Factors Influencing Copulation Duration in Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Stock Culture
2.2. Mating Condition
2.3. Trial 1: Copulation Duration Observation
2.4. Trial 2: Effect of Copulation Duration on Reproductive Outputs
2.5. Trial 3: Factors That Affect the Copulation Duration
2.5.1. Effect of Body Size
2.5.2. Effect of Mating History
2.5.3. Effect of Kinship
2.5.4. Effect of Sex Ratio
2.5.5. Effect of Mating Sequence
2.5.6. Effect of Feeding Status
2.5.7. Effect of Ambient Temperature
2.5.8. Effects of Photoperiod and Time of Day
2.6. Data Analysis
3. Results
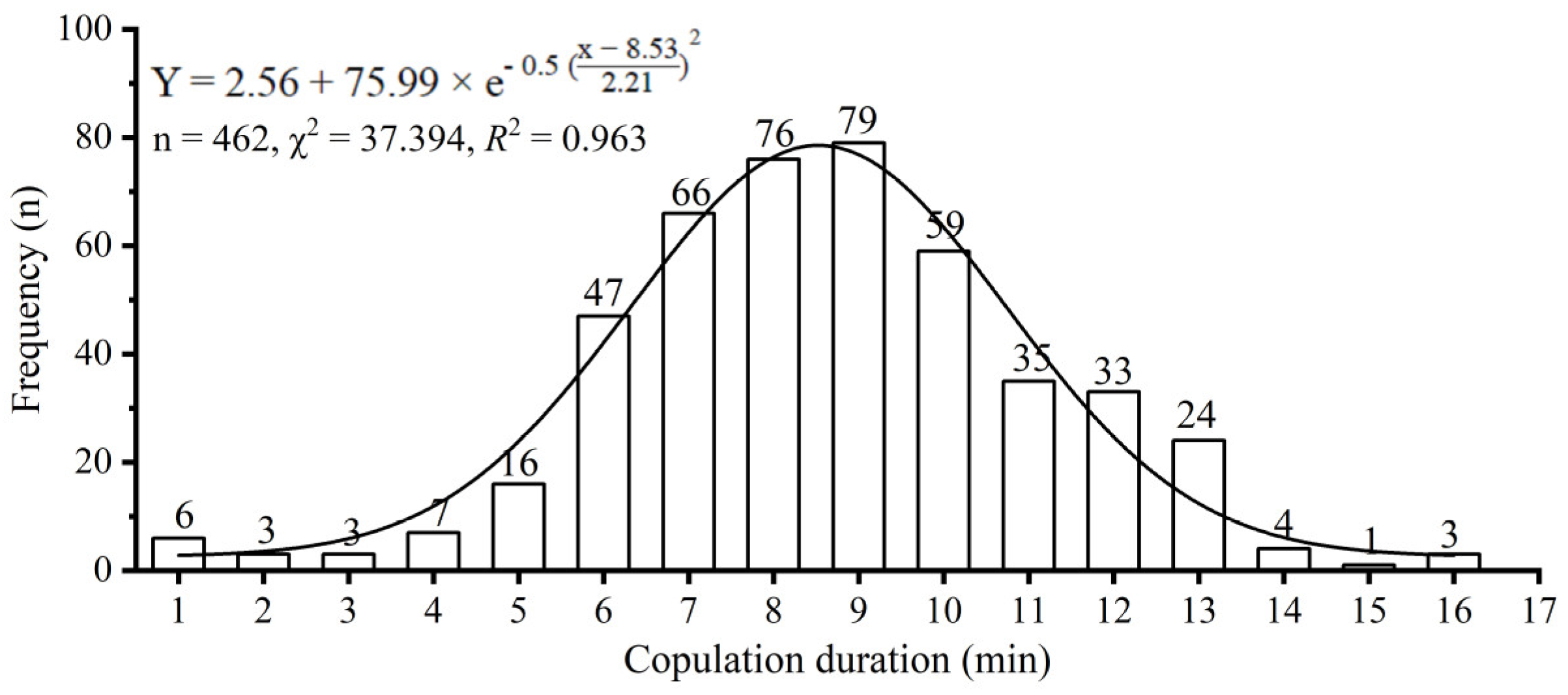
3.1. Copulation Duration and Its Effect on Reproductive Outputs
3.2. Influence Factors Influencing the Copulation Duration
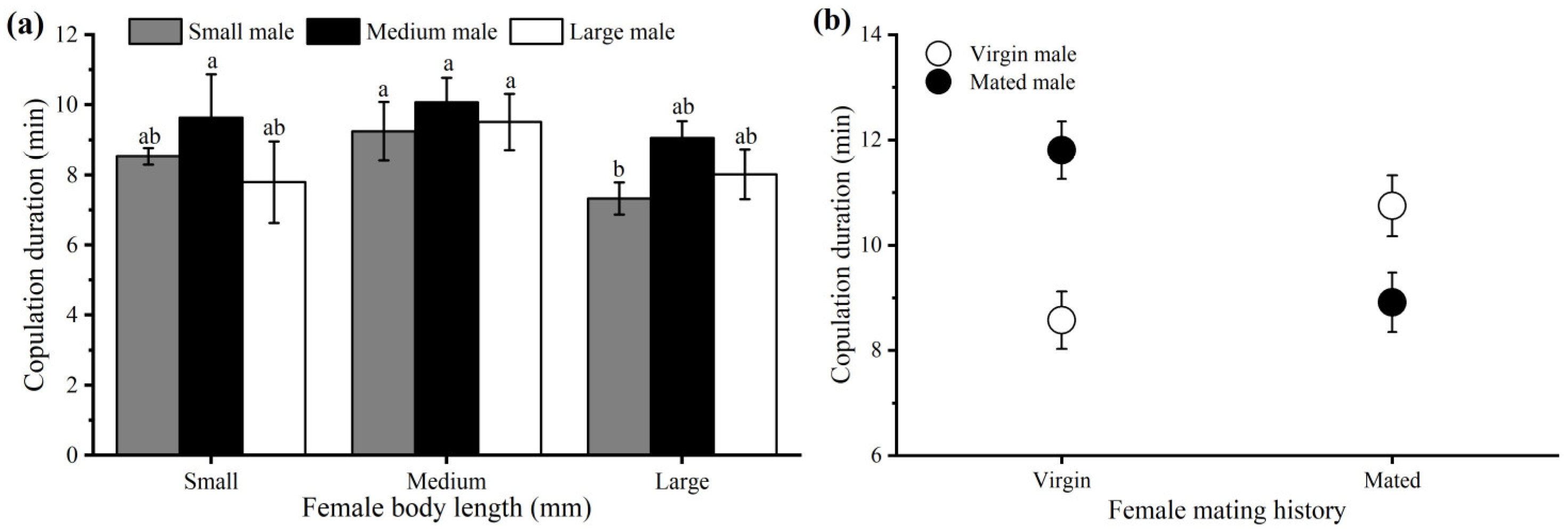
3.2.1. Body Size
3.2.2. Mating History
