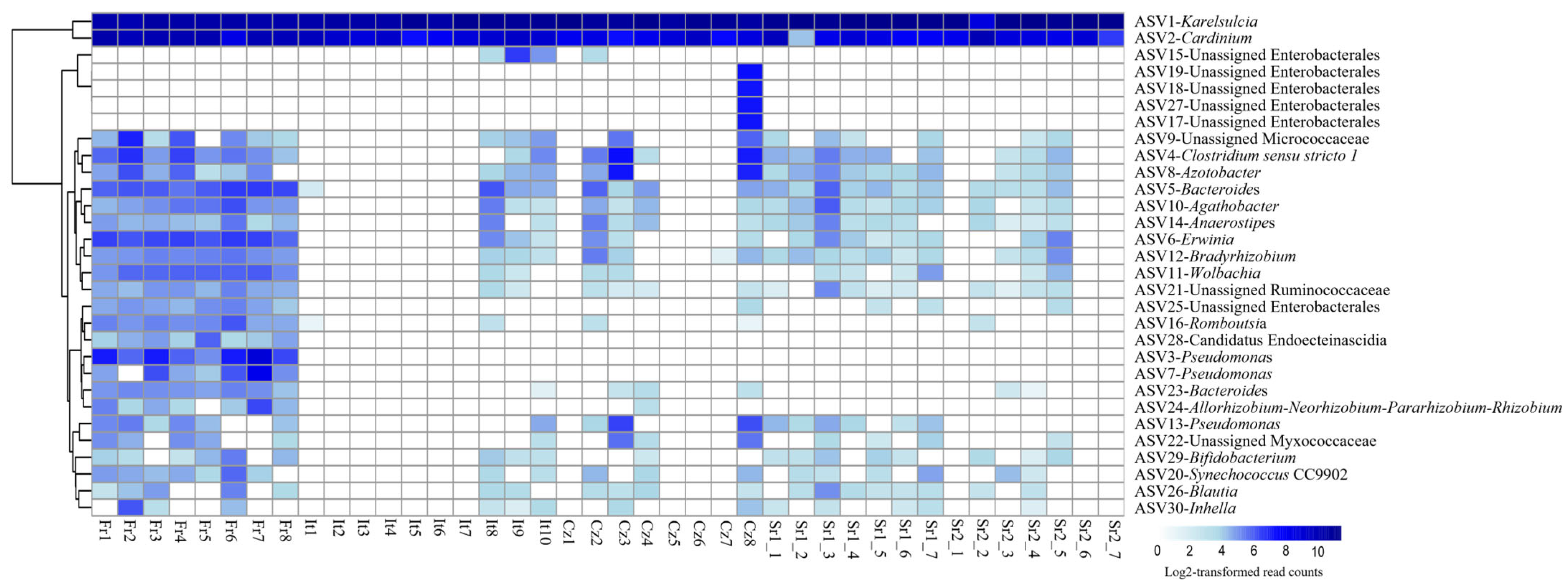
While Karelsulcia and Cardinium represented together 91% of all reads, all other ASVs were present with only low abundance rates, less than 1% of all reads each. Interestingly, these low-abundance ASVs were not equally distributed among the different individuals and populations (Figure 2). The individuals from the Fr group harbored several other taxa with higher relative abundance compared with the other populations. In particular, seven individuals from Italy (It), three from Czech Republic (Cz), and three individuals from Serbia (Sr2) had only reads belonging to Karelsulcia and Cardinium. Among the low-abundance taxa, Clostridium sensu stricto 1 was present in 65% of the samples. Furthermore, a BLAST search on four unidentified ASVs at the genus level showed that Unassigned Enterobacterales (ASV6) was identical to Erwinia sp. (KY856906) and Unassigned Pseudomonadaceae (ASV8) was identical to Azotobacter sp. (MN853548). ASV24, which was classified as Unidentified Myxococcaceae, showed 95% similarity with different genera, such as Corallococcus, Archangium, and Cytobacter. Two ASVs were classified as Pseudomonas (ASV3 and ASV7) and were exclusively found in adults from the Fr population. A third ASV13-Pseudomonas was present in individuals from the It and Sr1 groups (Figure 2).
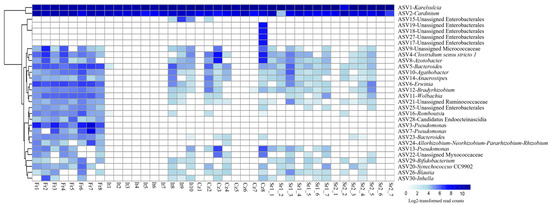
Figure 2.
