Ecological and Biological Studies of Two Larval Parasitoids on Two Monochamus Vectors of the Pinewood Nematode in South Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
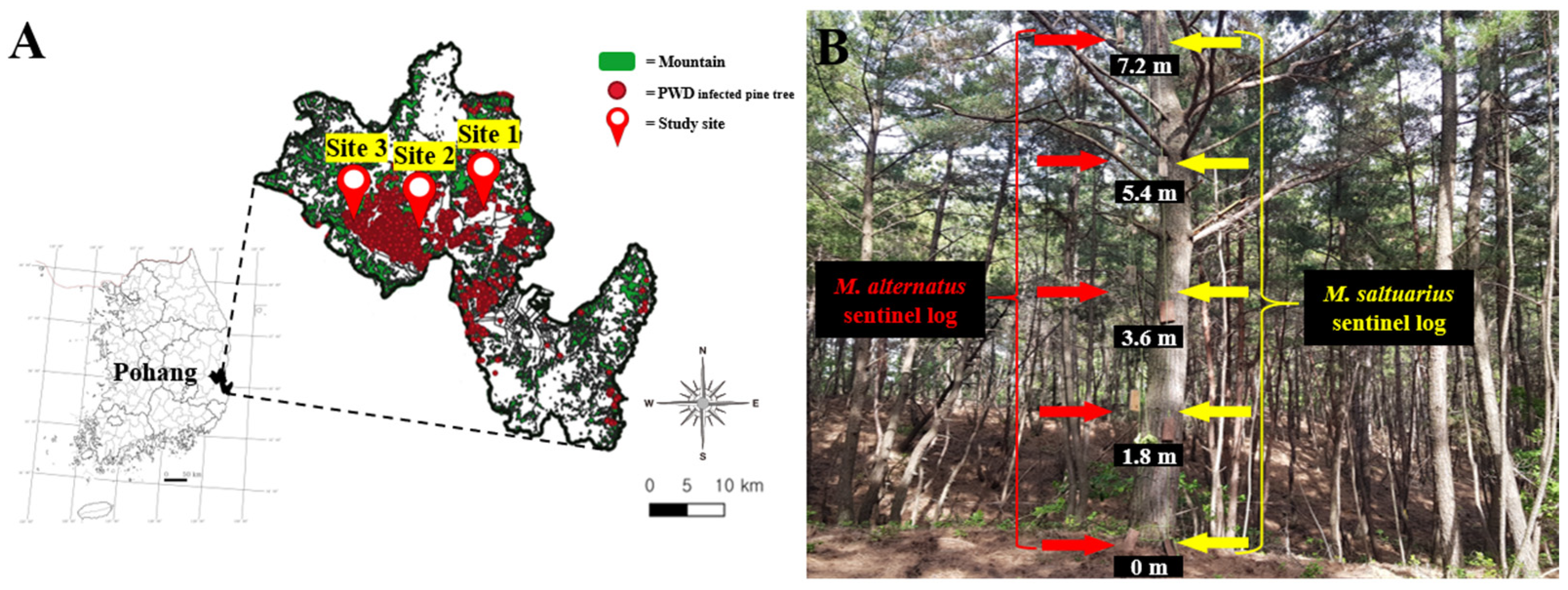
2.1. General Status of the Study Site
2.2. Production of Sentinel Logs
2.3. Installation of Sentinel Logs for Parasitoid Survey
2.4. Identification and Observation of Developmental Stages of Parasitoids and Survey of the Emergence Period of Vectors
2.5. Preliminary Survey for Selection of Dominant Biological Control Agents
2.6. Data Analysis
3. Results
3.1. Dominant Parasitoids Species of M. alternatus and M. saltuarius
3.2. Biological Characteristics of Parasitoids
3.3. Parasitism Rates of C. flavator and S. verustus According to Tree Height and Forest Depth
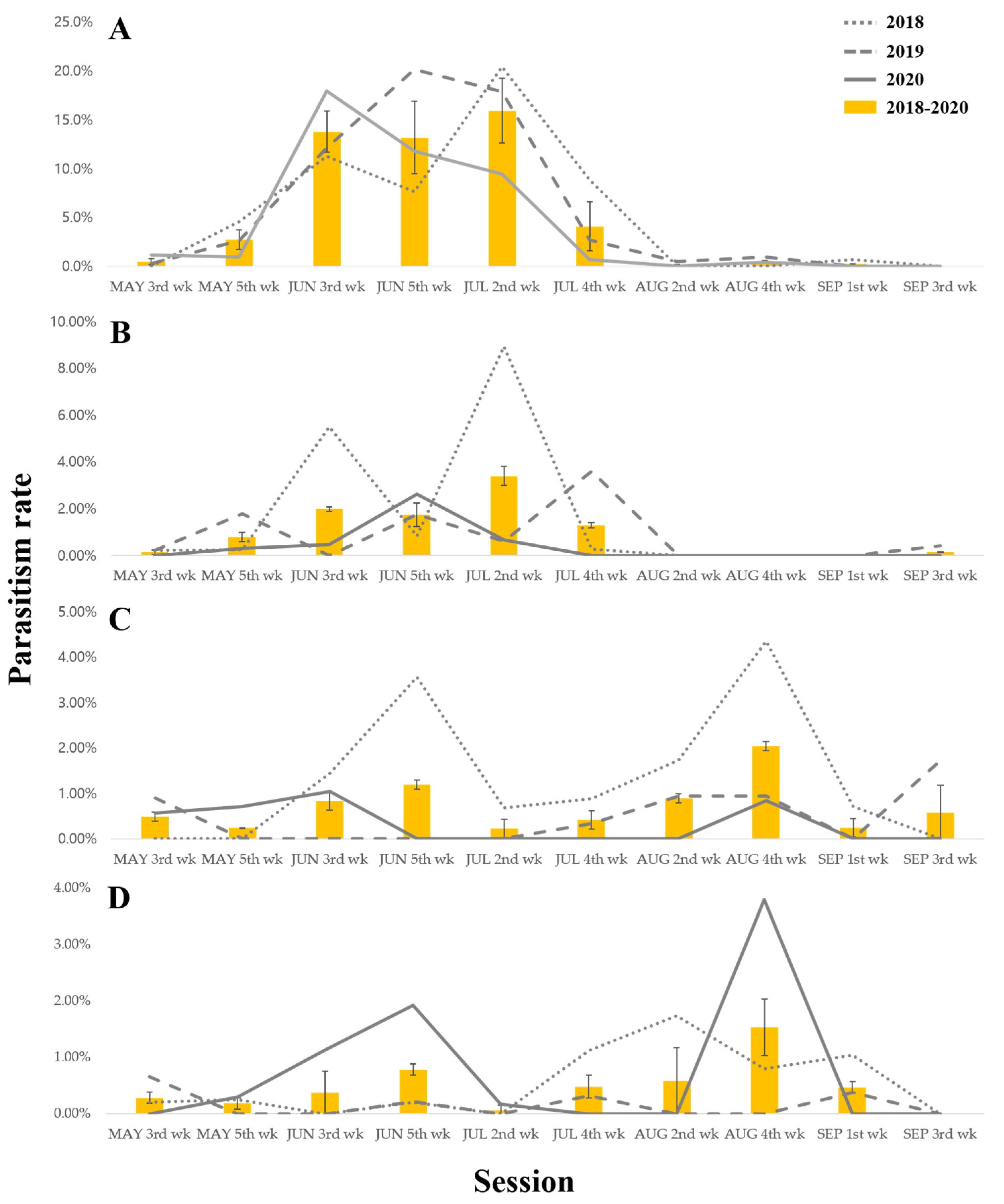
