New Strains of the Entomopathogenic Nematodes Steinernema scarabaei, S. glaseri, and S. cubanum for White Grub Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Laboratory Experiments
2.3. Greenhouse Experiments
2.4. Statistical Analysis
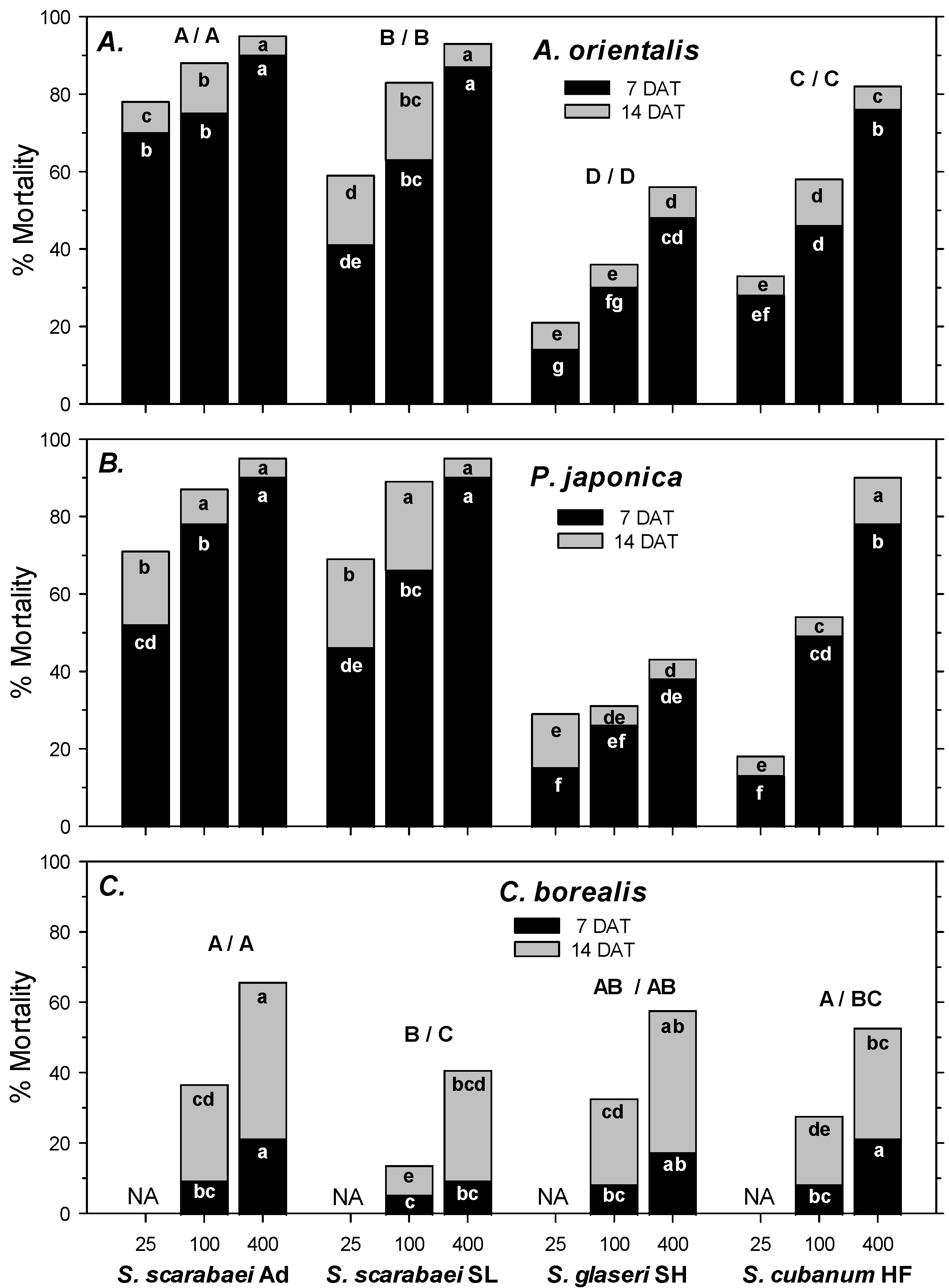
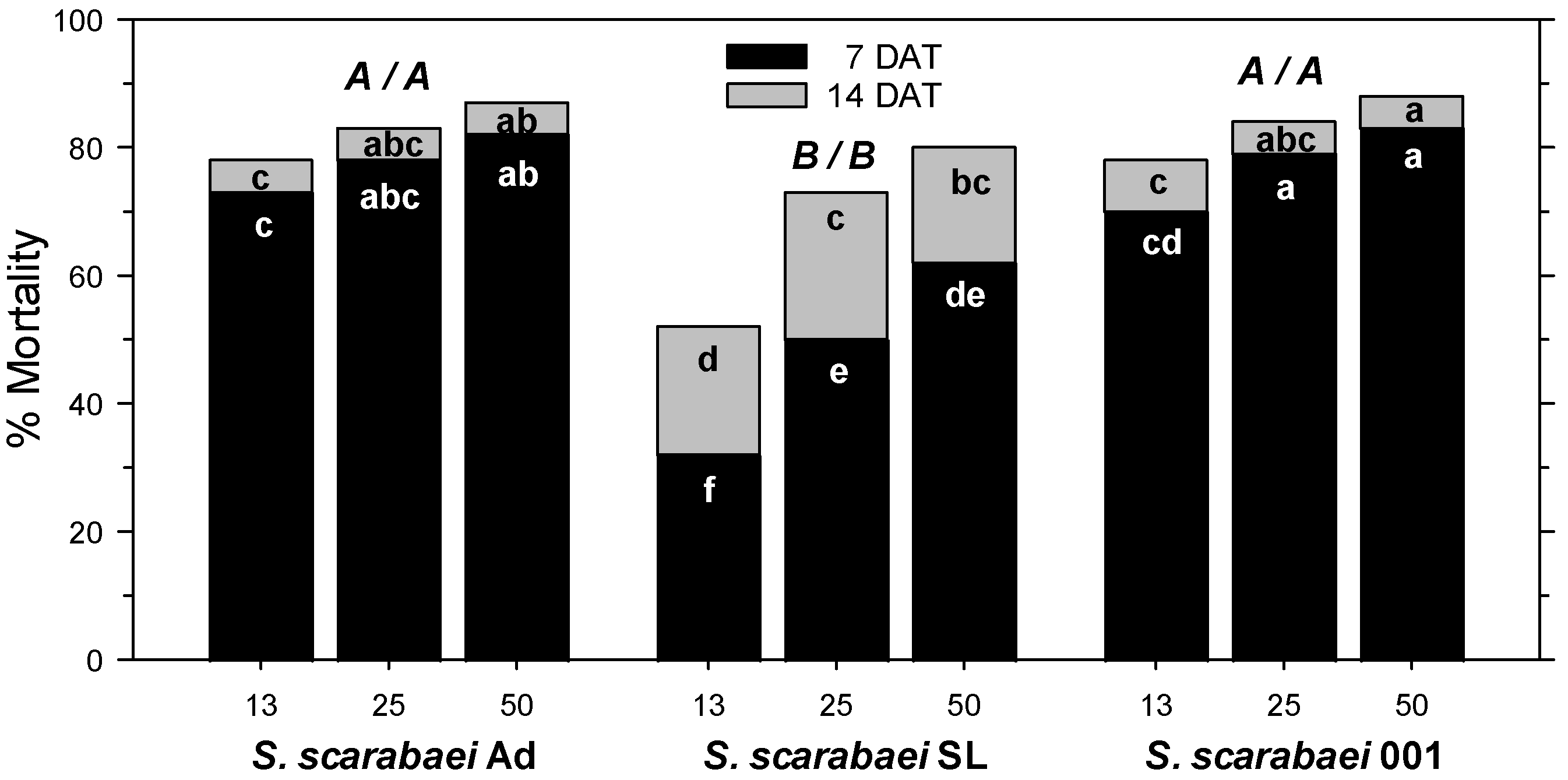
3. Results
3.1. Laboratory Experiments
3.2. Greenhouse Experiment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koppenhöfer, A.M.; Shapiro-Ilan, D.I.; Hiltpold, I. Advances in the use of entomopathogenic nematode biopesticides in suppressing crop insect pests. In Biopesticides for Sustainable Agriculture; Birch, N., Glare, T., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 1–38. [Google Scholar]
- Shapiro-Ilan, D.I.; Lewis, E.E. Entomopathogenic Nematodes as Biological Control Agents; CABI Publishing: Wallingford, UK, 2024. [Google Scholar]
- Klein, M.G.; Georgis, R. Persistence of control of Japanese beetle (Coleoptera: Scarabaeidae) larvae with steinernematid and heterorhabditid nematodes. J. Econ. Entomol. 1992, 85, 727–730. [Google Scholar] [CrossRef]
- Grewal, P.S.; Koppenhöfer, A.M.; Choo, H.Y. Lawn, turfgrass and pasture applications. In Nematodes as Biocontrol Agents; Grewal, P.S., Ehlers, R.-U., Shapiro-Ilan, D.I., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 115–146. [Google Scholar]
- Grewal, P.S.; Grewal, S.K.; Malik, V.S.; Klein, M.G. Differences in susceptibility of introduced and native white grub species to entomopathogenic nematodes from various geographic localities. Biol. Control 2002, 24, 230–237. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Fuzy, E.M.; Crocker, R.; Gelernter, W.; Polavarapu, S. Pathogenicity of Steinernema scarabaei, Heterorhabditis bacteriophora and S. glaseri to twelve white grub species. Biocontr. Sci. Technol. 2004, 14, 87–92. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Grewal, P.S.; Fuzy, E.M. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, Heterorhabditis zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biol. Control 2006, 38, 397–404. [Google Scholar] [CrossRef]