Heatmap showing the distribution of the 30 most abundant amplicon sequence variants (ASVs) in adult individuals of Scaphoideus titanus across Europe.
Bacterial species richness across the different populations was relatively low, ranging between 10 and 975 ASVs (Table S2). At the population level, the bacterial richness was higher among the Fr population, ranging between 423 and 975 ASVs, followed by the Cz population, with 20 to 898 ASVs, and the Sr1 population, with 131 to 348 ASVs. The It population had the lowest species richness, ranging from 8 to 403 ASVs. Moreover, bacterial species richness and diversity based on the Chao1 and Shannon indices showed significant differences among sampling sites, especially between the Fr population and the other localities (Chao1: ANOVA, F = 6.92, df = 4, p < 0.001; Shannon: ANOVA, F = 19.68, df = 4, p < 0.001) (Figure 1bc, Table S3). Additionally, we performed a principal coordinates analysis (PCoA) using Bray–Curtis dissimilarities to compare beta diversity in the different populations. This analysis revealed two major clusters: one comprising individuals from the Fr population and the other containing the remaining European populations (PERMANOVA, F = 8.39, df = 4, R2 = 0.48972, p < 0.001) (Figure 1d).
3.2. Bacterial Community of S. titanus Across Different Life Stages
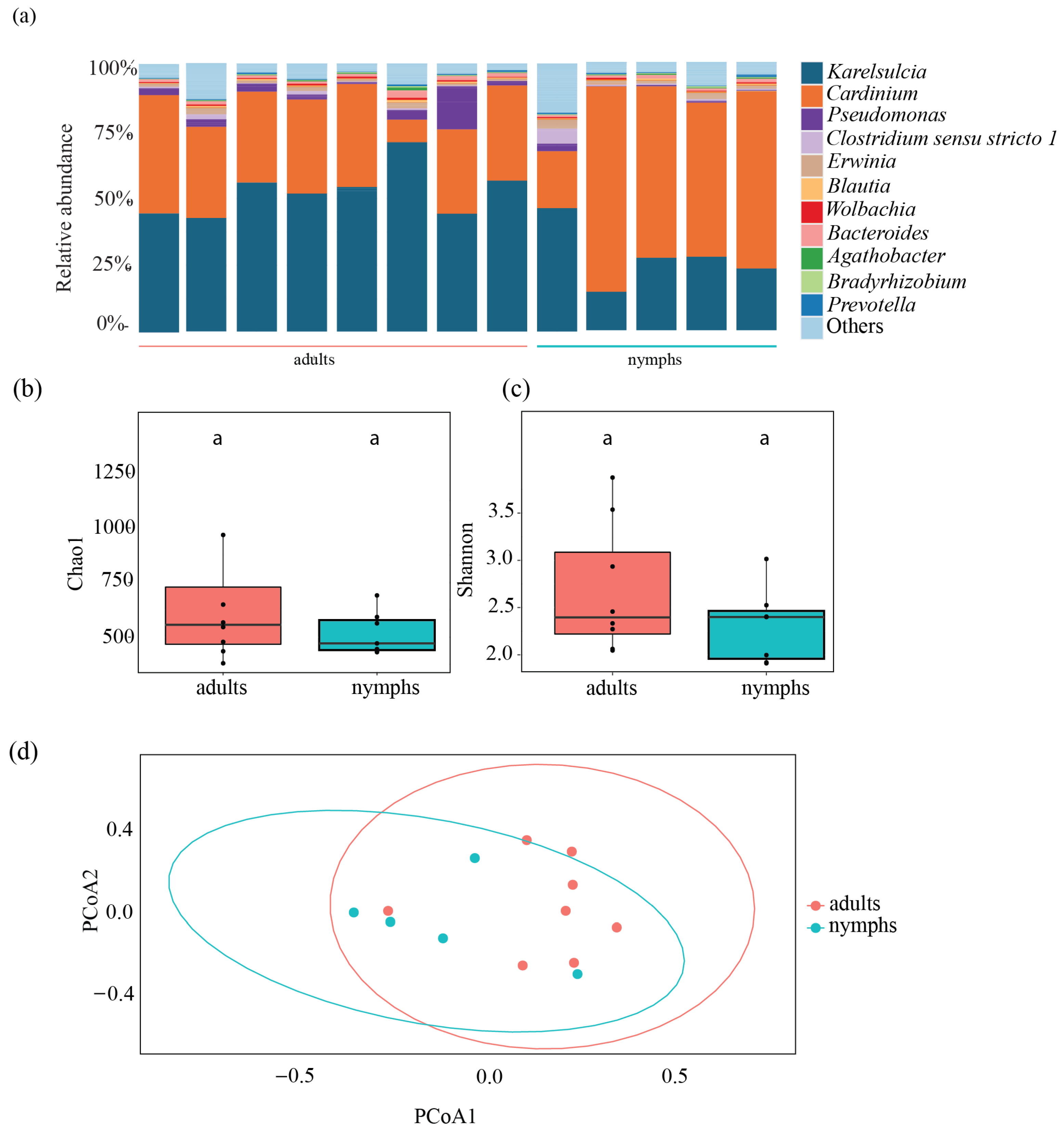
To assess differences between the different life stages, we analyzed five nymphs and compared them to eight adults from the Fr population. We obtained a total of 496,972 reads, which ranged from 61,836 to 105,982 reads per individual. Karelsulcia was found across both life stages, in nymphs (14,173 ± 4666 reads) and in adults (28,440 ± 4326 reads) (t-test: p < 0.001), with lower relative abundance in nymphs (12.63–35.64%) compared with adults (34.01–56.68%) (Figure 3a). In contrast, Cardinium showed a higher relative abundance in nymphs (30,307 ± 10,804 reads) compared with adults (18,053 ± 5710 reads) (t-test: p = 0.08). The third most abundant taxon was Pseudomonas, which was present in low abundance in every adult but only in some nymphs. Additionally, Bacteroides were detected in adults and nymphs (Figure 3a).