3.4. Seasonality in the Parasitism Rates of C. flavator and S. verustus
4. Discussion
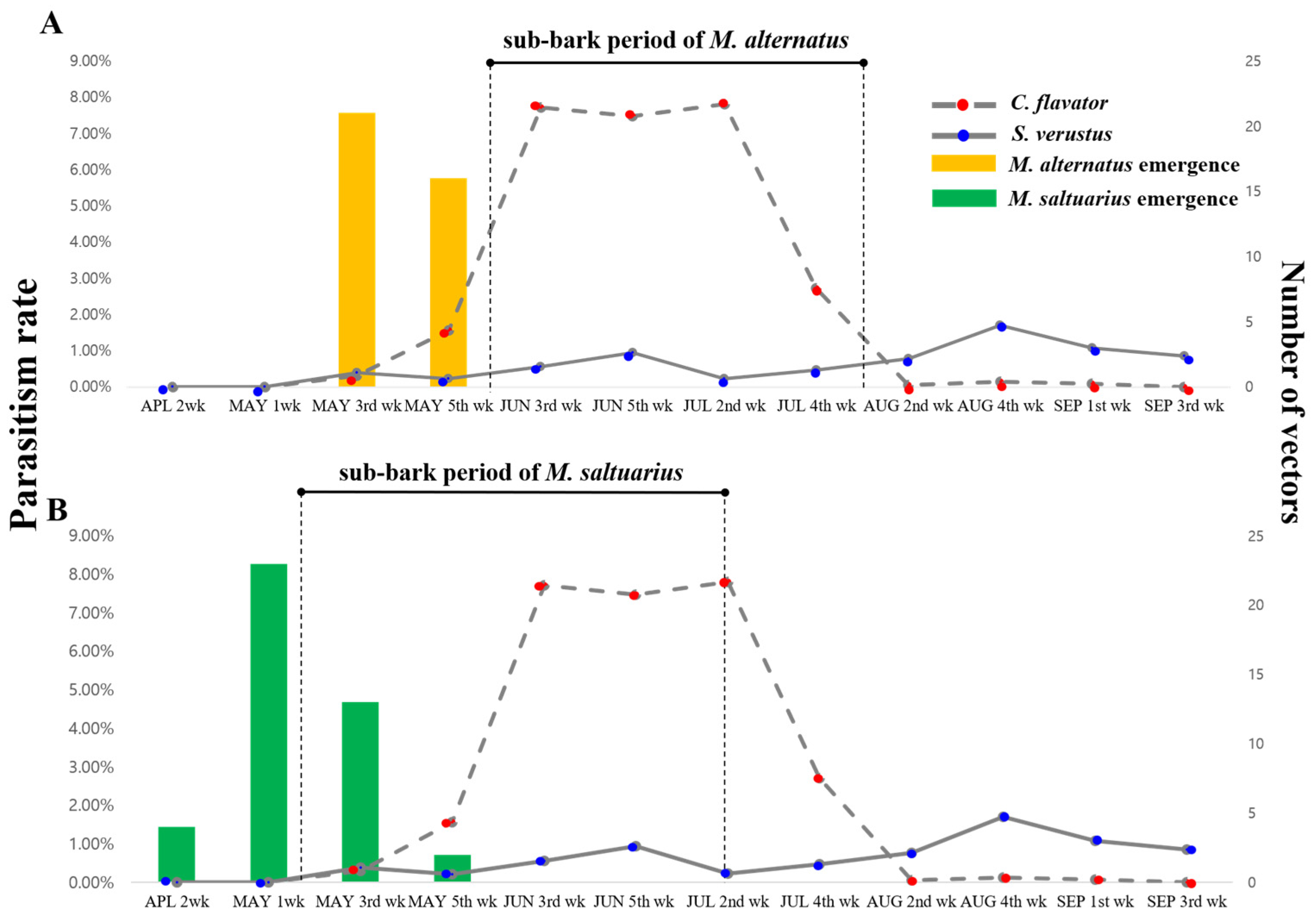
4.1. Development of the Vectors and Release Time of Parasitoids upon Field Application
4.2. Effective Release Locations for C. flavator
4.3. Sex Ratio of C. flavator
4.4. Host Size Preference of Parasitoids
4.5. Assessment of Natural Enemy Utilization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, W.I.; Park, Y.S. Dispersal patterns of exotic forest pests in South Korea. Insect Sci. 2012, 19, 535–548. [Google Scholar] [CrossRef]
- Dwinell, L.D. First report of pinewood nematode (Bursaphelenchus Xylophilus) in Mexico. Plant Dis. 1993, 77, 846. [Google Scholar] [CrossRef]
- Enda, N. The damage of pine wilt disease in Taiwan. For. Pests 1988, 37, 161–166. [Google Scholar]
- Malek, R.B.; Appleby, J.E. Epidemiology of pine wilt in Illinois. Dis. Distrib. Plant Dis. 1984, 68, 180–186. [Google Scholar] [CrossRef]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus Xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Zamora, P.; Rodríguez, V.; Renedo, F.; Sanz, A.V.; Domínguez, J.C.; Pérez-Escolar, G.; Miranda, J.; Álvarez, B.; González-Casas, A.; Mayor, E.; et al. First report of Bursaphelenchus Xylophilus causing pine wilt disease on Pinus radiata in Spain. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wei, Y.; Zhou, J.; Zhang, W.; Qin, P.; Chinta, S.; Kong, X.B.; Liu, Y.; Yu, H.; et al. Ascarosides coordinate the dispersal of a plant-parasitic nematode with the metamorphosis of its vector beetle. Nat. Commun. 2016, 7, 12341. [Google Scholar] [CrossRef] [PubMed]
- Korea Forest Service. Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Republic of Korea, 2023.
