Bumblebee Abundance in Species-Rich Grasslands in Southern Sweden Decreases with Increasing Amount of Arable Land at a Landscape Level
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Set
2.2. Data Handling
- (i)
- Tongue length: long-tongued or short-tongued [39];
- (ii)
- Habitat preference: agricultural or forested landscapes (www.artfakta.se);
- (iii)
- Colony size: small, medium or large [40];
- (iv)
- Queen emergence: early or late [40].
2.3. Statistical Analyses
2.3.1. Generalised Linear Mixed Model
2.3.2. Potential Confounding Factors
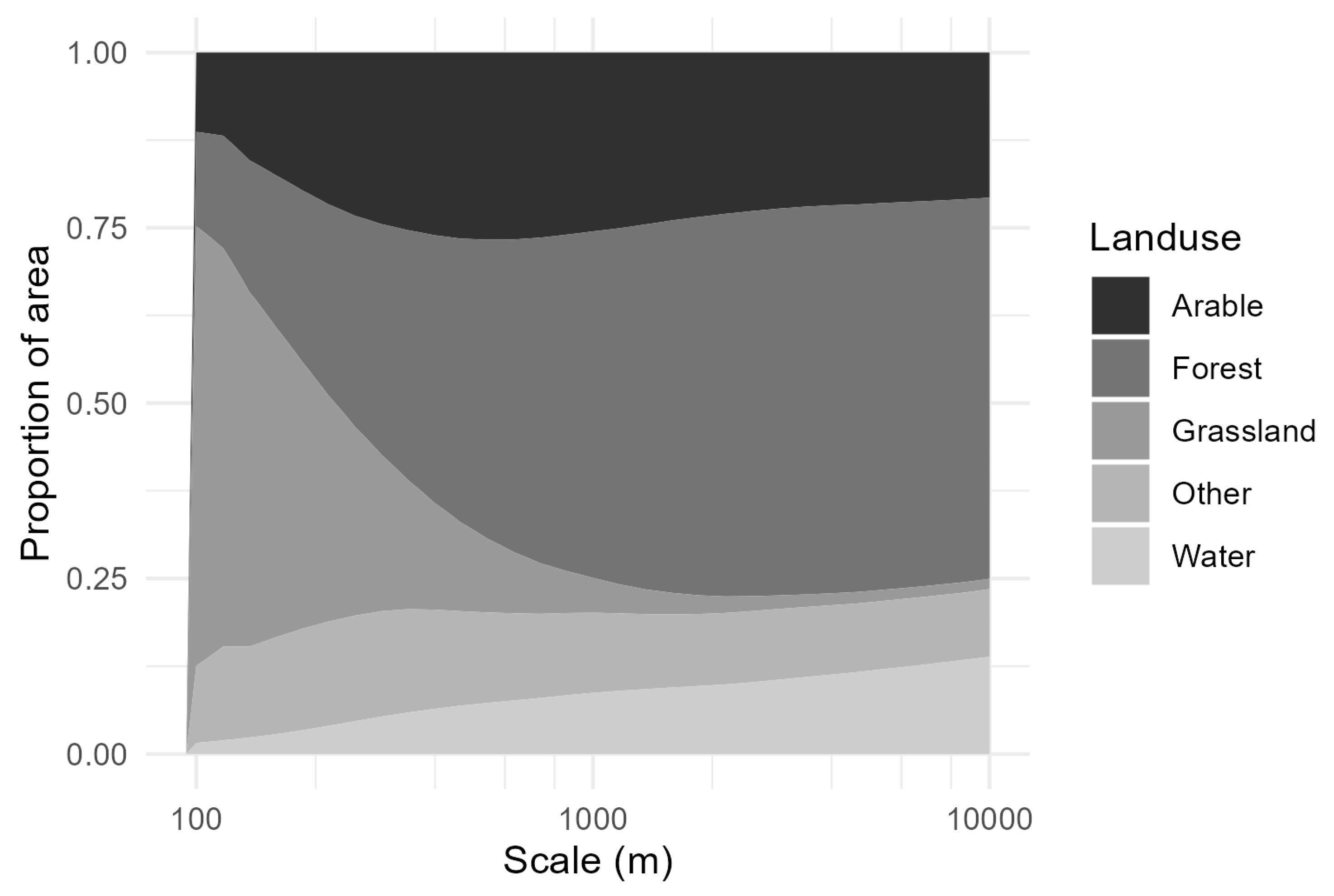
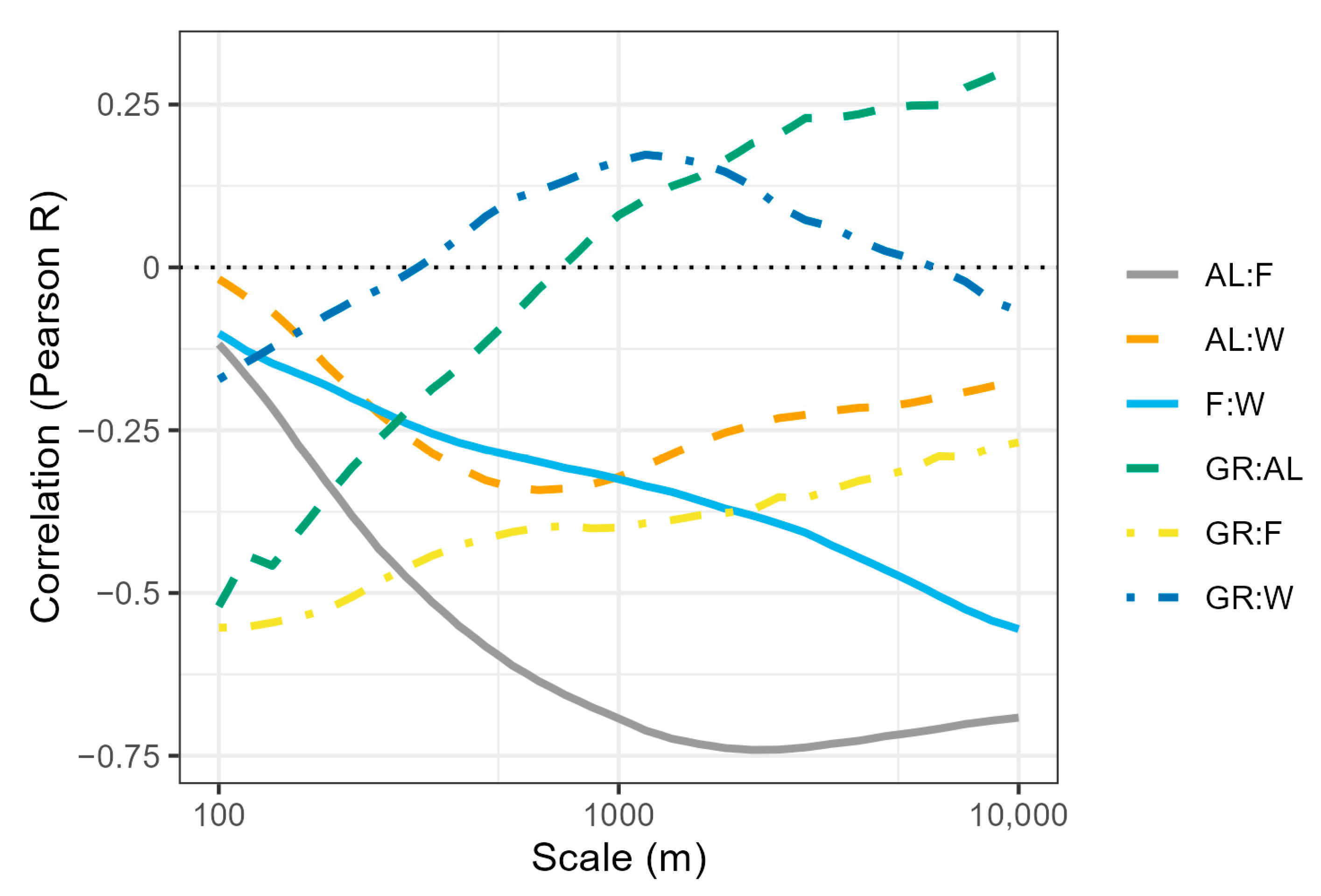
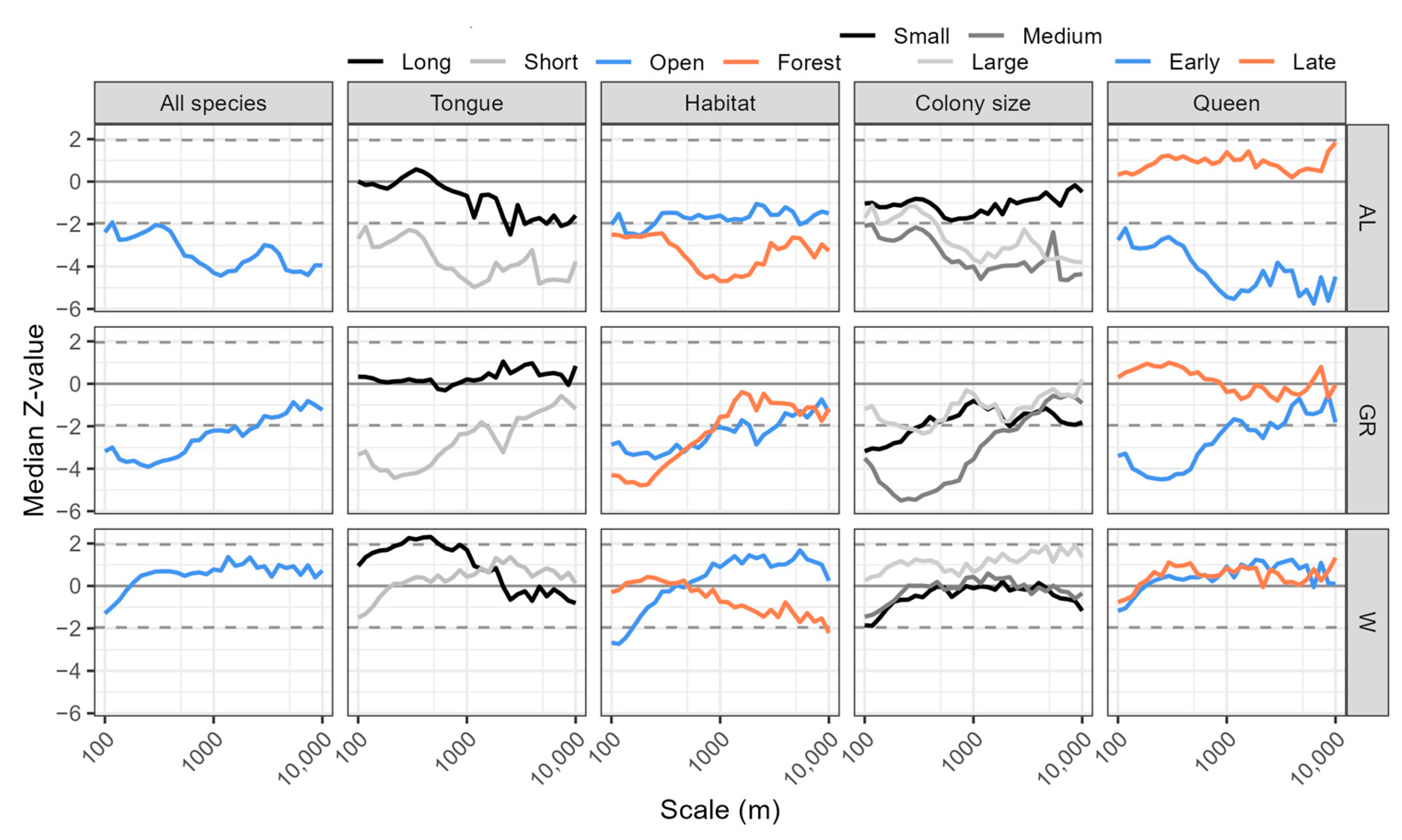
3. Results
4. Discussion
4.1. Effect of Land Use Type
Group-Wise Analyses
4.2. Effect of Scale
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 276, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Albrecht, M.; Schmid, B.; Hautier, Y.; Muller, C.B. Diverse pollinator communities enhance plant reproductive success. Proc. R. Soc. B Biol. Sci. 2012, 279, 4845–4852. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Marini, L.; Vaissière, B.E. Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 2012, 169, 1025–1032. [Google Scholar] [CrossRef]
- Winfree, R.; Williams, N.M.; Dushoff, J.; Kremen, C. Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 2007, 10, 1105–1113. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Williams, P.H.; Osborne, J.L. Bumblebee vulnerability and conservation world-wide. Apidologie 2009, 40, 367–387. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Bommarco, R.; Lundin, O.; Smith, H.G.; Rundlöf, M. Drastic historic shifts in bumble-bee community composition in Sweden. Proc. R. Soc. B Biol. Sci. 2011, 279, 309–315. [Google Scholar] [CrossRef]