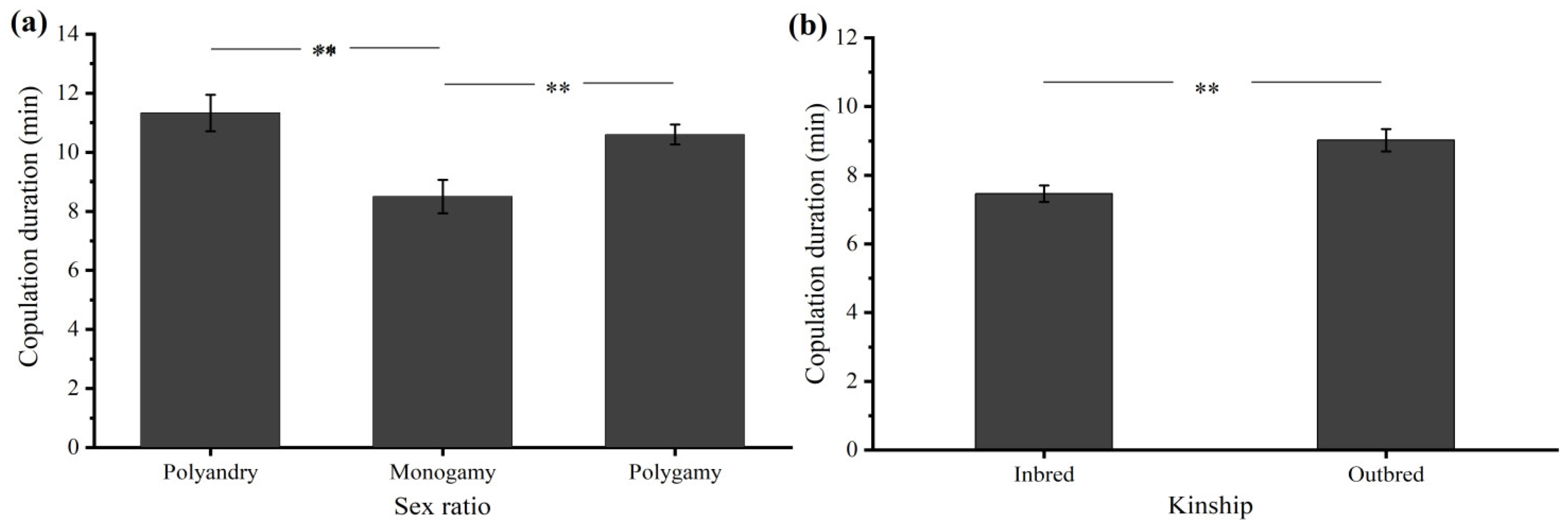
3.2.3. Sex Ratio
3.2.4. Kinship
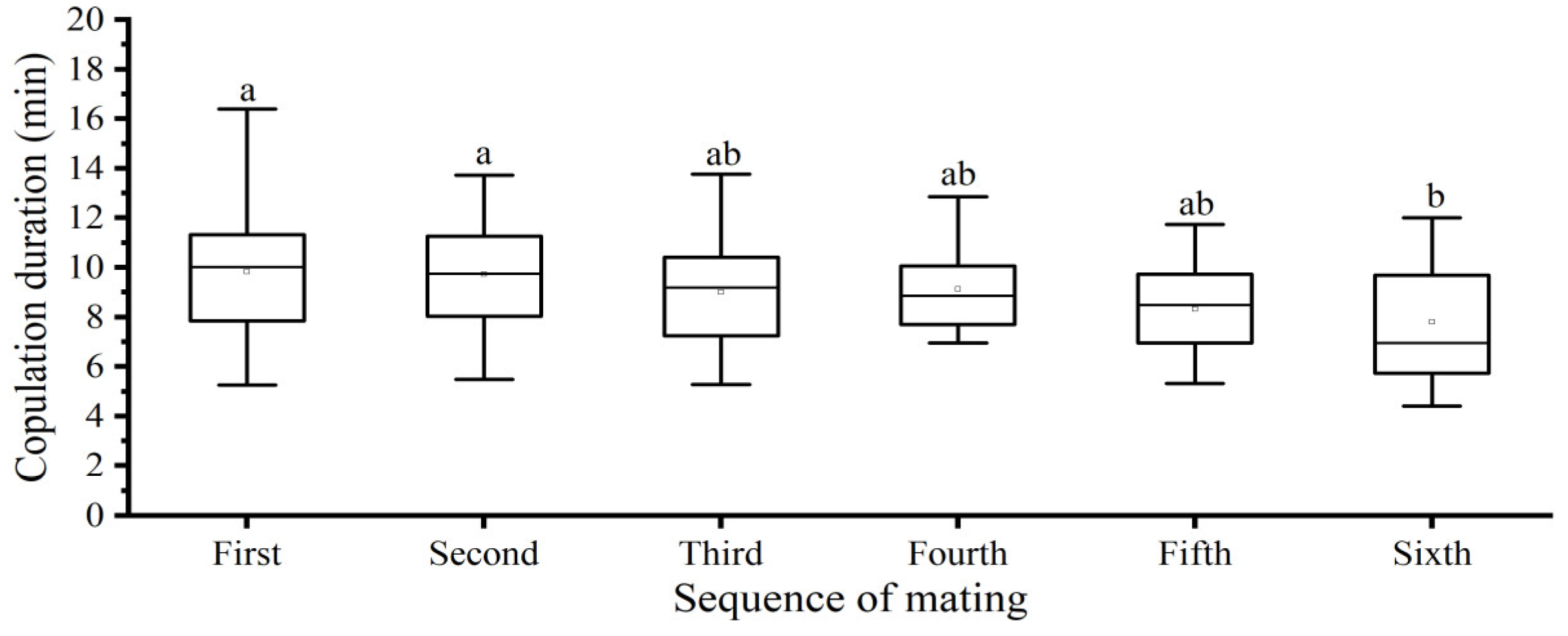
3.2.5. Mating Sequence
3.2.6. Feeding Status
3.2.7. Ambient Temperature
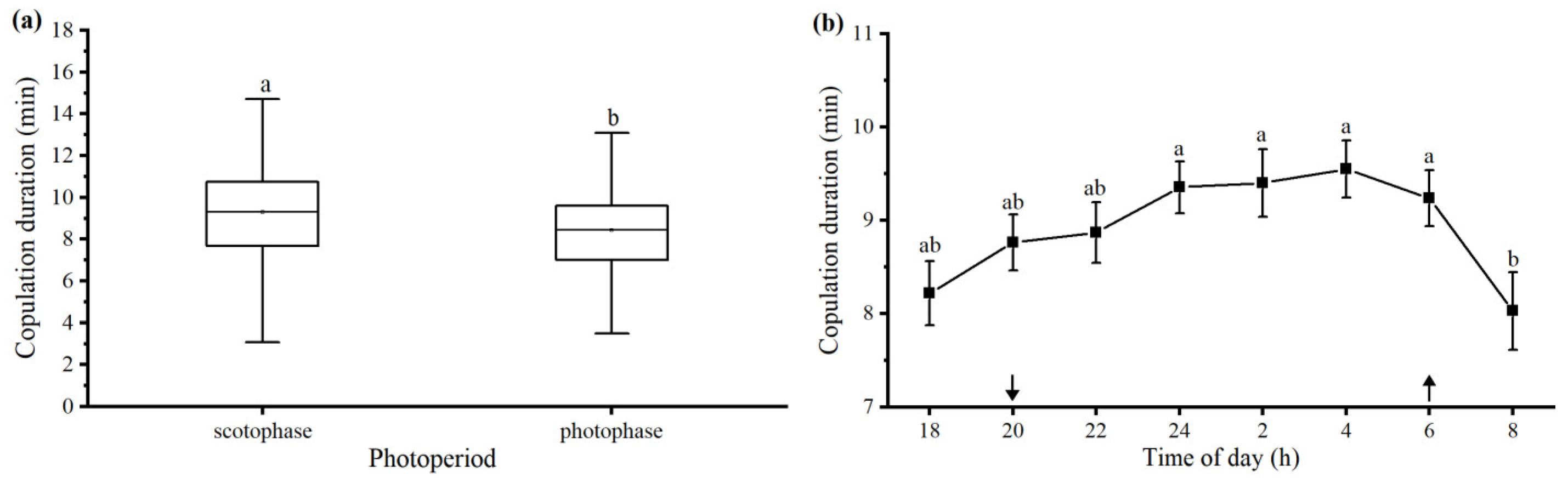
3.2.8. Photoperiod and Time of Day
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markow, T.A. Perspective: Female remating, operational sex ratio, and the arena of sexual selection in Drosophila species. Evolution 2002, 56, 1725–1734. [Google Scholar]
- Pavković-Lučić, S.; Lučić, L.; Miličić, D.; Tomić, V.; Savić, T. Mating success and copulation duration in Drosophila melanogaster flies having different mating experience: A brief experimental note. J. Biosci. Biotechnol. 2014, 153–159. Available online: https://biore.bio.bg.ac.rs/handle/123456789/643 (accessed on 19 January 2024).