- Yi, C.K.; Byun, B.H.; Park, J.D.; Yang, S.I.; Chang, K.H. First Finding of the Pinewood Nematode, Bursaphelenchus Xylophilus (Steiner et Buhrer) Nickle and Its Insect Vector in Korea. Res. Rep. For. Res. Inst. 1989, 38, 141–149. [Google Scholar]
- Shin, S.C. Pine wilt disease in Korea. In Pine Wilt Disease; Springer: Tokyo, Japan, 2008; pp. 32–36. [Google Scholar]
- Oku, H.; Shiraishi, T.; Ouchi, S.; Kurozumi, S.; Ohta, H. Pine wilt toxin, the metabolite of a bacterium associated with a nematode. Naturwissenschaften 1980, 67, 198–199. [Google Scholar] [CrossRef]
- Sasaki, S.; Odani, K.; Nishiyama, Y.; Hayashi, Y. Development and recovery of pine wilt disease studied by tracing ascending sap flow marked with water soluble stains. J. Jpn. Soc. 1984, 66, 141–148. [Google Scholar]
- Tamura, H.; Mineo, K.; Yamada, T. Blockage of water conduction in Pinus thunbergii inoculated with Bursaphelenchus xylophilus. Jpn. J. Nematol. 1987, 17, 23–30. [Google Scholar]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Kwon, T.S.; Lim, J.H.; Sim, S.J.; Kwon, Y.D.; Son, S.; Lee, K.Y.; Kim, Y.T.; Shin, C.H.; Ryu, S.B.; Lee, C.K. Distribution patterns of Monochamus alternatus and M. saltuarius (Coleoptera: Cerambycidae) in Korea. J. Korean Soc. For. Sci. 2006, 95, 543–550. [Google Scholar]
- Hong, J.I.; Koh, S.H.; Chung, Y.J.; Shin, S.C.; Kim, G.H.; Choi, K.S. Biological characteristics of Sclerodermus harmandi (Hymenoptera: Bethylidae) parasitized on cerambycid. Korean J. Appl. Entomol. 2008, 47, 133–139. [Google Scholar] [CrossRef]
- Jang, T.W.; Jeong, J.C.; Choi, J.K.; Jeong, C.S.; Kim, J.K. Biological characteristics of Dolichomitus cephalotes and Dolichomitus curticornis (Hymenoptera, Ichneumonidae), parasitoids of Monochamus saltuarius (Coleoptera, Cerambycidae). J. For. Environ. Sci. 2019, 35, 258–262. [Google Scholar]
- Kim, M.S.; Jung, J.K.; Hong, K.J.; Kim, C.J.; Lee, B.W.; Kim, I.K. Discovery and biology of Spathius verustus Chao (Hymenoptera: Braconidae), a potential biological agent on two Monochamus vectors of the pinewood nematode. Forests 2022, 13, 955. [Google Scholar] [CrossRef]
- Korea Forest Service. Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Republic of Korea, 2005.
