Blue Light Attracts More Spodoptera frugiperda Moths and Promotes Their Flight Speed
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. LED Light Sources
2.3. Phototactic Behavior Chamber
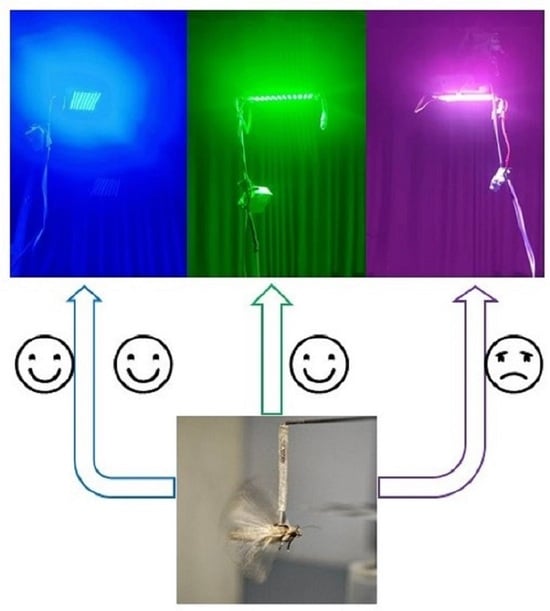
2.4. Phototactic Behavior Test
2.5. Flight Capability Determination
2.6. Statistical Analysis
3. Results
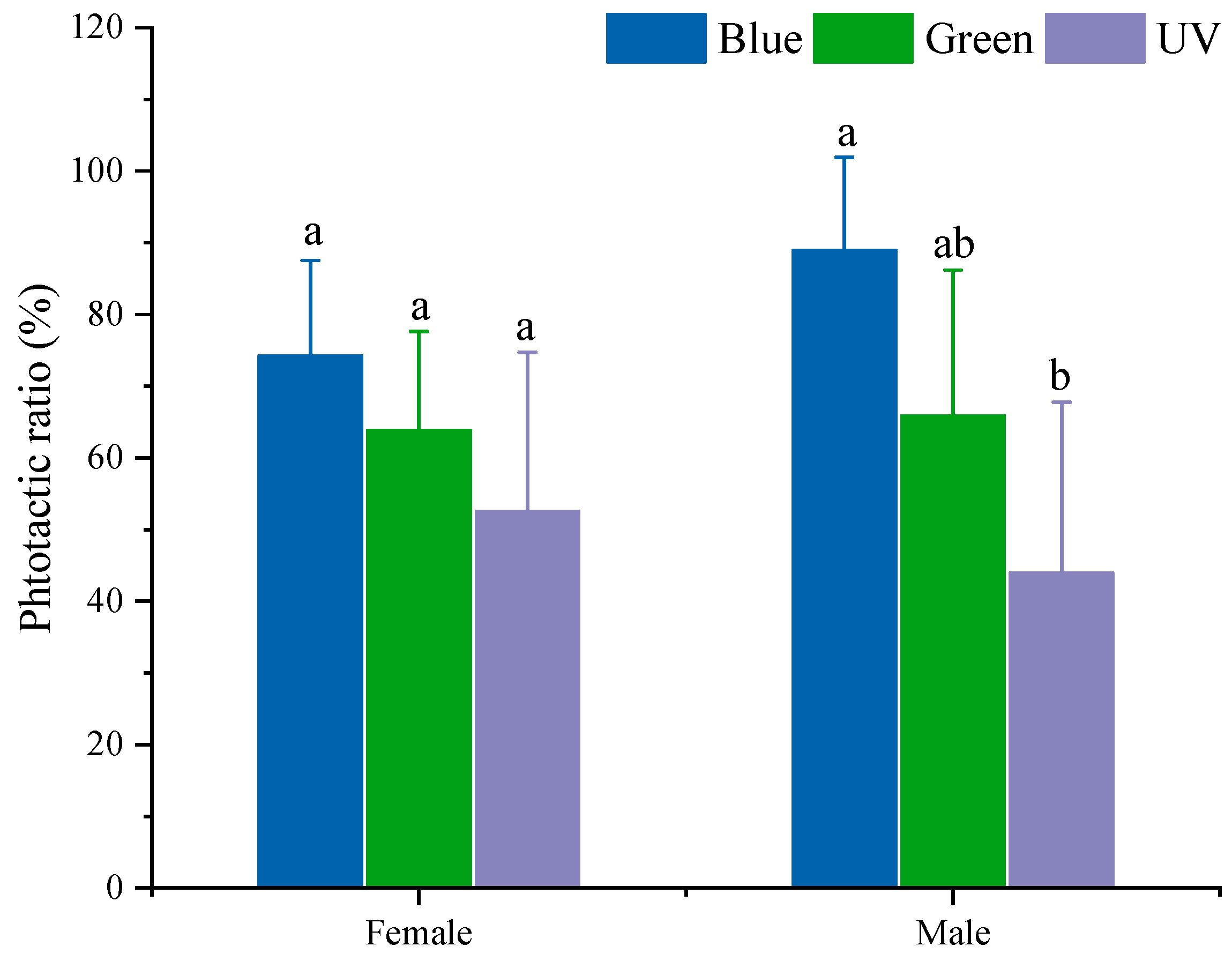
3.1. Phototactic Rates of S. frugiperda to Different Light Sources
3.2. Flight Performance of S. frugiperda in Different Light Conditions
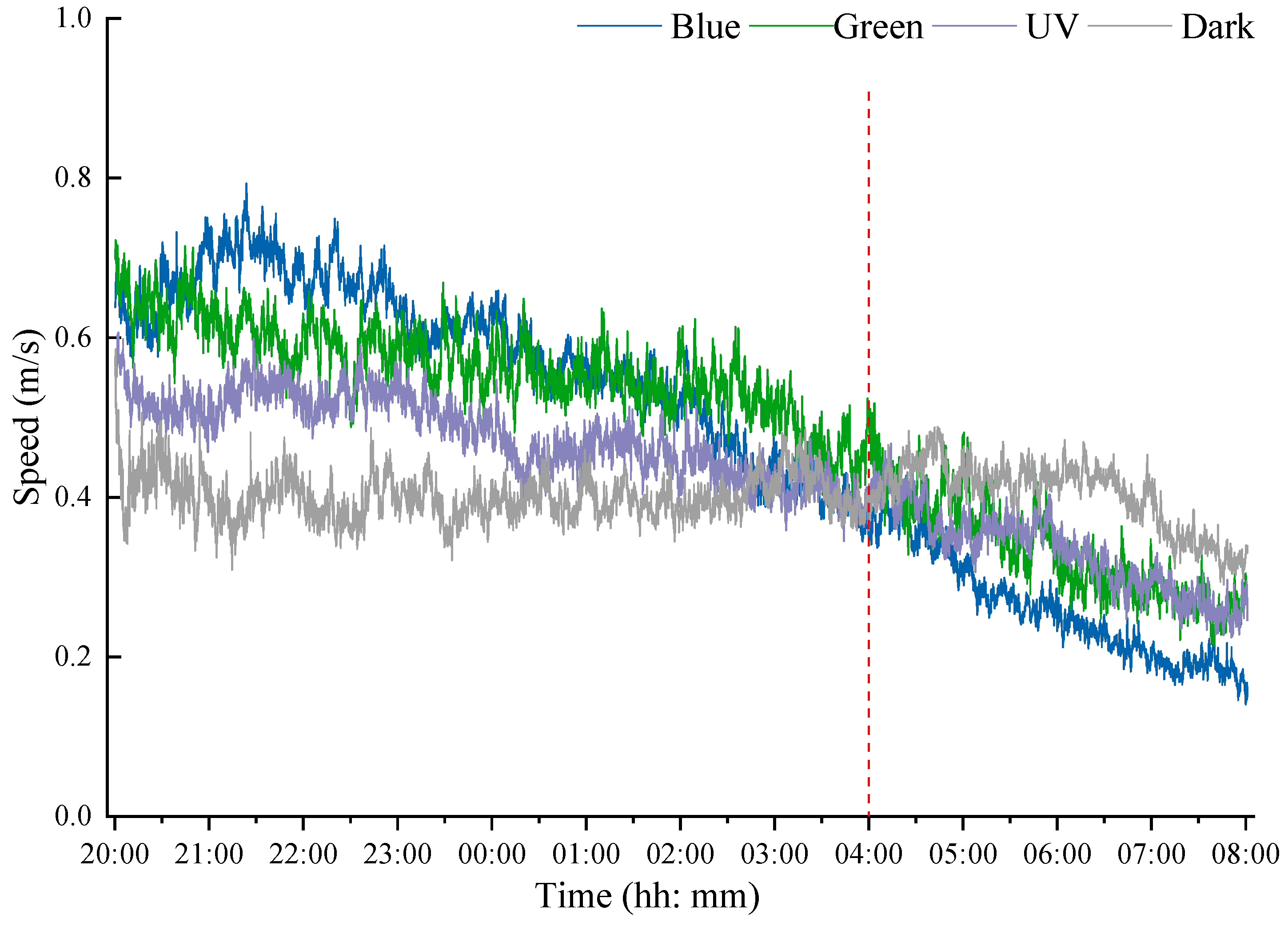
3.3. Flight Speed Dynamics in Different Light Conditions
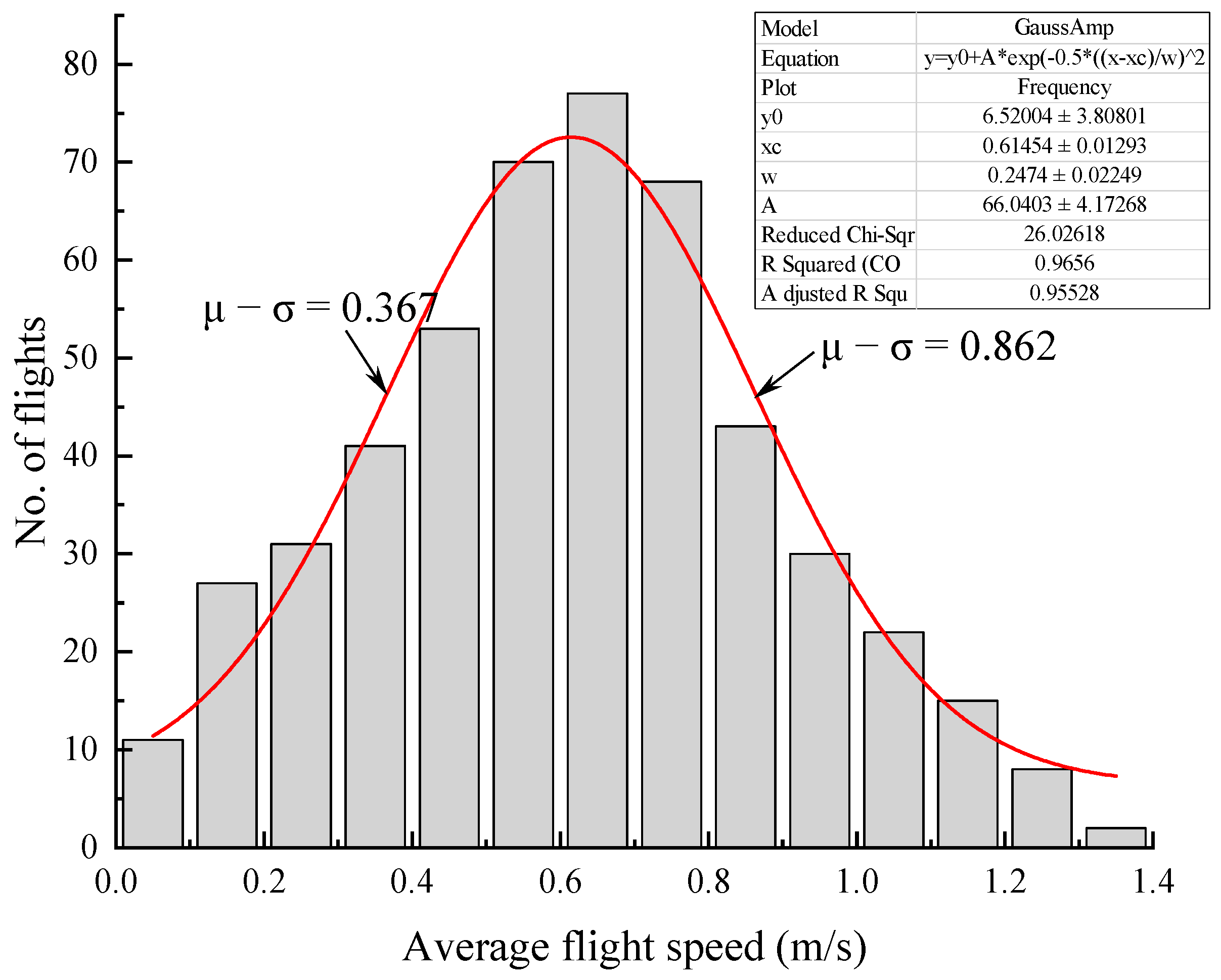
3.4. Flight Speed Types in Different Light Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82. [Google Scholar] [CrossRef]
- Togola, A.; Meseka, S.; Menkir, A.; Badu-Apraku, B.; Boukar, O.; Tamò, M.; Djouaka, R. Measurement of pesticide residues from chemical control of the invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a maize experimental field in Mokwa, Nigeria. Int. J. Environ. Res. Public Health 2018, 15, 849. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, M.; Ma, J.; Gao, B.; Wu, Q.; Chen, A.; Liu, J.; Jiang, Y.; Zhai, B.; Early, R.; et al. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2020, 76, 454–463. [Google Scholar] [CrossRef]
- Wan, J.; Huang, C.; Li, C.; Zhou, H.; Ren, Y.; Li, Z.; Xing, L.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Sun, X.; Hu, C.; Jia, H.; Wu, Q.; Shen, X.; Zhao, S.; Jiang, Y.; Wu, K. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Hasnain, A.; Cheng, Q.