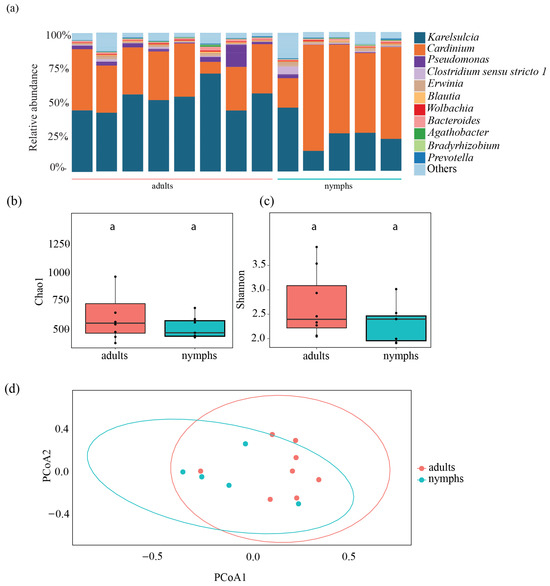
Figure 3.
Bacterial composition of the most common taxa detected in nymphs and adults of Scaphoideus titanus from France. (a) Relative abundance at genus level. Comparison of bacterial species richness based on Chao1 (b) and Shannon indices (c) of adults and nymphs in S. titanus. (d) Principal coordinates analysis based on Bray–Curtis dissimilarity matrix showing Shannon diversity index of nymphs and adults from Bordeaux. Different letters represent statistically significant differences.
Overall, there were no significant differences between nymphs and adults in terms of species richness, diversity, and evenness (Chao1: ANOVA, F = 1.604; df = 1, p = 0.227; Shannon: ANOVA, F = 0.785; df = 1, p = 0.395; Pioulu’s: ANOVA, F = 0.482; df = 1, p = 0.502). The Chao1 index in adults had a median value of 606.12 compared with 576.28 in nymphs (Figure 3b, Table S3), while Shannon diversity values for adults and nymphs were 2.56 and 2.45, respectively (Figure 3c, Table S3). The PCoA showed no clear segregation between nymphs and adults (PERMANOVA, df = 1, F = 2.2865, R2 = 0.172, p = 0.7; Figure 3d).
4. Discussion
The leafhopper Scaphoideus titanus is the most important vector of Flavescence dorée phytoplasma and represents one of the biggest threats to grapevine production in Europe [3,53]. Therefore, advances in understanding the relationship between this pest species and its associated microorganisms are crucial to getting a clear picture of its biology. Since symbionts have been proposed as ecological alternatives to control insect pests and their associated pathogens, knowledge about these organisms is key to developing effective symbiont-based application strategies [54,55].
Here, we characterized the bacterial community composition of S. titanus across Europe. While previous studies used pools of multiple individuals [40,41], our single-individual-based approach showed a predominance of two bacterial symbionts, Karelsulcia and Cardinium, in every specimen of S. titanus. Karelsulcia has been already described in S. titanus in the United States and in the invasive range in France and Italy [41]. This confirms the importance of this symbiont in this species due to its role in the supplementation of essential amino acids [12]. As shown in previous studies in S. titanus [33,41,56], Cardinium is commonly found in all populations across Europe. Every single individual analyzed harbored this symbiont, thus suggesting that Cardinium might be considered part of the core microbiome of S. titanus in Europe. While this bacterium is generally considered a facultative symbiont and interferes with the host’s reproduction, its function in S. titanus needs to be further investigated. Interestingly, the only study that analyzed a few individuals from a native population from the United States did not find Cardinium [41]. It is, therefore, possible that S. titanus acquired Cardinium after its introduction, as it was shown for Rickettsia in white flies [57] and Wolbachia in tephritid fruit flies [30].