- Vanbergen, A.J. The Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Paredes, D.; Rosenheim, J.A.; Chaplin-Kramer, R.; Winter, S.; Karp, D.S. Landscape simplification increases vineyard pest outbreaks and insecticide use. Ecol. Lett. 2021, 24, 73–83. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.; Pedersen, T.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on insecticides: A review of uses, molecular structures, targets, adverse effects, and alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Öckinger, E.; Smith, H.E. Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J. Appl. Ecol. 2006, 44, 50–59. [Google Scholar] [CrossRef]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008, 23, 191–208. [Google Scholar] [CrossRef]
- Proesmans, W.; Smagghe, G.; Meeus, I.; Bonte, D.; Verheyen, K. The effect of mass-flowering orchards and semi-natural habitat on bumblebee colony performance. Landsc. Ecol. 2019, 34, 1033–1044. [Google Scholar] [CrossRef]
- Sõber, V.; Leps, M.; Kaasik, A.; Mänd, M.; Teder, T. Forest proximity supports bumblebee species richness and abundance in hemi-boreal agricultural landscapes. Agric. Ecosyst. Environ. 2020, 298, 106961. [Google Scholar] [CrossRef]
- Gómez-Martínez, C.; Aase, A.L.T.; Totland, Ø.; Rodríguez-Pérez, J.; Birkemoe, T.; Sverdrup-Thygeson, A.; Lázaro, A. Forest fragmentation modifies the composition of bumblebee communities and modulates their trophic and competitive interactions for pollination. Sci. Rep. 2020, 10, 10872. [Google Scholar] [CrossRef]