- Lee, S.M.; Hong, D.K.; Park, J.; Lee, J.; Jang, S.H.; Lee, C. Field bioassay for longhorn pine sawyer beetle Monochamus alternatus (Coleoptera: Cerambycidae) in Korea based on aggregation pheromone 2-(undecyloxy)ethanol. J. Life Sci. 2015, 25, 1445–1449. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Hansen, J.A.; Abell, K.J.; Van Driesche, R. An improved method for monitoring parasitism and establishment of Oobius agrili (Hymenoptera: Encyrtidae), an egg parasitoid introduced for biological control of the emerald ash borer (Coleoptera: Buprestidae) in North America. Biol. Control 2012, 60, 255–261. [Google Scholar] [CrossRef]
- Futai, K.; Shirakikawa, S.; Nakai, I. The Suitability of Korean Pine (Pinus koraiensis SIEB. et ZUCC.) and Japanese Red Pine (P. densiflora SIEB. et ZUCC.) as a Host of the Japanese Pine Sawyer, Monochamus alternatus HOPE (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1994, 29, 167–177. [Google Scholar] [CrossRef]
- Hwang, I.C.; Kim, J.H.; Park, J.B.; Shin, S.C.; Chung, Y.J.; Cho, S.Y.; Park, Y.C. Growth, development, and reproduction of Monochamus saltuarius (Coleoptera: Cerambycidae) on conifers fed to larvae. Korean J. Appl. Entomol. 2008, 47, 385–394. [Google Scholar] [CrossRef]
- Ochi, K. Ecological studies on cerambycid injurious to pine trees (II) Biology of Two Monochamus (Coleoptera, Cerambycidae). J. Jpn. For. Soc. 1969, 51, 188–192. [Google Scholar]
- Kim, D.S.; Lee, S.M.; Moon, I.S.; Chung, Y.J.; Choi, K.S.; Park, J.G. Emergence ecology of Japanese pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae), a vector of pinewood nematode, Bursaphelenchus xylophilus. Korean J. Appl. Entomol. 2003, 42, 307–313. [Google Scholar]
- Han, J.H.; You, J.H.; Koo, C.D.; Yoon, C.M.; Choi, K.S.; Shin, S.C.; Kim, G.H. Emergence timing of the pine sawyer beetle, Monochamus saltuarius (Coleoptera: Cerambycidae) by tree species. Korean J. Appl. Entomol. 2009, 48, 189–195. [Google Scholar] [CrossRef]
- Petersen-Silva, R.; Pujade-Villar, J.; Naves, P.; Sousa, E.; Belokobylskij, S. Parasitoids of Monochamus galloprovincialis (Coleoptera, Cerambycidae), vector of the pine wood nematode, with identification key for the Palaearctic region. ZooKeys 2012, 251, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.A.; Huber, J.T.; Woolley, J.B. (Eds.) Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera); NRC Research Press: Ottawa, ON, Canada, 1997; Volume 40392. [Google Scholar]
- Lim, J.; Lyu, D.; Choi, G.; Jeong, Y.; Shin, S.; Lee, S. A Taxonomic Note on Sclerodermus Harmandi, Ectoparasite of Stem and Wood Boring Insect Larvae (Hymenoptera: Chrysidoidea: Bethylidae) in South Korea. J. Asia-Pac. Entomol. 2006, 9, 115–119. [Google Scholar]
- Broad, G. Identification Key to the Subfamilies of Ichneumonidae (Hymenoptera). 2011, pp. 1–52. Available online: https://api.semanticscholar.org/CorpusID:89431296 (accessed on 1 October 2024).