Apart from these two most common bacterial taxa, various additional bacteria were found in different localities across Europe with different frequencies. For instance, Pseudomonas and Clostridium sensu stricto 1 were the most abundant taxa after Karelsulcia and Cardinium. Pseudomonas was specifically found in adults from Bordeaux, France. This genus was also detected previously in nymphs of S. titanus occurring in the Trentino region (Italy) that fed on grapevine stems and roots [40]. Pseudomonas is a common bacterium found in the grapevine xylem sap and in sharpshooters that feed on these plants [58]. Members of this genus were shown to promote the fitness of their host in plants [59] and insects [60]. In the brown planthopper Nilaparvata lugens, this bacterium contributes to better tolerance of adverse climate conditions such as temperature and draught [61]. Clostridium sensu stricto 1 is a spore-forming bacterium commonly found in soil, water, and plants [62]. In grapevines, this genus occurred in the rhizosphere and in the flowering and early fruiting stages [63], suggesting that it might be acquired by S. titanus by feeding on the plant. Other taxa that were common in S. titanus were Azotobacter, Unassigned Enterobacterales, Agathobacter, and Bacteroides, which are part of the soil microbiome [64]. Although the Alphaproteobacteria Asaia was described to be associated to S. titanus from the Italian regions of Trentino and Piedmont [40,65], this genus was not detected in our dataset. Interestingly, it was shown that Asaia can interfere with Phytoplasma transmission in S. titanus individuals and thus might be a candidate for a future biological control agent [39].
Overall, the bacterial communities across the different populations in Europe were very homogenous. However, specimens collected from France showed a significantly different bacterial composition. While all the other insect samples were collected in summer in the field, the samples from France derived from grapevine logs harboring S. titanus eggs which had been kept overwintering under laboratory conditions and emerged in the laboratory in the following year. However, it is not clear why the samples collected as adults in nature show lower bacterial diversity than individuals collected as eggs and treated in generally more sterile conditions in the lab. Since the feeding habits can influence or change the microbial composition of the host [40,66] the use of broad bean plants as feeding plants for rearing might have influenced the bacterial community of S. titanus.
The ontogenetic development of insects alters the structure of bacterial communities [67]. For holometabolous insects such as fruit flies [68] and bark beetles [69], changes in the bacterial communities are very common between the different developmental stages. Changes in bacterial communities were also observed in hemimetabolous insects. In the brown planthopper N. lugens, significant changes in bacterial communities were described across different life stages. Especially, the relative abundance of the phyla Actinomycetota and Bacteroidota decreased through the life stages, showing lower abundance in the adult stages [70]. This contrasts with our study, where we did not find any significant differences in the microbial community of S. titanus across two life stages.
5. Conclusions
In this study, we characterized the bacterial community of S. titanus in four European countries and across different life stages. We found that Karelsulcia and Cardinium were the predominant taxa of the microbial communities in S. titanus present in all individuals and populations across the four European countries. While the omnipresence of Karelsulcia is not surprising, being a primary symbiont of almost every Deltocephalinae leafhopper, the presence of Cardinium in every single individual is remarkable. Especially the function and role of Cardinium, which is generally considered a facultative endosymbiont in other insects, needs to be investigated in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15110830/s1, Table S1: ASV table of adults from different populations of S. titanus in Europe, Table S2: ASV table of adults and nymphs from France (Fr), Table S3: Alpha diversity analysis of S. titanus from adults across Europe and nymphs in France (Fr).
Author Contributions
Conceptualization, J.S.E., E.C. and H.S.; methodology, J.S.E. and L.B.; software, J.S.E.; validation, H.S., E.C. and J.S.E.; formal analysis, J.S.E. and L.B.; investigation, J.S.E.; resources, H.S.; data curation, J.S.E.; writing—original draft preparation, J.S.E.; writing—review and editing, H.S., L.B., E.C. and J.S.E.; visualization, J.S.E.; supervision, H.S.; project administration, H.S.; funding acquisition, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by internal funds of the Free University of Bozen-Bolzano to H.S. We thank the Department of Innovation, Research and University of the Autonomous Province of Bozen-Bolzano for covering the open access publication charges.