- Mola, J.M.; Hemberger, J.; Kochanski, J.; Richardson, L.L.; Pearse, I.S. The importance of forests in bumble bee biology and conservation. BioScience 2021, 71, 1234–1248. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Munzenberg, U.; Burger, C.; Thies, C.; Tscharntke, T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 2002, 83, 1421–1432. [Google Scholar] [CrossRef]
- Jauker, F.; Diekötter, T.; Schwarzbach, F.; Wolters, V. Pollinator dispersal in an agricultural matrix: Opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landsc. Ecol. 2009, 24, 547–555. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Verboven, H.A.F.; Uyttenbroeck, R.; Brys, R.; Hermy, M. Different responses of bees and hoverflies to land use in an urban–rural gradient show the importance of the nature of the rural land use. Landsc. Urban Plan. 2014, 126, 31–41. [Google Scholar] [CrossRef]
- Senapathi, D.; Goddard, M.A.; Kunin, W.E.; Baldock, K.C. Landscape impacts on pollinator communities in temperate systems: Evidence and knowledge gap. Funct. Ecol. 2017, 31, 26–37. [Google Scholar] [CrossRef]
- Andersson, G.K.; Boke-Olén, N.; Roger, F.; Ekroos, J.; Smith, H.G.; Clough, Y. Landscape-scale diversity of plants, bumblebees and butterflies in mixed farm-forest landscapes of Northern Europe: Clear-cuts do not compensate for the negative effects of plantation forest cover. Biol. Conserv. 2022, 274, 109728. [Google Scholar] [CrossRef]
- Smith, A.C.; Fahrig, L.; Francis, C.M. Landscape size affects the relative importance of habitat amount, habitat fragmentation, and matrix quality on forest birds. Ecography 2011, 34, 103–113. [Google Scholar] [CrossRef]
