Use of Chemical and Colorimetric Changes to Age Cryptotermes brevis Frass for Termite Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Termite Collection
2.2. Frass Collection and Ageing
2.3. Frass Color Analysis
2.4. Chemical Analysis
2.5. Data Analysis
3. Results
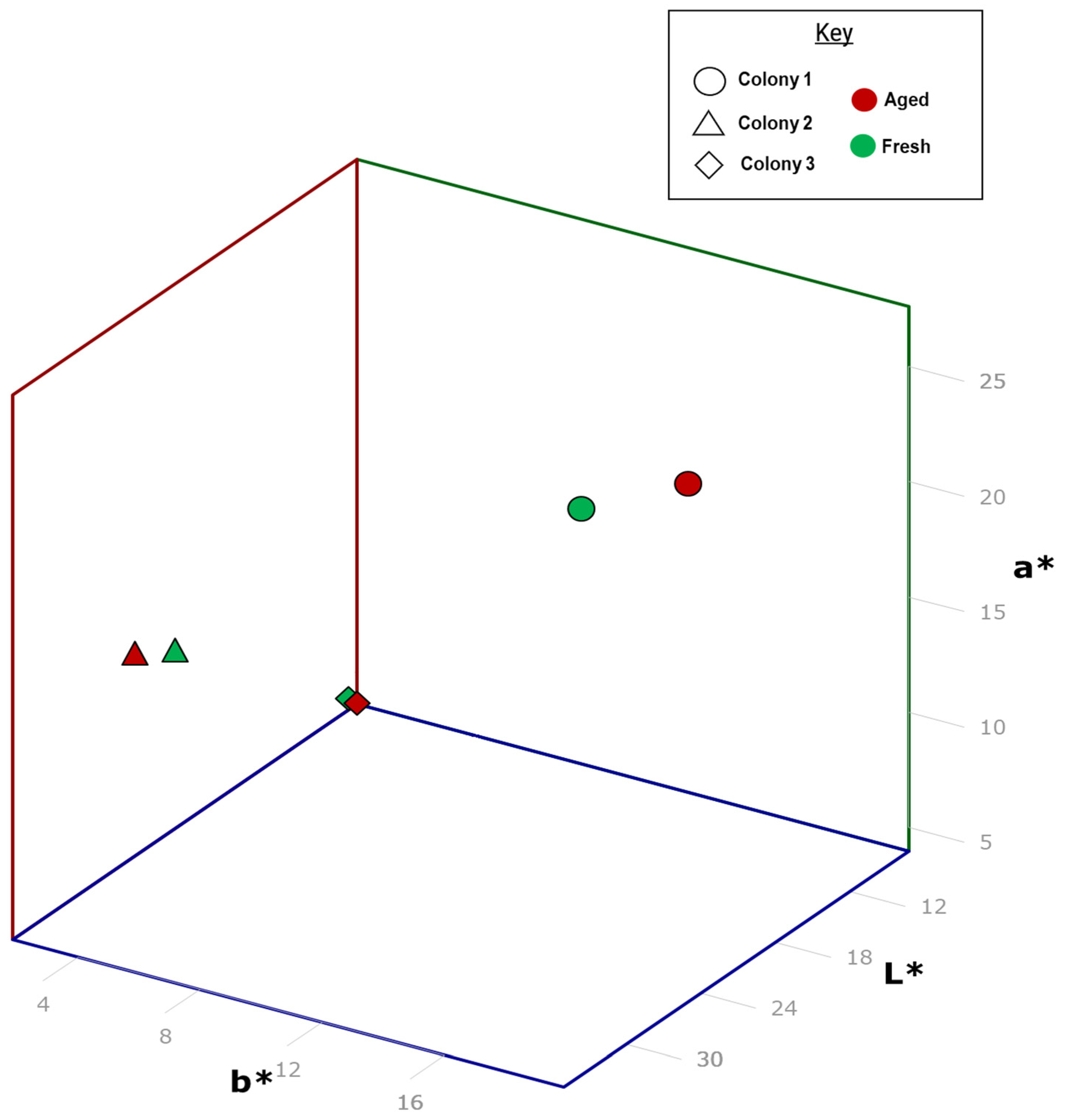
3.1. Color Analysis
3.2. Chemical Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, G.J.; Miller, L.R.; Lenz, M.; Crozier, R.H. Phylogenetic analysis and trait evolution in Australian lineages of drywood termites (Isoptera, Kalotermitidae). Mol. Phylogenet. Evol. 2000, 17, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Horwood, M. West Indian Drywood Termite; New South Wales Department of Primary Industries: Orange, Australia, 2008; Primefact 826.