Data Availability Statement
The data used in this study are available at NCBI under BioProject PRJNA1155196.
Acknowledgments
We thank Sandrine Eveillard for sample collection in France, Andrea Kosovac for collection in Serbia, Nicola Mori and Enea Guerrieri for sending specimens from the Veneto region in Italy, and Gudrun Strauss for the collection of specimens from Czech Republic. We also thank Jessica Dittmer for helpful discussion and input.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Hogenhout, S.A.; Oshima, K.; Ammar, E.-D.; Kakizawa, S.; Kingdom, H.N.; Namba, S. Phytoplasmas: Bacteria That Manipulate Plants and Insects. Mol. Plant Pathol. 2008, 9, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Alma, A.; Lessio, F.; Nickel, H. Insects as Phytoplasma Vectors: Ecological and Epidemiological Aspects. In Phytoplasmas: Plant Pathogenic Bacteria—II: Transmission and Management of Phytoplasma—Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 1–25. ISBN 9789811328329. [Google Scholar]
- Gonella, E.; Benelli, G.; Arricau-Bouvery, N.; Bosco, D.; Duso, C.; Dietrich, C.H.; Galetto, L.; Rizzoli, A.; Jović, J.; Mazzoni, V.; et al. Scaphoideus titanus Up-to-the-Minute: Biology, Ecology, and Role as a Vector. Entomologia 2024, 44, 481–496. [Google Scholar] [CrossRef]
- Bonfils, J.; Schvester, D. The Leafhoppers (Homoptera: Auchenorrhyncha) and Their Relationship with Vineyards in South-Western France. Ann. Epiphyt. 1960, 11, 325–336. [Google Scholar]
- Aguin-Pombo, D.; Aguiar, A.M.F.; Cravo, D. First Report of Scaphoideus titanus for Madeira Island. EPPO Bull. 2020, 50, 564–567. [Google Scholar] [CrossRef]
- McCutcheon, J.P.; McDonald, B.R.; Moran, N.A. Convergent Evolution of Metabolic Roles in Bacterial Co-Symbionts of Insects. Proc. Natl. Acad. Sci. USA 2009, 106, 15394–15399. [Google Scholar] [CrossRef]
- Douglas, A.E. Nutritional Interactions in Insect-Microbial Symbioses: Aphids and Their Symbiotic Bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef]
- Shigenobu, S.; Watanabe, H.; Hattori, M.; Sakaki, Y.; Ishikawa, H. Genome Sequence of the Endocellular Bacterial Symbiont of Aphids Buchnera sp. APS. Nature 2000, 407, 81–86. [Google Scholar] [CrossRef]
- Dittmer, J.; Corretto, E.; Štarhová Serbina, L.; Michalik, A.; Nováková, E.; Schuler, H. Division of Labor within Psyllids: Metagenomics Reveals an Ancient Dual Endosymbiosis with Metabolic Complementarity in the Genus Cacopsylla. mSystems 2023, 8, e00578-23. [Google Scholar] [CrossRef]
- Sloan, D.B.; Moran, N.A. Endosymbiotic Bacteria as a Source of Carotenoids in Whiteflies. Biol. Lett. 2012, 8, 986–989. [Google Scholar] [CrossRef]
- Baumann, P. Biology of Bacteriocyte-Associated Endosymbionts of Plant Sap-Sucking Insects. Annu. Rev. Microbiol. 2005, 59, 155–189. [Google Scholar] [CrossRef]
- Moran, N.A.; Tran, P.; Gerardo, N.M. Symbiosis and Insect Diversification: An Ancient Symbiont of Sap-Feeding Insects from the Bacterial Phylum Bacteroidetes. Appl. Environ. Microbiol. 2005, 71, 8802–8810. [Google Scholar] [CrossRef] [PubMed]
- Koga, R.; Bennett, G.M.; Cryan, J.R.; Moran, N.A. Evolutionary Replacement of Obligate Symbionts in an Ancient and Diverse Insect Lineage. Environ. Microbiol. 2013, 15, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Moriyama, M.; Łukasik, P.; Vanderpool, D.; Tanahashi, M.; Meng, X.-Y.; McCutcheon, J.P.; Fukatsu, T. Recurrent Symbiont Recruitment from Fungal Parasites in Cicadas. Proc. Natl. Acad. Sci. USA 2018, 115, E5970–E5979. [Google Scholar] [CrossRef]
- McCutcheon, J.P.; Moran, N.A. Parallel Genomic Evolution and Metabolic Interdependence in an Ancient Symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 19392–19397. [Google Scholar] [CrossRef]