-H.; Xia, L.-J.; Cai, Y.-H.; Hu, R.; Gong, C.-W.; Liu, X.-M.; Pu, J.; Zhang, L.; et al. Resistance monitoring and mechanism in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) for chlorantraniliprole from Sichuan Province, China. Front. Physiol. 2023, 14, 1180655. [Google Scholar] [CrossRef]
- Kim, K.; Huang, Q.; Lei, C. Advances in insect phototaxis and application to pest management: A review. Pest Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef]
- Mitchell, E.R. Monitoring adult populations of the fall armyworm. Fla. Entomol. 1979, 62, 91. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Yang, J.J.; Zhao, W.X.; Yin, L.; Liu, Y.; Ye, S.F.; Qin, B.Q.; Song, L.D. Trapping effect of searchlight-trap and light trap for the moth of Spodoptera frugiperda in 2019. Plant Prot. 2020, 46, 118–122. [Google Scholar]
- Liu, Y.; Zhang, D.; Yang, L.; Dong, Y.; Liang, G.; Philip, D.; Ren, G.; Xu, P.; Wu, K. Analysis of phototactic responses in Spodoptera frugiperda using Helicoverpa Armigera as control. J. Integr. Agric. 2021, 20, 821–828. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Y.; Zhang, S.; Jiang, X.; Yang, B.; Wang, G. Comparison of phototactic behavior between two migratory pests, Helicoverpa armigera and Spodoptera frugiperda. Insects 2022, 13, 917. [Google Scholar] [CrossRef]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef]
- Van Der Kooi, C.J.; Stavenga, D.G.; Arikawa, K.; Belušič, G.; Kelber, A. Evolution of insect color vision: From spectral sensitivity to visual ecology. Annu. Rev. Entomol. 2021, 66, 435–461. [Google Scholar] [CrossRef]