- Mazzi, D.; Kesäniemi, J.; Hoikkala, A.; Klappert, K. Sexual conflict over the duration of copulation in Drosophila montana: Why is longer better? BMC Evol. Biol. 2009, 9, 132. [Google Scholar] [CrossRef]
- Andrés, J.A.; Rivera, A.C. Copulation duration and fertilization success in a damselfly: An example of cryptic female choice? Anim. Behav. 2000, 59, 695–703. [Google Scholar] [CrossRef]
- Himuro, C.; Fujisaki, K. Effects of mating duration on female reproductive traits of the seed bug Togo hemipterus (Heteroptera: Lygaeidae). Appl. Entomol. Zool. 2015, 50, 491–496. [Google Scholar] [CrossRef]
- Gupta, K.K.; Shazad, M.; Kumar, S. Relevance of prolonged first mating in reproductive bioactivities of Dysdercus koenigii (Fabricius, 1775) (Heteroptera: Pyrrhocoridae). Pol. J. Entomol. 2019, 88, 63–77. [Google Scholar] [CrossRef]
- De Lima, C.H.M.; Nόbrega, R.L.; Ferraz, M.L.; Pontes, W.J.T. Mating duration and spermatophore transfer in Cryptolaemus montrouzieri (Coccinellidae). Biologia 2022, 77, 149–155. [Google Scholar] [CrossRef]
- Östrand, F.; Anderbrant, O. Mating duration and frequency in a pine sawfly. J. Insect Behav. 2001, 14, 595–606. [Google Scholar] [CrossRef]
- Lü, J.L.; Zhang, B.H.; Jiang, X.H.; Wang, E.D. Quantitative impact of mating duration on reproduction and offspring sex ratio of Phytoseiulus persimilis (Acari: Phytoseiidae). J. Integr. Agric. 2019, 18, 884–892. [Google Scholar] [CrossRef]
- Abdel-Azim, M.M.; Aldosari, S.A.; Shukla, P. Factors influencing mating behavior and success in the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Neotrop. Entomol. 2019, 48, 25–37. [Google Scholar] [CrossRef]
- Wong-Muñoz, J.; Anderson, C.N.; Munguía-Steyer, R.; Córdoba-Aguilar, A. Body size and morph as drivers of copulation duration in a male dimorphic damselfly. Ethology 2013, 119, 407–416. [Google Scholar] [CrossRef]
- Lüpold, S.; Manier, M.K.; Ala-Honkola, O.; Belote, J.M.; Pitnick, S. Male Drosophila melanogaster adjust ejaculate size based on female mating status, fecundity, and age. Behav. Ecol. 2011, 22, 184–191. [Google Scholar] [CrossRef]
- De Morais, R.M.; Redaelli, L.R.; Sant’Ana, J. Age and multiple mating effects on reproductive success of Grapholita molesta (Busck) (Lepidoptera, Tortricidae). Rev. Bras. Entomol. 2012, 56, 319–324. [Google Scholar] [CrossRef]
- Lai, M.; Zhang, S.Y.; Zhang, Y.F.; Liu, X.P. Male age affects female mating preference but not fitness in the monandrous moth Dendrolimus punctatus Walker (Lepidoptera: Lasiocampidae). Physiol. Entomol. 2020, 45, 22–29. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Liu, Z.; Lin, Y.Q.; Liu, S.Z. Effects of delayed mating on mating performance and reproductive fitness of the willow leaf beetle (Coleoptera: Chrysomelidae) under laboratory conditions. Insects 2021, 12, 481. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Tu, X.Y.; He, H.M.; Chen, C.; Xue, F.S. Evidence for inbreeding depression and pre-copulatory, but not post copulatory inbreeding avoidance in the cabbage beetle Colaphellus bowringi. PLoS ONE 2014, 9, e94389. [Google Scholar] [CrossRef] [PubMed]
- Bouchebti, S.; Durier, V.; Pasquaretta, C.; Rivault, C.; Lihoreau, M. Subsocial cockroaches Nauphoeta cinerea mate indiscriminately with kin despite high costs of inbreeding. PLoS ONE 2016, 11, e0162548. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.P.; Amano, H. Temperature at immature and adult stages differentially affects mating duration and egg production of Neoseiulus californicus females mated once (Acari: Phytoseiidae). J. Asia-Pac. Entomol. 2010, 13, 65–68. [Google Scholar] [CrossRef]