- Bergman, K.-O.; Dániel-Ferreira, J.; Milberg, P.; Öckinger, E.; Westerberg, L. Butterflies in Swedish grasslands benefit from forest and respond to landscape composition at different spatial scales. Landsc. Ecol. 2018, 33, 2189–2204. [Google Scholar] [CrossRef]
- Goulson, D.; Hanley, M.E.; Darvill, B.; Ellis, J.S.; Knight, M.E. Causes of rarity in bumblebees. Biol. Conserv. 2005, 122, 1–8. [Google Scholar] [CrossRef]
- Ståhl, G.; Allard, A.; Esseen, P.-A.; Glimskär, A.; Ringvall, A.; Svensson, J.; Sundquist, S.; Christensen, P.; Gallegos Torell, Å.; Högström, M.; et al. National Inventory of Landscapes in Sweden (NILS): Scope, design, and experiences from establishing a multiscale biodiversity monitoring system. Environ. Monit. Assess. 2011, 173, 579–595. [Google Scholar] [CrossRef]
- Persson, K. Ängs-Och Betesmarksinventeringen 2002–2004 [Survey of Semi-Natural Pastures and Meadows 2002–2004]; Board of Agriculture: Jönköping, Sweden, 2005; (In Swedish with English Summary). [Google Scholar]
- Persson, K. Ängs-Och Betesmarksinventeringen: Inventeringsmetod [Survey of Semi-Natural Pastures and Meadows: Methodology]; Board of Agriculture: Jönköping, Sweden, 2005. [Google Scholar]
- Öster, M.; Persson, K.; Eriksson, O. Validation of plant diversity indicators in semi-natural grasslands. Agric. Ecosyst. Environ. 2008, 125, 65–72. [Google Scholar] [CrossRef]
- Milberg, P.; Franzen, M.; Karpaty Wickbom, A.; Svelander, S.; Johansson, V. Temporal pattern of pollinator flight and flowering of agricultural weeds in Sweden. Ecol. Evol. 2024, 14, e11725. [Google Scholar] [CrossRef]