- Nascimento, I.N.D.; Oliveira, G.M.D.; Souza, M.D.S.D.; Nunes, G.D.S.; Alves, A.C.L.; Araújo, H.M.D.; Batista, J.D.L. Light-emitting diodes (LED) as luminous lure for adult Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae). J. Exp. Agric. Int. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Liu, S.M.; Wang, Y.Q.; Tang, J.R.; Zhang, Y.J.; Fu, X.W.; Liang, G.M. Effects of different wavelengths of light on the phototactic behavior and opsin expression in Spodoptera frugiperda adults. Plant Prot. 2023, 49, 176–183. [Google Scholar]
- Zhang, Y.N.; Liu, Y.X.; Li, M.Y.; Yao, M.Y.; Wei, G.S. Effects of light source, sex and light processing time on phototaxis of fall armyworm Spodoptera frugiperda. J. Plant Prot. 2023, 50, 1010–1015. [Google Scholar]
- Cronin, T.W.; Järvilehto, M.; Weckström, M.; Lall, A.B. Tuning of photoreceptor spectral sensitivity in fireflies (Coleoptera: Lampyridae). J. Comp. Physiol. A 2000, 186, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Möller, R. Insects could exploit UV–green contrast for landmark navigation. J. Theor. Biol. 2002, 214, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef]
- Gaston, K.J.; Davies, T.W.; Bennie, J.; Hopkins, J. Review: Reducing the ecological consequences of night-time light pollution: Options and developments. J. Appl. Ecol. 2012, 49, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Altermatt, F.; Ebert, D. Reduced flight-to-light behaviour of moth populations exposed to long-term urban light pollution. Biol. Lett. 2016, 12, 20160111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Xu, H.X.; Hu, Y.; Han, H.L.; Qian, J.N.; Yin, S.; Lyu, Z.X. Growth development and reproduction of Spodoptera frugiperda reared on an artificial diet. Chin. J. Appl. Entomol. 