- Go, M.S.; Kwon, S.H.; Kim, S.B.; Kim, D.S. The developmental characteristics for the head capsule width of Monochamus alternatus (Coleoptera: Cerambycidae) larvae and determination of the number of instars. J. Insect Sci. 2019, 19, 26. [Google Scholar] [CrossRef]
- Kojima, K.; Katagiri, K. On the larval instar and changes of its composition of Monochamus alternalus Hope. J. Jpn. Soc. 1964, 46, 307–310. [Google Scholar]
- Fan, L.; Wang, J.; Wang, W.; Zheng, Y. Larval instars and adult flight period of Monochamus saltuarius (Coleoptera: Cerambycidae). Forests 2022, 13, 910. [Google Scholar] [CrossRef]
- Moreira, M.J. A conditional likelihood ratio test for structural models. Econometrica 2003, 71, 1027–1048. [Google Scholar] [CrossRef]
- Douglas, B.; Martin, M.; Ben, B.; Steve, W. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Segoli, M.; Kishinevsky, M.; Rozenberg, T.; Hoffmann, I. Parasitoid abundance and community composition in desert vineyards and their adjacent natural habitats. Insects 2020, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Jikumaru, S.; Togashi, K.; Taketsune, A.; Takahashi, F. Oviposition biology of Monochamus saltuarius (Coleoptera: Cerambycidae) at a constant temperature. Appl. Entomol. Zool. 1994, 29, 555–561. [Google Scholar] [CrossRef]
- Jikumaru, S.; Togashi, K. A weak deleterious effect of the avirulent pinewood nematode, Bursaphelenchus mucronatus (Nematoda: Aphelenchoididae), on the longevity of its vector, Monochamus saltuarius (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1995, 30, 9–16. [Google Scholar] [CrossRef]
- Anbutsu, H.; Togashi, K. Oviposition deterrent by female reproductive gland secretion in Japanese pine sawyer, Monochamus alternatus. J. Chem. Ecol. 2001, 27, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, J.K.; Nam, Y.; Koh, S.H. Temperature-dependent oviposition models for Monochamus saltuarius (Coleoptera: Cerambycidae). Insects 2024, 15, 597. [Google Scholar] [CrossRef]
- Nakayama, Y.; Jikumaru, S.; Togashi, K. Reproductive traits and diel activity of adult Monochamus saltuarius (Coleoptera: Cerambycidae) at two different temperatures. J. Res. 1998, 3, 61–65. [Google Scholar] [CrossRef]
- Zhang, X.; Linit, M.J. Comparison of oviposition and longevity of Monochamus alternatus and M. carolinensis (Coleoptera: Cerambycidae) under laboratory conditions. Environ. Entomol. 1998, 27, 885–891. [Google Scholar] [CrossRef]
- Morimoto, K.; Mamiya, Y. Pine Disease and Its Control; Japanese Forestry Association: Tokyo, Japan, 1977; p. 66. [Google Scholar]
- Li, M.; Dai, Y.; Wang, Y.; Wang, L.; Sun, S.; Chen, F. New insights into the life history of Monochamus saltuarius (Cerambycidae: Coleoptera) can enhance surveillance strategies for pine wilt disease. J. Res. 2021, 32, 2699–2707. [Google Scholar] [CrossRef]
- Yu, D.S.; van Achterberg, C.; Horstmann, K. Taxapad 2016, Ichneumonoidea 2015, Database on Flash-Drive; Nepean: Narellan, NSW, Australia, 2016. [Google Scholar]
- Fulmek, L. Insect parasites of insect galls of Europe. Beitr. Zur. Entomol. 1968, 18, 719–752. [Google Scholar]
- Kittel, R.N.; Quicke, D.L.; Maeto, K. Recision of braconine wasps of Japan (Hymenoptera: Braconidae) with revised generic records. Jpn. J. Syst. Entomol. 2019, 25, 132–153. [Google Scholar]