- Mamiya, Y. Comparison of the infectivity of Steinernema kushidai (Nematoda: Steinernematidae) and other steinernematid and heterorhabditid nematodes for three different insects. Appl. Entomol. Zool. 1989, 24, 302–308. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Fuzy, E.M. Steinernema scarabaei for the control of white grubs. Biol. Control 2003, 28, 47–59. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Fuzy, E.M. Ecological characterization of Steinernema scarabaei: A natural pathogen of scarab larvae. J. Invertebr. Pathol. 2003, 83, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.A. Destructive Turfgrass Insects: Biology, Diagnosis, and Control; Ann Arbor Press: Chelsea, MI, USA, 1998. [Google Scholar]
- Vittum, P.J. Turfgrass Insects of the United States and Canada; Cornell University Press: Ithaca, NY, USA; London, UK, 2020. [Google Scholar]
- Georgis, R.; Gaugler, R. Predictability in biological control using entomopathogenic nematodes. J. Econ. Entomol. 1991, 84, 713–720. [Google Scholar] [CrossRef]
- Grewal, P.S.; Power, K.T.; Grewal, S.K.; Suggars, A.; Haupricht, S. Enhanced consistency in biological control of white grubs (Coleoptera: Scarabaeidae) with new strains of entomopathogenic nematodes. Biol. Control 2004, 30, 73–82. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Fuzy, E.M. Long-term effects and persistence of Steinernema scarabaei applied for suppression of Anomala orientalis (Coleoptera: Scarabaeidae). Biol. Control 2009, 48, 63–72. [Google Scholar] [CrossRef]
- Stock, S.P.; Koppenhöfer, A.M. Steinernema scarabaei n. sp. (Rhabditida: Steinernematidae), a natural pathogen of scarab larvae (Coleoptera: Scarabaeidae) from New Jersey. Nematol. 2003, 5, 191–204. [Google Scholar]