- Scheffrahn, R.; Křeček, J.; Ripa, R.; Luppichini, P. Endemic origin and vast anthropogenic dispersal of the West Indian drywood termite. Biol. Invasions 2009, 11, 787–799. [Google Scholar] [CrossRef]
- Haigh, W.; Hassan, B.; Hayes, R.A. West Indian drywood termite, Cryptotermes brevis, in Australia: Current understanding, ongoing issues, and future needs. Aust. For. 2023, 85, 211–223. [Google Scholar] [CrossRef]
- Rust, M.K.; Su, N.-Y. Managing social insects of urban importance. Ann. Rev. Entomol. 2012, 57, 355–375. [Google Scholar] [CrossRef]
- Najjari, A.; Taheri, A.; Hernández-Teixidor, D.; Wetterer, J.K. First outdoor records in the Old World of the invasive drywood termite, Cryptotermes brevis (Walker, 1853) (Kalotermitidae). J. Appl. Entomol. 2023, 147, 875–877. [Google Scholar] [CrossRef]
- Osbrink, W.L.A.; Scheffrahn, R.H.; Su, N.-Y.; Rust, M.K. Laboratory comparisons of sulfuryl fluoride toxicity and mean time of mortality among ten termite species (Isoptera: Hodotermitidae, Kalotermitidae, Rhinotermitidae). J. Econ. Entomol. 1987, 80, 1044–1047. [Google Scholar] [CrossRef]
- McDonald, J.; Fitzgerald, C.; Hassan, B.; Morrell, J.J. Thermal tolerance of an invasive drywood termite, Cryptotermes brevis (Blattodea: Kalotermitidae). J. Therm. Biol. 2022, 104, 103199. [Google Scholar] [CrossRef]
- Hassan, B.; Fitzgerald, C. Potential of gas-propelled aerosol containing synergized pyrethrins for localized treatment of Cryptotermes brevis (Kalotermitidae: Blattodea). Insects 2023, 14, 522. [Google Scholar] [CrossRef]
- Hassan, B.; Fitzgerald, C.; Minett, R. Toxicity, repellency, and horizontal transfer of foam insecticides for remedial control of an invasive drywood termite, Cryptotermes brevis (Blattodea: Kalotermitidae). BioResources 2023, 18, 2589–2610. [Google Scholar] [CrossRef]
- McDonald, J.; Fitzgerald, C.; Hassan, B.; Morrell, J. Non-destructive detection of an invasive drywood termite, Cryptotermes brevis (Blattodea: Kalotermitidae), in timber. Sociobiology 2022, 69, e7881. [Google Scholar] [CrossRef]
- Minnick, D.R.; Kerr, S.H.; Wilkinson, R.C. Control of Cryptotermes brevis. J. Econ. Entomol. 1972, 65, 1577–1579. [Google Scholar] [CrossRef]
- Oi, F.M.; Scheffrahn, R.; Kern, W.; Ruppert, K.C. Drywood and dampwood termites: ENY-211/IG098. EDIS 2008. [Google Scholar] [CrossRef]
- Bobadilla, I.; Martínez, R.D.; Martínez-Ramírez, M.; Arriaga, F. Identification of Cryptotermes brevis (Walker, 1853) and Kalotermes flavicollis (Fabricius, 1793) termite species by detritus analysis. Forests 2020, 11, 408. [Google Scholar] [CrossRef]