- Cordero, A. The adaptive significance of the prolonged copulations of the damselfly, Ischnura graellsii (Odonata: Coenagrionidae). Anim. Behav. 1990, 40, 43–48. [Google Scholar] [CrossRef]
- Droney, D.C.; Thaker, M. Factors influencing mating duration and male choice in the red milkweed beetle, Tetraopes tetrophthalmus (Forster) (Coleoptera Cerambycidae). Ethol. Ecol. Evol. 2006, 18, 173–183. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, G.L. Mating frequency, duration, and circadian mating rhythm of New Zealand wheat bug Nysius huttoni White (Heteroptera: Lygaeidae). N. Z. Entomol. 2004, 27, 113–117. [Google Scholar] [CrossRef]
- Molleman, F.; Zwaan, B.J.; Brakefield, P.M. The effect of male sodium diet and mating history on female reproduction in the puddling squinting bush brown Bicyclus anynana (Lepidoptera). Behav. Ecol. Sociobiol. 2004, 56, 404–411. [Google Scholar] [CrossRef]
- Pröhl, H. Population differences in female resource abundance, adult sex ratio, and male mating success in Dendrobates pumilio. Behav. Ecol. 2002, 13, 175–181. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, G.; Omkar. Are the effects of hunger stage-specific? A case study in an aphidophagous ladybird beetle. Bull. Entomol. Res. 2021, 111, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, R.M.; Seiedy, M. Effect of starvation on the mating behavior of an aphidophagous ladybird beetle, Hippodamia variegata Goeze (Coleoptera: Coccinellidae) in laboratory conditions. J. Entomol. Soc. Iran 2022, 42, 81–85. [Google Scholar]
- Arya, H.; Toltesi, R.; Eng, M.; Garg, D.; Merritt, T.J.S.; Rajpurohit, S. No water, no mating: Connecting dots from behaviour to pathways. PLoS ONE 2021, 16, e0252920. [Google Scholar] [CrossRef]
- Chaudhary, D.D.; Mishra, G.; Omkar. Strategic mate-guarding behaviour in ladybirds. Ethology 2017, 123, 376–385. [Google Scholar] [CrossRef]
- Wei, J.R.; Yang, Z.Q.; Poland, T.M.; Du, J.W. Parasitism and olfactory responses of Dastarcus helophoroides (Coleoptera: Bothrideridae) to different Cerambycid hosts. BioControl 2009, 54, 733–742. [Google Scholar] [CrossRef]
- Ren, L.L.; Balakrishnan, K.; Luo, Y.Q.; Schütz, S. EAG response and behavioral orientation of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae) to synthetic host-associated volatiles. PLoS ONE 2017, 12, e0190067. [Google Scholar] [CrossRef]
- Urano, T. Effects of host stage and number of feeding larvae on parasitism success and fitness in the coleopteran parasitoid, Dastarcus longulus Sharp (Coleoptera: Bothrideridae). Appl. Entomol. Zool. 2010, 45, 215–223. [Google Scholar] [CrossRef]
- Lim, J.; Oh, H.; Park, S.; Koh, S.; Lee, S. First record of the family Bothrideridae (Coleoptera) in Korea represented by the wood-boring beetle ectoparasite, Dastarcus helophoroides. J. Asia-Pac. Entomol. 2012, 15, 273–275. [Google Scholar] [CrossRef]
- Wei, J.R.; Yang, Z.Q.; Ma, J.H.; Tang, H. Progress on the research of Dastarcus helophoroides. For. Pest Dis. 2007, 26, 23–25, (In Chinese with English summary). [Google Scholar]