- McCutcheon, J.P.; Moran, N.A. Functional Convergence in Reduced Genomes of Bacterial Symbionts Spanning 200 My of Evolution. Genome Biol. Evol. 2010, 2, 708–718. [Google Scholar] [CrossRef]
- Kobiałka, M.; Michalik, A.; Szwedo, J.; Szklarzewicz, T. Diversity of Symbiotic Microbiota in Deltocephalinae Leafhoppers (Insecta, Hemiptera, Cicadellidae). Arthropod Struct. Dev. 2018, 47, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Michalik, A.; Castillo Franco, D.; Kobiałka, M.; Szklarzewicz, T.; Stroiński, A.; Łukasik, P. Alternative Transmission Patterns in Independently Acquired Nutritional Cosymbionts of Dictyopharidae Planthoppers. mBio 2021, 12, e0122821. [Google Scholar] [CrossRef]
- Kobiałka, M.; Michalik, A.; Walczak, M.; Szklarzewicz, T. Dual “Bacterial-Fungal” Symbiosis in Deltocephalinae Leafhoppers (Insecta, Hemiptera, Cicadomorpha: Cicadellidae). Microb. Ecol. 2017, 75, 771. [Google Scholar] [CrossRef]
- Sandström, J.P.; Russell, J.A.; White, J.P.; Moran, N.A. Independent Origins and Horizontal Transfer of Bacterial Symbionts of Aphids. Mol. Ecol. 2001, 10, 217–228. [Google Scholar] [CrossRef]
- Zhang, Q.; Lan, R.; Ji, D.; Tan, Y.; Zhou, X.; Tan, X.; Wu, Q.; Jin, L. The Detection of Wolbachia in Tea Green Leafhopper (Empoasca onukii Matsuda) and Its Influence on the Host. Agriculture 2022, 12, 36. [Google Scholar] [CrossRef]
- Ishii, Y.; Matsuura, Y.; Kakizawa, S.; Nikoh, N.; Fukatsu, T. Diversity of Bacterial Endosymbionts Associated with Macrosteles Leafhoppers Vectoring Phytopathogenic Phytoplasmas. Appl. Environ. Microbiol. 2013, 79, 5013–5022. [Google Scholar] [CrossRef]
- Zhang, K.-J.; Han, X.; Hong, X.-Y. Various Infection Status and Molecular Evidence for Horizontal Transmission and Recombination of Wolbachia and Cardinium among Rice Planthoppers and Related Species. Insect Sci. 2013, 20, 329–344. [Google Scholar] [CrossRef]
- Negri, I.; Pellecchia, M.; Mazzoglio, P.J.; Patetta, A.; Alma, A. Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a Leafhopper with an XX/X0 Sex-Determination System. Proc. R. Soc. B 2006, 273, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yukuhiro, F.; Matsumura, M.; Noda, H. Cytoplasmic Incompatibility Involving Cardinium and Wolbachia in the White-Backed Planthopper Sogatella furcifera (Hemiptera: Delphacidae). Appl. Entomol. Zool. 2012, 47, 273–283. [Google Scholar] [CrossRef]
- Fan, Z.-Y.; Liu, Y.; He, Z.-Q.; Wen, Q.; Chen, X.-Y.; Khan, M.M.; Osman, M.; Mandour, N.S.; Qiu, B.-L. Rickettsia Infection Benefits Its Whitefly Hosts by Manipulating Their Nutrition and Defense. Insects 2022, 13, 1161. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and Virus Protection in Insects. Science 2008, 322, 702. [Google Scholar] [CrossRef]
- Dale, C.; Moran, N.A. Molecular Interactions between Bacterial Symbionts and Their Hosts. Cell 2006, 126, 453–465. [Google Scholar] [CrossRef]
- Engelstädter, J.; Hurst, G.D.D. The Ecology and Evolution of Microbes That Manipulate Host Reproduction. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 127–149. [Google Scholar] [CrossRef]
- Schuler, H.; Bertheau, C.; Egan, S.P.; Feder, J.L.; Riegler, M.; Schlick-Steiner, B.C.; Steiner, F.M.; Johannesen, J.; Kern, P.; Tuba, K.; et al. Evidence for a Recent Horizontal Transmission and Spatial Spread of Wolbachia from Endemic Rhagoletis cerasi (Diptera: Tephritidae) to Invasive Rhagoletis cingulata in Europe. Mol. Ecol. 2013, 22, 4101–4111. [Google Scholar] [CrossRef]
- Gonella, E.; Pajoro, M.; Marzorati, M.; Crotti, E.; Mandrioli, M.; Pontini, M.; Bulgari, D.; Negri, I.; Sacchi, L.; Chouaia, B.; et al. Plant-Mediated Interspecific Horizontal Transmission of an Intracellular Symbiont in Insects. Sci. Rep. 2015, 5, 15811. [Google Scholar] [CrossRef]