- Gordon, J.M.; Scheffrahn, R.H.; Su, N.-Y. West Indian drywood termite Cryptotermes brevis (Walker) (Insecta: Isoptera: Kalotermitidae). EDIS 2021, 7, 79. [Google Scholar] [CrossRef]
- Poulos, N.A.; Lee, C.-Y.; Rust, M.K.; Choe, D.-H. Potential use of pinenes to improve localized insecticide injections targeting the western drywood termite (Blattodea: Kalotermitidae). J. Econ. Entomol. 2024, 117, 1628–1635. [Google Scholar] [CrossRef]
- Keesey, I.W.; Koerte, S.; Retzke, T.; Haverkamp, A.; Hansson, B.S.; Knaden, M. Adult frass provides a pheromone signature for Drosophila feeding and aggregation. J. Chem. Ecol. 2016, 42, 739–747. [Google Scholar] [CrossRef]
- Chuche, J.; Xuéreb, A.; Thiéry, D. Attraction of Dibrachys cavus (Hymenoptera: Pteromalidae) to its host frass volatiles. J. Chem. Ecol. 2006, 32, 2721–2731. [Google Scholar] [CrossRef]
- Lewis, V.R.; Nelson, L.J.; Haverty, M.I.; Baldwin, J.A. Quantitative changes in hydrocarbons over time in fecal pellets of Incisitermes minor may predict whether colonies are alive or dead. J. Chem. Ecol. 2010, 36, 1199–1206. [Google Scholar] [CrossRef][Green Version]
- Sharma, G.; Bala, R. Colour fundamentals for digital imaging. In Digital Color Imaging Handbook; Sharma, G., Ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 31. [Google Scholar]
- Haverty, M.I.; Woodrow, R.J.; Nelson, L.J.; Grace, J.K. Identification of termite species by the hydrocarbons in their feces. J. Chem. Ecol. 2005, 31, 2119–2151. [Google Scholar] [CrossRef]
- Stein, S.E. Chemical substructure identification by mass spectral library searching. J. Am. Soc. Mass Spectrom. 1995, 6, 644–655. [Google Scholar] [CrossRef]
- Chakraborty, A.; Šobotník, J.; Votýpková, K.; Hradecký, J.; Stiblik, P.; Synek, J.; Bourguignon, T.; Baldrian, P.; Engel, M.S.; Novotný, V.; et al. Impact of wood age on termite microbial assemblages. Appl. Environ. Microbiol. 2023, 89, e00361-23. [Google Scholar] [CrossRef] [PubMed]
- Andjic, V.; Maxwell, A.; Gorton, M. A loop-mediated isothermal amplification (LAMP) assay for the detection of Cryptotermes brevis West Indian drywood termite (Blattodea: Kalotermitidae). Sci. Rep. 2022, 12, 15111. [Google Scholar] [CrossRef] [PubMed]
- Kalleshwaraswamy, C.M. Potential invasive termites in India and importance of integrative taxonomy. Ind. J. Entomol. 2023, 85, 1088–1104. [Google Scholar] [CrossRef]
- Tolossa, M. Termite, a hidden enemy of crops: A review. Am. J. Biosci. 2022, 10, 172–179. [Google Scholar]