- Wang, Q.Z.; Guo, Z.; Zhang, J.T.; Chen, Y.S.; Zhou, J.Y.; Pan, Y.L.; Liu, X.P. Phototactic behavioral response of the ectoparasitoid beetle Dastarcus helophoroides (Coleoptera: Bothrideridae): Evidence for attraction by near-infrared light. J. Econ. Entomol. 2021, 114, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; Ren, Z.; Hai, X.X.; Zhang, L.; Wang, Z.G.; Lyu, F. Exposure to artificial light at night mediates the locomotion activity and oviposition capacity of Dastarcus helophoroides (Fairmaire). Front. Physiol. 2023, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N. Recent advances in biological control of important native and invasive forest pests in China. Biol. Control 2014, 68, 117–128. [Google Scholar] [CrossRef]
- Rim, K.; Golec, J.R.; Duan, J.J. Host selection and potential non-target risk of Dastarcus helophoroides, a larval parasitoid of the Asian longhorned beetle, Anoplophora glabripennis. Biol. Control 2018, 123, 120–126. [Google Scholar] [CrossRef]
- Li, X.J.; Dong, G.P.; Yang, L.; Guo, W.L. Fecundity and egg hatchability of Dastarcus helophoroides adults fed on different types of artificial diets. J. For. Res. 2015, 26, 219–224. [Google Scholar] [CrossRef]
- Shi, H.N.; Zhou, J.Y.; Chen, Y.S.; Wang, Q.Z.; Pan, Y.L.; Zhang, J.T.; Liu, X.P. A comparison of fitness-related traits in the Coleopteran parasitoid Dastarcus helophoroides (Coleoptera: Bothrideridae) reared on two factitious hosts. J. Econ. Entomol. 2020, 113, 2634–2640. [Google Scholar] [CrossRef]
- Tang, H.; Yang, Z.Q.; Zhang, Y.N.; Li, G.W. Technical researches on distinguishing female and male alive adults of the main parasite of longhorn beetles Dastarcus helophoroides (Coleoptera: Bethrideriidae) without injuring. Acta Zootax. Sin. 2007, 32, 649–654, (In Chinese with English summary). [Google Scholar]
- Qiu, L.F.; Zhong, L.; Shao, J.L.; Che, S.C.; Li, G.; Wang, J.H.; Wei, J.R. Influence of environmental temperature and adult body size on the mortality and fecundity of Dastarcus helophoroides. Chin. J. Appl. Entomol. 2021, 58, 959–965, (In Chinese with English summary). [Google Scholar]
- Sivinski, J. Intrasexual aggression in the stick insects Diapheromera veliei and D. covilleae and sexual dimorphism in the Phasmatodea. Psyche J. Entomol. 1978, 85, 395–405. [Google Scholar] [CrossRef]
- Aluja, M.; Rull, J.; Sivinski, J.; Trujillo, G.; Perez-Staples, D. Male and female condition influence mating performance and sexual receptivity in two tropical fruit flies (Diptera: Tephritidae) with contrasting life histories. J. Insect Physiol. 2009, 55, 1091–1098. [Google Scholar] [CrossRef]
- Shuker, D.M.; Simmons, L.W. (Eds.) The Evolution of Insect Mating Systems (No. 27); Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Edvardsson, M.; Canal, D. The effects of copulation duration in the bruchid beetle Callosobruchus maculatus. Behav. Ecol. 2006, 17, 430–434. [Google Scholar] [CrossRef]
- Ortigosa, A.; Rowe, L. The role of mating history and male size in determining mating behaviours and sexual conflict in a water strider. Anim. Behav. 2003, 65, 851–858. [Google Scholar] [CrossRef]
- Katsuki, M.; Miyatake, T. Effects of temperature on mating duration, sperm transfer and remating frequency in Callosobruchus chinensis. J. Insect Physiol. 2009, 55, 112–115. [Google Scholar] [CrossRef]