- Stock, S.P.; Goodrich-Blair, H. Nematode parasites, pathogens and associates of insects and invertebrates of economic importance. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 373–426. [Google Scholar] [CrossRef]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Rodríguez Hernández, M.G. Entomopathogenic nematodes in Cuba: From laboratories to popular biological control agents for pest management in a developing country. In Nematode Pathogenesis of Insects and Other Pests: Ecology and Applied Technologies for Sustainable Plant and Crop Protection; Campos Herrera, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 309–327. [Google Scholar]
- Koppenhöfer, A.M.; Sousa, A.L. Long-term suppression of turfgrass insect pests with native persistent entomopathogenic nematodes. J. Invertebr. Pathol. 2024, 204, 108123. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, A.M.; Ebssa, L.; Fuzy, E.M. Storage temperature and duration affect Steinernema scarabaei dispersal and attraction, virulence, and infectivity to a white grub host. J. Invertebr. Pathol. 2013, 112, 129–137. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koppenhöfer, A.M.; Sousa, A.L. New Strains of the Entomopathogenic Nematodes Steinernema scarabaei, S. glaseri, and S. cubanum for White Grub Management. Insects 2024, 15, 1022. https://doi.org/10.3390/insects15121022
Koppenhöfer AM, Sousa AL. New Strains of the Entomopathogenic Nematodes Steinernema scarabaei, S. glaseri, and S. cubanum for White Grub Management. Insects. 2024; 15(12):1022. https://doi.org/10.3390/insects15121022
Chicago/Turabian StyleKoppenhöfer, Albrecht M., and Ana Luiza Sousa. 2024. "New Strains of the Entomopathogenic Nematodes Steinernema scarabaei, S. glaseri, and S. cubanum for White Grub Management" Insects 15, no. 12: 1022. https://doi.org/10.3390/insects15121022
APA StyleKoppenhöfer, A. M., & Sousa, A. L. (2024). New Strains of the Entomopathogenic Nematodes Steinernema scarabaei, S. glaseri, and S. cubanum for White Grub Management. Insects, 15(12), 1022. https://doi.org/10.3390/insects15121022