- SLU. Fältinstruktion för Fjärilar, Humlor, Grova Träd Och Lavar i Ängs-Och Betesmarker. Institutionen för Skoglig Resurshållning, Sveriges Lantbruksuniversitet. 2011. (In Swedish). Available online: https://www.slu.se/globalassets/ew/org/centrb/nils/publikationer/2011/fjaril_2011.pdf (accessed on 22 March 2017).
- Wolf, S.; Rohde MMoritz, R.F. The reliability of morphological traits in the differentiation of Bombus terrestris and B. lucorum (Hymenoptera: Apidae). Apidologie 2010, 41, 45–53. [Google Scholar] [CrossRef]
- Jordbruksverket. Ängs-Och Betesmarksinventeringen 2002–2004 [Result of the Survey of Semi-Natural Pastures and Meadows]. Jordbruksverket, Rapport 1, 44 p. (in Swedish with English Summary). 2005. Available online: http://www2.jordbruksverket.se/webdav/files/SJV/trycksaker/Pdf_rapportler/ra05_1.pdf (accessed on 15 March 2024).
- Bergman, K.-O.; Jansson, N.; Claesson, K.; Palmer, M.W.; Milberg, P. How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles. For. Ecol. Manag. 2012, 265, 133–141. [Google Scholar] [CrossRef]
- Pekkarinen, A. Morphometric, colour and enzyme variation in bumblebees (Hymenoptera, Apidae, Bombus) In Fennoscandia and Denmark. Acta Zool. Fenn. 1979, 158, 1–60. [Google Scholar]
- Persson, A.S.; Rundlöf, M.; Clough, Y.; Smith, H.G. Bumble bees show trait-dependent vulnerability to landscape simplification. Biodivers. Conserv. 2015, 24, 3469–3489. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 March 2024).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 15 March 2024).