- Sontowski, R.; van Dam, N.M. Functional Variation in Dipteran Gut Bacterial Communities in Relation to Their Diet, Life Cycle Stage and Habitat. Insects 2020, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, L.; Genchi, M.; Clementi, E.; Bigliardi, E.; Avanzati, A.M.; Pajoro, M.; Negri, I.; Marzorati, M.; Gonella, E.; Alma, A.; et al. Multiple Symbiosis in the Leafhopper Scaphoideus titanus (Hemiptera: Cicadellidae): Details of Transovarial Transmission of Cardinium Sp. and Yeast-like Endosymbionts. Tissue Cell 2008, 40, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Penz, T.; Schmitz-Esser, S.; Kelly, S.E.; Cass, B.N.; Müller, A.; Woyke, T.; Malfatti, S.A.; Hunter, M.S.; Horn, M. Comparative Genomics Suggests an Independent Origin of Cytoplasmic Incompatibility in Cardinium hertigii. PLoS Genet. 2012, 8, e1003012. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Perlman, S.J. Distribution of the Bacterial Symbiont Cardinium in Arthropods. Mol. Ecol. 2004, 13, 2009–2016. [Google Scholar] [CrossRef]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The Incidence of Bacterial Endosymbionts in Terrestrial Arthropods. Proc. R. Soc. B 2015, 282, 20150249. [Google Scholar] [CrossRef]
- Tarlachkov, S.V.; Efeykin, B.D.; Castillo, P.; Evtushenko, L.I.; Subbotin, S.A. Distribution of Bacterial Endosymbionts of the Cardinium Clade in Plant-Parasitic Nematodes. Int. J. Mol. Sci. 2023, 24, 2905. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhao, D.-X.; Hong, X.-Y. Cardinium—The Leading Factor of Cytoplasmic Incompatibility in the Planthopper Sogatella furcifera Doubly Infected with Wolbachia and Cardinium. Environ. Entomol. 2012, 41, 833–840. [Google Scholar] [CrossRef]
- Gonella, E.; Crotti, E.; Mandrioli, M.; Daffonchio, D.; Alma, A. Asaia Symbionts Interfere with Infection by Flavescence Dorée Phytoplasma in Leafhoppers. J. Pest. Sci. 2018, 91, 1033–1046. [Google Scholar] [CrossRef]
- Lòpez-Fernàndez, S.; Mazzoni, V.; Pedrazzoli, F.; Pertot, I.; Campisano, A. A Phloem-Feeding Insect Transfers Bacterial Endophytic Communities between Grapevine Plants. Front. Microbiol. 2017, 8, 834. [Google Scholar] [CrossRef]
- Abbà, S.; Rossi, M.; Vallino, M.; Galetto, L.; Marzachì, C.; Turina, M. Metatranscriptomic Assessment of the Microbial Community Associated With the Flavescence Dorée Phytoplasma Insect Vector Scaphoideus titanus. Front. Microbiol. 2022, 13, 866523. [Google Scholar] [CrossRef]
- Eveillard, S.; Jollard, C.; Labroussaa, F.; Khalil, D.; Perrin, M.; Desqué, D.; Salar, P.; Razan, F.; Hévin, C.; Bordenave, L.; et al. Contrasting Susceptibilities to Flavescence Dorée in Vitis vinifera, Rootstocks and Wild Vitis Species. Front. Plant Sci. 2016, 7, 1762. [Google Scholar] [CrossRef] [PubMed]
- Papura, D.; Burban, C.; van Helden, M.; Giresse, X.; Nusillard, B.; Guillemaud, T.; Kerdelhué, C. Microsatellite and Mitochondrial Data Provide Evidence for a Single Major Introduction for the Neartic Leafhopper Scaphoideus titanus in Europe. PLoS ONE 2012, 7, e36882. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; Springer: New York, NY, USA, 2015. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps; Springer: New York, NY, USA, 2018. [Google Scholar]
- Chuche, J.; Thiéry, D. Biology and Ecology of the Flavescence Dorée Vector Scaphoideus titanus: A Review. Agron. Sustain. Dev. 2014, 34, 381–403. [Google Scholar] [CrossRef]
- Wang, G.-H.; Du, J.; Chu, C.Y.; Madhav, M.; Hughes, G.L.; Champer, J. Symbionts and Gene Drive: Two Strategies to Combat Vector-Borne Disease. Trends Genet. 2022, 38, 708–723. [Google Scholar] [CrossRef]