- Blumenfeld, A.J.; Vargo, E.L. Geography, opportunity and bridgeheads facilitate termite invasions to the United States. Biol. Invasions 2020, 22, 3269–3282. [Google Scholar] [CrossRef]


| Age Class | Age Range (Days) | Frass Mass (mg) | ||
|---|---|---|---|---|
| Colony 1 | Colony 2 | Colony 3 | ||
| Fresh | 0–39 | 28.8 | 48.2 | 28.1 |
| 40–66 | 17.8 | 54.5 | 25.3 | |
| 67–105 | 16.7 | 36.2 | 2.00 | |
| 106–133 | 23.3 | 0.20 | 2.60 | |
| 134–165 | 11.0 | 3.90 | 5.60 | |
| 166–196 | 41.1 | 47.9 | 2.10 | |
| Medium | 197–228 | 54.1 | 19.9 | 21.9 |
| 229–259 | 30.7 | 4.60 | 19.1 | |
| 260–288 | 110.3 | 12.3 | 11.9 | |
| 289–312 | 10.9 | 36.0 | 5.80 | |
| 313–353 | 31.2 | 239.3 | 70.1 | |
| 354–381 | 213.3 | 3.90 | 85.0 | |
| Old | 382–416 | 68.4 | 3.20 | 25.2 |
| 417–442 | 50.8 | 2.90 | 5.40 | |
| 443–499 | 35.6 | 14.4 | 228.2 | |
| 500–533 | 53.7 | 42.4 | 48.1 | |
| 534–567 | 56.8 | 62.9 | 139.7 | |
| 568–599 | 30.6 | 31.8 | 119.9 | |
| 600–690 | 255.5 | 183.3 | 453.3 | |
| Colony | Sample | Days Aged | L* | a* | b* |
|---|---|---|---|---|---|
| Colony 1 | Fresh * | 0 | 31.85 | 19.24 | 27.62 |
| Aged | 102 | 24.92 | 19.91 | 26.38 | |
| Colony 2 | Fresh * | 0 | 34.98 | 7.21 | 18.42 |
| Aged | 84 | 34.46 | 5.66 | 17.52 | |
| Colony 3 | Fresh * | 0 | 9.17 | 2.38 | 5.08 |
| Aged | 84 | 7.27 | 1.86 | 3.96 |
| Sample 1 | Sample 2 | ∆E* |
|---|---|---|
| Within samples, across time | ||
| Colony 1, Day 0 | Colony 1, Day 102 | 7.068 |
| Colony 2, Day 0 | Colony 2, Day 84 | 1.857 |
| Colony 3, Day 0 | Colony 3, Day 84 | 2.271 |
| Between samples | ||
| Colony 1, Day 0 | Colony 2, Day 0 | 15.469 |
| Colony 1, Day 0 | Colony 3, Day 0 | 36.142 |
| Colony 2, Day 0 | Colony 3, Day 0 | 29.443 |
| Ret. Time (min) | Compound | Fresh (0–6 Months), 16 Results | Medium (6–12 Months), 19 Results | Old (12+ Months), 19 Results | Friedman |
|---|---|---|---|---|---|
| 6.55 | tetradecanal (C14-al) | 0.04 ± 0.04 (5) a | 0.32 ± 0.12 (47) b | 0.16 ± 0.06 (32) ab | Χ22 = 7.32, p = 0.026 |
| 10.05 | heptadecanal (C16-al) | 0.02 ± 0.02 (5) | 0.05 ± 0.03 (16) | 0.04 ± 0.03 (16) | Χ22 = 1.14, n.d. |
| 17.13 | nonadecane or eicosane | 0.02 ± 0.02 (5) a | 0.24 ± 0.10 (32) ab | 0.35 ± 0.11 (42) b | Χ22 = 6.33, p = 0.042 |
| 17.79 | pentadecanal (C15-al) | 0 (0) a | 0.03 ± 0.03 (5) b | 0.07 ± 0.03 (26) b | Χ22 = 6.5, p = 0.039 |
| 18.48 | 7-hexyldocosane | 0.61 ± 0.21 (32) | 1.56 ± 0.50 (47) | 2.73 ± 0.63 (74) | Χ22 = 3.93, n.d. |
| 19.32 | pentacosane (C25) | 14.25 ± 3.06 (58) | 20.03 ± 2.92 (68) | 19.17 ± 2.69 (79) | Χ22 = 2.61, n.d. |