2020, 57, 1341–1344. [Google Scholar]
- Ge, S.S.; He, L.M.; He, W.; Xu, R.B.; Sun, X.T.; Wu, K.M. Determination on moth flight capacity of Spodoptera frugiperda. Plant Prot. 2019, 45, 28–33. [Google Scholar]
- Kim, K.N.; Song, H.S.; Li, C.S.; Huang, Q.Y.; Lei, C.L. Effect of several factors on the phototactic response of the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2018, 21, 952–957. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Huang, L.; Xu, C.F.; Li, J.H.; Wang, F.Y.; Cheng, W.; Gao, B.Y.; Chapman, J.W.; Hu, G. Flight capability and the low temperature threshold of a Chinese field population of the fall armyworm Spodoptera frugiperda. Insects 2022, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.S. Phototactic behavioral response of agricultural insects and stored-product insects to light-emitting diodes (LEDs). Appl. Biol. Chem. 2017, 60, 137–144. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Chen, Y.; Wang, Y.; Chen, J.; Fu, S.; Ma, W.; Xia, S.; Liu, D.; Wu, T.; et al. CRISPR/Cas9-mediated knockout of LW-opsin reduces the efficiency of phototaxis in the diamondback moth Plutella xylostella. Pest Manag. Sci. 2021, 77, 3519–3528. [Google Scholar] [CrossRef]
- Frentiu, F.D.; Yuan, F.; Savage, W.K.; Bernard, G.D.; Mullen, S.P.; Briscoe, A.D. Opsin clines in butterflies suggest novel roles for insect photopigments. Mol. Biol. Evol. 2015, 32, 368–379. [Google Scholar] [CrossRef]