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Kruess, A.; Tcharntke, T. Grazing intensity and the diversity of grasshoppers, butterflies, and trap-nesting bees and wasps. Conserv. Biol. 2002, 16, 1570–1580. [Google Scholar] [CrossRef]
- Kleijn, D.; Van Langevelde, F. Interacting effects of landscape context and habitat quality on flower visiting insects in agricultural landscapes. Basic Appl. Ecol. 2006, 7, 201–214. [Google Scholar] [CrossRef]
- Sjödin, N.E. Pollinator behavioural response to grazing intensity. Biodivers. Conserv. 2006, 16, 2103–2121. [Google Scholar] [CrossRef]
- Diaz-Forero, I.; Kuusemets, V.; Mänd, M.; Liivamägi, A.; Kaart, T.; Luig, J. Influence of local and landscape factors on bumblebees in semi-natural meadows: A multiple-scale study in a forested landscape. J. Insect Conserv. 2012, 17, 113–125. [Google Scholar] [CrossRef]
- Le Féon, V.; Schermann-Legionnet, A.; Delettre, Y.; Aviron, S.; Billeter, R.; Bugter, R.; Hendrickx, F.; Burel, F. Intensification of agriculture, landscape composition and wild bee communities: A large scale study in four European countries. Agric. Ecosyst. Environ. 2010, 137, 143–150. [Google Scholar] [CrossRef]
- Milberg, P.; Bergman, K.-O.; Cronvall, E.; Eriksson, Å.I.; Glimskär, A.; Islamovic, A.; Jonason, D.; Löfqvist, Z.; Westerberg, L. Flower abundance and vegetation height as predictors for nectar-feeding insect occurrence in Swedish semi-natural grasslands. Agric. Ecosyst. Environ. 2016, 230, 47–54. [Google Scholar] [CrossRef]
- Öckinger, E.; Lindborg, R.; Sjödin, N.E.; Bommarco, R. Landscape matrix modifies richness of plants and insects in grassland fragments. Ecography 2012, 35, 259–267. [Google Scholar] [CrossRef]
- Toivanen, M.; Peltonen, A.; Herzon, I.; Heliölä, J.; Leikola, N.; Kuussaari, M. High cover of forest increases the abundance of most grassland butterflies in boreal farmland. Insect Conserv. Divers. 2017, 10, 321–330. [Google Scholar] [CrossRef]
- Goulson, D. Bumblebees: Behaviour, Biology and Conservation, 2nd ed.; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Milberg, P.; Bergman, K.-O.; Björklund, L.; Westerberg, L. The potential of weeds in arable fields to support pollinator assemblages. Weed Res. 2024; in press. [Google Scholar] [CrossRef]
- Persson, A.S.; Smith, H.G. Seasonal persistence of bumblebee populations is affected by landscape context. Agric. Ecosyst. Environ. 2013, 165, 201–209. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dainese, M.; González-Varo, J.P.; Mudri-Stojnić, S.; Riedinger, V.; Rundlöf, M.; Scheper, J.; Wickens, J.B.; Wickens, V.J.; Bommarco, R.; et al. Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 2016, 19, 1228–1236. [Google Scholar] [CrossRef]
- Jordbruksverket. Publikation JO10 SM 1001: Jordbruksmarkens Användning 2009—Slutlig Statistik. 2010. (In Swedish). Available online: https://jordbruksverket.se/om-jordbruksverket/jordbruksverkets-officiella-statistik/jordbruksverkets-statistikrapporter/statistik/2020-09-11-jordbruksmarkens-anvandning-2010.--preliminar-statistik (accessed on 6 December 2024).