- Naves, P.; Kenis, M.; Sousa, E. Parasitoids associated with Monochamus galloprovincialis (Oliv.) (Coleoptera: Cerambycidae) within the pine wilt nematode-affected zone in Portugal. J. Pest Sci. 2005, 78, 57–62. [Google Scholar] [CrossRef]
- Kwon, S.H.; Go, M.S.; Park, J.; Han, T.W.; Kim, K.B.; Shin, C.H.; Kim, D.S. The bimodal adult activity of Monochamus alternatus (Coleoptera: Cerambycidae) caught in pheromone traps in Jeju can be explained by the competitive attractiveness of dying pine trees. Entomol. Res. 2019, 49, 172–178. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, L.C.; Zhang, Y.L.; Wang, J.; Zheng, Y.A. Distribution rule of Monochamus saltuarius larvae in the trunk of Pinus koraiensis. Sci. Silvae Sin. 2022, 58, 128–133. [Google Scholar] [CrossRef]
- Charnov, E.L.; Los-den Hartogh, R.L.; Jones, W.T.; Van den Assem, J. Sex ratio evolution in a variable environment. Nature 1981, 289, 27–33. [Google Scholar] [CrossRef]
- Boulton, R.A.; Collins, L.A.; Shuker, D.M. Beyond sex allocation: The role of mating systems in sexual selection in parasitoid wasps. Biol. Rev. Camb. Philos. Soc. 2015, 90, 599–627. [Google Scholar] [CrossRef]
- Lin, L.A.; Ives, A.R. The effect of parasitoid host-size preference on host population growth rates: An example of Aphidius colemani and Aphis glycines. Ecol. Entomol. 2003, 28, 542–550. [Google Scholar] [CrossRef]
- Pekas, A.; Tena, A.; Harvey, J.A.; Garcia-Marí, F.; Frago, E. Host size and spatiotemporal patterns mediate the coexistence of specialist parasitoids. Ecology 2016, 97, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Krimmel, B.A.; Morse, D.H. Host-size decisions of female parasitoid wasps seeking hidden hosts. Ecol. Entomol. 2019, 44, 552–559. [Google Scholar] [CrossRef]
- Kim, J.K.; Won, D.S.; Park, Y.C.; Koh, S.H. Natural enemies of wood borers and seasonal occurrence of major natural enemies of Monochamus saltuarius on pine trees. J. Korean Soc. For. Sci. 2010, 99, 439–445. [Google Scholar]
- Liu, H.; Bauer, L.S.; Miller, D.L.; Zhao, T.; Gao, R.; Song, L.; Luan, Q.; Jin, R.; Gao, C. Seasonal abundance of Agrilus planipennis (Coleoptera: Buprestidae) and its natural enemies Oobius agrili (Hymenoptera: Encyrtidae) and Tetrastichus planipennisi (Hymenoptera: Eulophidae) in China. Biol. Control 2007, 42, 61–71. [Google Scholar] [CrossRef]

| Host | Parasitoids | Total Parasitism Rate (Number of Parasitized Larvae/ Total Number of Host Larvae) |
|---|---|---|
| M. alternatus (Total number of larvae: 2715) | Cyanopterus flavator | 5.8% |
| Spathius verustus | 1.3% | |
| Doryctes strialellus | 0.2% | |
| Rhaconotus formosanus | 0.1% | |
| Xorides sepulchralis | 0.03% | |
| Ichneumonidae sp. 1 | 0.03% | |
| Ichneumonidae sp. 2 | 0.03% | |
| M. saltualius (Total number of larvae: 4076) | Cyanopterus flavator | 1.6% |
| Doryctes strialellus | 0.6% | |
| Spathius verustus | 0.5% | |
| Rhaconotus formosanus | 0.3% | |
| Sclerodermus harmandi | 0.1% | |
| Heydenia sp. 1 | 0.1% | |
| Heydenia sp. 2 | 0.1% | |
| Pteromalidae sp. 1 | 0.1% | |
| Pteromalidae sp. 2 | 0.1% | |
| Pteromalidae sp. 3 | 0.1% | |
| Pteromalidae sp. 4 | 0.02% | |
| Pteromalidae sp. 5 | 0.02% |
| Parasitoid (Parasitism Type) | Host | Total Parasitism Rate (n) | p-Value | Sex Ratio |
|---|---|---|---|---|
| C. flavator (solitary) | M. alternatus | 6.3% (1350) | 2.2 × 10−16 *** | 0.8 |
| M. saltuarius | 1.0% (1350) | 0.9 | ||
| M. alternatus + M. saltuarius | 3.4% (2700) | - | 0.8 | |