- Arora, A.K.; Douglas, A.E. Hype or Opportunity? Using Microbial Symbionts in Novel Strategies for Insect Pest Control. J. Insect Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, E.; Sacchi, L.; Genchi, M.; Alma, A.; Pajoro, M.; Daffonchio, D.; Marzorati, M.; Avanzati, A.M. Ultrastructure of a Novel Cardinium Sp. Symbiont in Scaphoideus titanus (Hemiptera: Cicadellidae). Tissue Cell 2006, 38, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Fein, E.; et al. Rapid Spread of a Bacterial Symbiont in an Invasive Whitefly Is Driven by Fitness Benefits and Female Bias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef]
- Welch, E.W.; Macias, J.; Bextine, B. Geographic Patterns in the Bacterial Microbiome of the Glassy-Winged Sharpshooter, Homalodisca vitripennis (Hemiptera: Cicadellidae). Symbiosis 2015, 66, 1–12. [Google Scholar] [CrossRef]
- Shalev, O.; Karasov, T.L.; Lundberg, D.S.; Ashkenazy, H.; Pramoj Na Ayutthaya, P.; Weigel, D. Commensal Pseudomonas Strains Facilitate Protective Response against Pathogens in the Host Plant. Nat. Ecol. Evol. 2022, 6, 383–396. [Google Scholar] [CrossRef]
- Peral-Aranega, E.; Saati-Santamaría, Z.; Kolařik, M.; Rivas, R.; García-Fraile, P. Bacteria Belonging to Pseudomonas typographi Sp. Nov. from the Bark Beetle Ips typographus Have Genomic Potential to Aid in the Host Ecology. Insects 2020, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sinha, D.K.; Nair, S. Shifts in Pseudomonas Species Diversity Influence Adaptation of Brown Planthopper to Changing Climates and Geographical Locations. iScience 2022, 25, 104550. [Google Scholar] [CrossRef]
- Zeiller, M.; Rothballer, M.; Iwobi, A.N.; Böhnel, H.; Gessler, F.; Hartmann, A.; Schmid, M. Systemic Colonization of Clover (Trifolium repens) by Clostridium botulinum Strain 2301. Front. Microbiol. 2015, 6, 1207. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Niu, Z.; Song, S.; Zhao, Y. Water Stress Affects the Frequency of Firmicutes, Clostridiales and Lysobacter in Rhizosphere Soils of Greenhouse Grape. Agric. Water Manag. 2019, 226, 105776. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, C.; Long, M. Variation of Soil Nutrients and Bacterial Community Diversity of Different Land Utilization Types in Yangtze River Basin, Chongqing Municipality. PeerJ 2020, 8, e9386. [Google Scholar] [CrossRef]
- Crotti, E.; Damiani, C.; Pajoro, M.; Gonella, E.; Rizzi, A.; Ricci, I.; Negri, I.; Scuppa, P.; Rossi, P.; Ballarini, P.; et al. Asaia, a Versatile Acetic Acid Bacterial Symbiont, Capable of Cross-Colonizing Insects of Phylogenetically Distant Genera and Orders. Environ. Microbiol. 2009, 11, 3252–3264. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.-W.; Xia, X.-J.; Feng, Y.-L.; Wu, W.; Li, H.-J.; Sun, Z.-T.; Li, J.-M.; Chen, J.-P. The Plant-Sucking Insect Selects Assembly of the Gut Microbiota from Environment to Enhance Host Reproduction. NPJ Biofilms Microbiomes 2024, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H.; et al. Insect Gut Bacterial Diversity Determined by Environmental Habitat, Diet, Developmental Stage, and Phylogeny of Host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Andongma, A.A.; Wan, L.; Dong, Y.-C.; Li, P.; Desneux, N.; White, J.A.; Niu, C.-Y. Pyrosequencing Reveals a Shift in Symbiotic Bacteria Populations across Life Stages of Bactrocera dorsalis. Sci. Rep. 2015, 5, 9470. [Google Scholar] [CrossRef]
- Briones-Roblero, C.I.; Hernández-García, J.A.; Gonzalez-Escobedo, R.; Soto-Robles, L.V.; Rivera-Orduña, F.N.; Zúñiga, G. Structure and Dynamics of the Gut Bacterial Microbiota of the Bark Beetle, Dendroctonus rhizophagus (Curculionidae: Scolytinae) across Their Life Stages. PLoS ONE 2017, 12, e0175470. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wang, T.-Z.; Zhu, H.-F.; Pan, H.-B.; Yu, X.-P. Diversity and Dynamics of Microbial Communities in Brown Planthopper at Different Developmental Stages Revealed by High-Throughput Amplicon Sequencing. Insect Sci. 2020, 27, 883–894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).