- Jordbruksverket. Publikation JO 10 SM1201: Jordbruksmarkens Användning 2011—Slutlig Statistik. 2012. (In Swedish). Available online: https://jordbruksverket.se/om-jordbruksverket/jordbruksverkets-officiella-statistik/jordbruksverkets-statistikrapporter/statistik/2020-09-11-jordbruksmarkens-anvandning-2011.--slutlig-statistik (accessed on 6 December 2024).
- Rundlöf, M.; Persson, A.S.; Smith, H.G.; Bommarco, R. Late-season mass-flowering red clover increases bumble bee queen and male densities. Biol. Conserv. 2014, 172, 138–145. [Google Scholar] [CrossRef]
- Kallioniemi, E.; Åström, J.; Rusch, G.M.; Dahle, S.; Åström, S.; Gjershaug, J.O. Local resources, linear elements and mass-flowering crops determine bumblebee occurrences in moderately intensified farmlands. Agric. Ecosyst. Environ. 2017, 239, 90–100. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. OJ L 2013, 139, 12–26. Available online: http://eur-lex.europa.eu/eli/reg_impl/2013/485/oj (accessed on 2 June 2017).
- Holzschuh, A.; Dormann, C.F.; Tscharntke, T.; Steffan-Dewenter, I. Expansion of mass-flowering crops leads to transient pollinator dilution and reduced wild plant pollination. Proc. R. Soc. B-Biol. Sci. 2011, 278, 3444–3451. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.F.; Phillips, B.B.; Doyle, T.; Pell, J.P.; Redhead, J.W.; Savage, J.; Woodcock, B.A.; Bullock, J.M.; Osborne, J.L. Mass-flowering crops have a greater impact than semi-natural habitat on crop pollinators and pollen deposition. Landsc. Ecol. 2020, 35, 513–527. [Google Scholar] [CrossRef]
- Sadovy de Mitcheson, Y.J.; Linardich, C.; Barreiros, J.P.; Ralph, G.M.; Aguilar-Perera, A.; Afonso, P.; Erisman, B.E.; Pollard, D.A.; Fennessy, S.T.; Bertoncini, A.A.; et al. Valuable but vulnerable: Over-fishing and under-management continue to threaten groupers so what now? Mar. Policy 2020, 116, 103909. [Google Scholar] [CrossRef]
- Cooke, R.; Mancini, F.; Boyd, R.J.; Evans, K.L.; Shaw, A.; Webb, Y.J.; Isaac, N.J.B. Protected areas support more species than unprotected areas in Great Britain, but lose them equally rapidly. Biol. Conserv. 2023, 278, 109884. [Google Scholar] [CrossRef]
- Musa, N.; Andersson, K.; Burman, J.; Andersson, F.; Hedenström, E.; Jansson, N.; Paltto, H.; Westerberg, L.; Winde, I.; Larsson, M.C.; et al. Using sex pheromone and a multi-scale approach to predict the distribution of a rare saproxylic beetle. PLoS ONE 2013, 8, e66149. [Google Scholar] [CrossRef]
- Riva, F.; Haddadd, N.; Fahrig, L.; Banks-Leite, C. Principles for area-based biodiversity conservation. Ecol. Lett. 2024, 27, e14459. [Google Scholar] [CrossRef]
- Tscharntke, T.; Grass, I.; Wanger, T.C.; Westphal, C.; Batáry, P. Beyond organic farming–harnessing biodiversity-friendly landscapes. Trends Ecol. Evol. 2021, 36, 919–930. [Google Scholar] [CrossRef]
- Stein-Bachinger, K.; Gottwald, F.; Haub, A.; Schmidt, E. To what extent does organic farming promote species richness and abundance in temperate climates? A review. Org. Agric. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Hopfenmüller, S.; Steffan-Dewenter, I.; Holzschuh, A. Trait-specific responses of wild bee communities to landscape composition, configuration and local factors. PLoS ONE 2014, 9, e104439. [Google Scholar] [CrossRef] [PubMed]