- Lefranc, A.; Bungaard, J. The influence of male and female body size on copulation duration and fecundity in Drosophila melanogaster. Hereditas 2000, 132, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Harvey, D.J.; Gange, A.C. Size variation and mating success in the stag beetle, Lucanus cervus. Physiol. Entomol. 2006, 31, 218–226. [Google Scholar] [CrossRef]
- Kumano, N.; Haraguchi, D.; Kohama, T. Female mating status does not affect male mating behavior in the West Indian sweetpotato weevil, Euscepes postfasciatus. Entomol. Exp. Appl. 2009, 131, 39–45. [Google Scholar] [CrossRef]
- Walgenbach, C.A.; Burkholder, W.E. Mating behavior of the maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 1987, 80, 578–583. [Google Scholar] [CrossRef]
- ter Haar, S.; Francuski, L.; Billeter, J.C.; Schenkel, M.A.; Beukeboom, L.W. Adult sex ratios affect mating behaviour in the common housefly Musca domestica L. (Diptera; Muscidae). Behaviour 2023, 160, 1329–1357. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Hedderley, D. Function of prolonged copulation in Nysius huttoni White (Heteroptera: Lygaeidae) under male-biased sex ratio and high population density. J. Insect Behav. 2008, 21, 89–99. [Google Scholar] [CrossRef]
- Vitta, A.C.R.; Lorenzo, M.G. Copulation and mate guarding behavior in Triatoma brasiliensis (Hemiptera: Reduviidae). J. Med. Entomol. 2009, 46, 789–795. [Google Scholar] [CrossRef]
- Fedina, T.Y.; Lewis, S.M. Proximal traits and mechanisms for biasing paternity in the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae). Behav. Ecol. Sociobiol. 2006, 60, 844–853. [Google Scholar] [CrossRef]
- Papanastasiou, S.A.; Nakas, C.T.; Carey, J.R.; Papadopoulos, N.T. Condition-dependent effects of mating on longevity and fecundity of female medflies: The interplay between nutrition and age of mating. PLoS ONE 2013, 8, e70181. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J. The effects of operational sex ratio and food deprivation on copulation duration in the water strider (Gerris remiges Say). Behav. Ecol. Sociobiol. 1988, 23, 317–322. [Google Scholar] [CrossRef]
- Sillén-Tullberg, B. Prolonged copulation: A male ‘postcopulatory’strategy in a promiscuous species, Lygaeus equestris (Heteroptera: Lygaeidae). Behav. Ecol. Sociobiol. 1981, 9, 283–289. [Google Scholar] [CrossRef]
- Jiao, X.G.; Wu, J.; Chen, Z.Q.; Chen, J.; Liu, F.X. Effects of temperature on courtship and copulatory behaviours of a wolf spider Pardosa astrigera (Araneae: Lycosidae). J. Therm. Biol. 2009, 34, 348–352. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Jiao, X.G.; Wu, J.; Chen, J.; Liu, F.X. Effects of copulation temperature on female reproductive output and longevity in the wolf spider Pardosa astrigera (Araneae: Lycosidae). J. Therm. Biol. 2010, 35, 125–128. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.-H.; Li, C.-Q.; Zhang, J.-T.; Wei, L.-F.; Liu, X.-P. Factors Influencing Copulation Duration in Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). Insects 2024, 15, 104. https://doi.org/10.3390/insects15020104
Zhong H-H, Li C-Q, Zhang J-T, Wei L-F, Liu X-P. Factors Influencing Copulation Duration in Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). Insects. 2024; 15(2):104. https://doi.org/10.3390/insects15020104
Chicago/Turabian StyleZhong, Hui-Hui, Chao-Qun Li, Jiang-Tao Zhang, Li-Feng Wei, and Xing-Ping Liu. 2024. "Factors Influencing Copulation Duration in Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae)" Insects 15, no. 2: 104. https://doi.org/10.3390/insects15020104