| S. verustus (gregarious) | M. alternatus | 0.7% (1350) | 0.49 | 0.2 |
| M. saltuarius | 0.5% (1350) | 0.2 | ||
| M. alternatus + M. saltuarius | 0.6% (2700) | - | 0.2 |
| Parasitoid | Host | Larval Instar (Range of Head Capsule Width, mm) | Mean ± SD Head Capsule Width (mm) with Sample Size n in Bracket (OR ± SE) | Parasitism Rate % * |
|---|---|---|---|---|
| C. flavator | M. alternatus | 1st (0–1.1) | 0.54 ± 0.02 (2) | 1.7 |
| 2nd (1.1–1.4) | 1.03 ± 0.12 (4) | 3.4 | ||
| 3rd (1.4–1.9) | 1.39 ± 0.15 (15) | 12.7 | ||
| 4th (1.9–2.4) | 2.24 ± 0.11 (61) | 51.7 | ||
| 5th (2.4–2.8) | 1.11 ± 0.07 (25) | 21.2 | ||
| 8th (3.3–3.6) | 3.75 ± 0.18 (11) | 9.3 | ||
| M. saltuarius | 1st (0.64–1.00) | 1.11 ± 0.17 (2) | 4.1 | |
| 2nd (1.03–1.5) | 1.75 ± 0.14 (28) | 57.1 | ||
| 3rd (1.52–2.3) | 2.19 ± 0.22 (12) | 24.5 | ||
| 4th (2.31–3.81) | 2.85 ± 0.07 (7) | 14.3 | ||
| S. verustus | M. alternatus | 2nd (1.1–1.4) | 1.11 ± 0.01 (2) | 7.1 |
| 3rd (1.4–1.9) | 1.14 ± 0.1 (7) | 25.0 | ||
| 4th (1.9–2.4) | 2.22 ± 0.12 (14) | 50.0 | ||
| 5th (2.4–2.8) | 2.55 ± 0.11 (5) | 17.9 | ||
| M. saltuarius | 1st (0.64–1.00) | 1.13 ± 0.12 (13) | 59.1 | |
| 2nd (1.03–1.5) | 1.72 ± 0.12 (8) | 36.4 | ||
| 3rd (1.52–2.3) | 1.99 ± 0.00 (1) | 4.5 |
| M. alternatus | Sex of C. flavator (n) | t-Test | |
|---|---|---|---|
| Larval Instar | Average Head Capsule Width ± SD (mm) | ||
| 1st | 1.22 ± 0.18 | Female (2) | 0.09 |
| 0.93 ± 0.11 | Male (4) | ||
| 2nd | 1.45 ± 0.04 | Female (2) | 0.12 |
| 1.34 ± 0.09 | Male (30) | ||
| 3rd | 1.73 ± 0.33 | Female (2) | 0.08 |
| 1.7 ± 0.18 | Male (16) | ||
| 4th | 2.36 ± 0.18 | Female (41) | 0.09 |
| 2.31 ± 0.15 | Male (156) | ||
| 5th | 3.69 ± 0.2 | Female (9) | 0.06 |
| 3.66 ± 0.21 | Male (19) | ||
| Parasitoid | Variable | Interaction Effect Between Height and Depth (p-Value) | Distance (m) from the Ground on Trunks/from the Edge in Forest | Total Parasitism Rate † | p-Value |
|---|---|---|---|---|---|
| C. flavator | Height of sentinel log | 0.06 | 0 | 1.7% a | 2.2 × 10−16 *** |
| 1.8 | 2.6% ab | ||||
| 3.6 | 3.5% bc | ||||
| 5.4 | 4.2% bc | ||||
| 7.2 | 5.2% c | ||||
| Forest depth | 0 | 3.4% | 0.65 | ||
| 20 | 3.6% | ||||
| 40 | 3.3% | ||||
| S. verustus | Height of sentinel log | 0.12 | 0 | 0.4% ab | 0.003 ** |
| 1.8 | 1.0% c | ||||
| 3.6 | 0.4% ab | ||||
| 5.4 | 0.7% b | ||||
| 7.2 | 0.2% a | ||||
| Forest depth | 0 | 0.6% | 0.55 | ||
| 20 | 0.5% | ||||
| 40 | 0.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Kim, I.-K. Ecological and Biological Studies of Two Larval Parasitoids on Two Monochamus Vectors of the Pinewood Nematode in South Korea. Insects 2024, 15, 943. https://doi.org/10.3390/insects15120943
Kim M-S, Kim I-K. Ecological and Biological Studies of Two Larval Parasitoids on Two Monochamus Vectors of the Pinewood Nematode in South Korea. Insects. 2024; 15(12):943. https://doi.org/10.3390/insects15120943
Chicago/Turabian StyleKim, Moo-Sung, and Il-Kwon Kim. 2024. "Ecological and Biological Studies of Two Larval Parasitoids on Two Monochamus Vectors of the Pinewood Nematode in South Korea" Insects 15, no. 12: 943. https://doi.org/10.3390/insects15120943
APA StyleKim, M.-S., & Kim, I.-K. (2024). Ecological and Biological Studies of Two Larval Parasitoids on Two Monochamus Vectors of the Pinewood Nematode in South Korea. Insects, 15(12), 943. https://doi.org/10.3390/insects15120943