| 20.92 | 3-ethyltetracosane | 2.16 ± 0.65 (47) a | 3.62 ± 0.74 (68) ab | 5.73 ± 0.88 (79) b | Χ22 = 7.65, p = 0.022 |
| 21.50 | hexacosane (C26) | 0.58 ± 0.31 (21) a | 1.90 ± 0.34 (63) b | 2.31 ± 0.35 (79) b | Χ22 = 10.58, p = 0.005 |
| 22.27 | oxygenated hydrocarbon | 0 (0) a | 0.21 ± 0.09 (37) ab | 0.41 ± 0.24 (42) b | Χ22 = 9.25, p = 0.01 |
| 23.73 | heptacosane (C27) | 21.59 ± 4.40 (63) | 29.54 ± 3.10 (84) | 29.74 ± 2.93 (100) | Χ22 = 4.63, n.d. |
| 25.31 | 9-octylhexacosane | 0.12 ± 0.07 (16) | 0.37 ± 0.10 (42) | 0.31 ± 0.07 (53) | Χ22 = 5.78, n.d. |
| 28.06 | octacosane (C28) | 0.46 ± 0.24 (21) a | 1.44 ± 0.26 (63) b | 1.71 ± 0.28 (74) b | Χ22 = 10.33, p = 0.012 |
| 32.37 | cholesterol | 0.20 ± 0.11 (16) | 0.20 ± 0.09 (37) | 0.11 ± 0.09 (16) | Χ22 = 2.44, n.d. |
| 32.54 | cholestanol | 0.13 ± 0.10 (11) | 0.34 ± 0.13 (37) | 0.26 ± 0.11 (42) | Χ22 = 3.21, n.d. |
| 33.77 | steroid 1 | 22.91 ± 3.11 (84) | 17.08 ± 3.84 (84) | 14.61 ± 2.98 (95) | Χ22 = 3.88, n.d. |
| 36.56 | stigmastanol | 0.27 ± 0.13 (21) | 0.65 ± 0.28 (42) | 1.00 ± 0.31 (79) | Χ22 = 5.65, n.d. |
| 37.55 | steroid 2 | 25.66 ± 3.89 (84) | 14.17 ± 2.44 (84) | 14.24 ± 2.64 (100) | Χ22 = 3.50, n.d. |
| 42.07 | steroid 3 | 10.90 ± 1.87 (84) | 8.06 ± 1.50 (84) | 6.68 ± 1.18 (100) | Χ22 = 2.62, n.d. |
| Compound | % Contribution to Dissimilarity 1 | Cumulative % |
|---|---|---|
| heptacosane (C27) | 14.24 | 14.24 |
| pentacosane (C25) | 13.79 | 28.03 |
| steroid 2 | 11.40 | 39.43 |
| steroid 1 | 11.21 | 50.64 |
| 3-ethyltetracosane | 8.49 | 59.13 |
| steroid 3 | 7.83 | 66.97 |
| hexacosane (C26) | 6.34 | 73.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haigh, W.; Hassan, B.; Yi, T.; Hayes, R.A. Use of Chemical and Colorimetric Changes to Age Cryptotermes brevis Frass for Termite Management. Insects 2024, 15, 924. https://doi.org/10.3390/insects15120924
Haigh W, Hassan B, Yi T, Hayes RA. Use of Chemical and Colorimetric Changes to Age Cryptotermes brevis Frass for Termite Management. Insects. 2024; 15(12):924. https://doi.org/10.3390/insects15120924
Chicago/Turabian StyleHaigh, William, Babar Hassan, Tengfei Yi, and R. Andrew Hayes. 2024. "Use of Chemical and Colorimetric Changes to Age Cryptotermes brevis Frass for Termite Management" Insects 15, no. 12: 924. https://doi.org/10.3390/insects15120924
APA StyleHaigh, W., Hassan, B., Yi, T., & Hayes, R. A. (2024). Use of Chemical and Colorimetric Changes to Age Cryptotermes brevis Frass for Termite Management. Insects, 15(12), 924. https://doi.org/10.3390/insects15120924