- McHugh, N.M.; Bown, B.; McVeigh, A.; Powell, R.; Swan, E.; Szczur, J.; Wilson, P.; Holland, J. The value of two agri-environment scheme habitats for pollinators: Annually cultivated margins for arable plants and floristically enhanced grass margins. Agric. Ecosyst. Environ. 2022, 326, 107773. [Google Scholar] [CrossRef]
- Blumgart, D.; Botham, M.S.; Menéndez, R.; Bell, J.R. Floral enhancement of arable field margins increases moth abundance and diversity. J. Insect Conserv. 2023, 27, 455–465. [Google Scholar] [CrossRef] [PubMed]

| Bombus Species | Number of Specimens | Tongue Length (Proboscis < 8 mm: Short) 1 | Habitat Preference 2 | Colony Size 3 | Queen Emergence 3 |
|---|---|---|---|---|---|
| Non-parasitic spp. | |||||
| B. distinguendus | 2 | Long | Open | Nd | Nd |
| B. hortorum | 116 | Long | Forest | Medium | Late |
| B. humilis | 60 | Long | Open | Nd | Nd |
| B. hypnorum | 165 | Short | Open | Medium | Early |
| B. jonellus | 65 | Nd | Forest | Small | Late |
| B. lapidarius | 252 | Short | Open | Large | Early |
| B. lucorum & B. terrestris | 1521 | Short | * | Large | Early |
| B. lucorum/terrestris/sporadicus/soroeensis | 28 | Short | * | * | * |
| B. muscorum | 5 | Short | Open | Small | Late |
| B. pascuorum | 672 | Short | Open | Medium | Early |
| B. pratorum | 298 | Short | Forest | Small | Early |
| B. ruderarius | 65 | Nd | Open | Small | Late |
| B. soroeensis | 69 | Short | Open | Medium | Late |
| B. sporadicus | 2 | Short | Forest | Nd | Nd |
| B. subterraneus | 13 | Long | Open | Medium | Late |
| B. sylvarum | 224 | Short | Open | Small | Late |
| Parasitic spp. | |||||
| B. barbutellus | 7 | Nd | Forest | Nd | Nd |
| B. bohemicus | 45 | Nd | Forest | Nd | Nd |
| B. campestris | 18 | Nd | Forest | Nd | Nd |
| B. norvegicus | 2 | Nd | Forest | Nd | Nd |
| B. quadricolor | 4 | Nd | Forest | Nd | Nd |
| B. rupestris | 24 | Nd | Open | Nd | Nd |
| B. sylvestris | 21 | Nd | Forest | Nd | Nd |
| B. vestalis | 1 | Nd | Open | Nd | Nd |
| Sum of Specimen | 3679 | Long: 191 Short: 3236 | Open: 1552 Forest: 578 | Small: 657 Medium: 1035 Large: 1773 | Early: 2908 Late: 557 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milberg, P.; Bergman, K.-O.; Fjellander, G.; Tälle, M.; Westerberg, L. Bumblebee Abundance in Species-Rich Grasslands in Southern Sweden Decreases with Increasing Amount of Arable Land at a Landscape Level. Insects 2024, 15, 982. https://doi.org/10.3390/insects15120982
Milberg P, Bergman K-O, Fjellander G, Tälle M, Westerberg L. Bumblebee Abundance in Species-Rich Grasslands in Southern Sweden Decreases with Increasing Amount of Arable Land at a Landscape Level. Insects. 2024; 15(12):982. https://doi.org/10.3390/insects15120982
Chicago/Turabian StyleMilberg, Per, Karl-Olof Bergman, Gabriella Fjellander, Malin Tälle, and Lars Westerberg. 2024. "Bumblebee Abundance in Species-Rich Grasslands in Southern Sweden Decreases with Increasing Amount of Arable Land at a Landscape Level" Insects 15, no. 12: 982. https://doi.org/10.3390/insects15120982
APA StyleMilberg, P., Bergman, K.-O., Fjellander, G., Tälle, M., & Westerberg, L. (2024). Bumblebee Abundance in Species-Rich Grasslands in Southern Sweden Decreases with Increasing Amount of Arable Land at a Landscape Level. Insects, 15(12), 982. https://doi.org/10.3390/insects15120982