- Cheng, W.J.; Zheng, X.L.; Wang, P.; Lei, C.L.; Wang, X.P. Sexual difference of insect phototactic behavior and related affecting factors. Chin. J. Appl. Ecol. 2011, 22, 3351–3357. [Google Scholar]
- Qiu, K.R.; Li, J.G.; Liu, W.; Wang, X.P. Research progress on the measurement of insect sensitive wavelength. Chin. J. Appl. Ecol. 2023, 34, 1430–1440. [Google Scholar]
- Yang, H.B.; Hu, G.; Zhang, G.; Chen, X.; Zhu, Z.R.; Liu, S.; Liang, Z.L.; Zhang, X.X.; Cheng, X.N.; Zhai, B.P. Effect of light colours and weather conditions on captures of Sogatella furcifera (Horváth) and Nilaparvata lugens (STål). J. Appl. Entomol. 2014, 138, 743–753. [Google Scholar] [CrossRef]
- Stukenberg, N.; Pietruska, M.; Waldherr, A.; Meyhöfer, R. Wavelength-specific behavior of the western flower thrips (Frankliniella Occidentalis): Evidence for a blue-green chromatic mechanism. Insects 2020, 11, 423. [Google Scholar] [CrossRef]
- Somers-Yeates, R.; Hodgson, D.; McGregor, P.K.; Spalding, A.; ffrench-Constant, R.H. Shedding light on moths: Shorter wavelengths attract noctuids more than geometrids. Biol. Lett. 2013, 9, 20130376. [Google Scholar] [CrossRef]
- Wu, K.M. Management strategies of fall armymorm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 1–5. [Google Scholar]
- Cinzano, P.; Falchi, F.; Elvidge, C.D. The first world atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 2001, 328, 689–707. [Google Scholar] [CrossRef]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef]
- Davies, T.W.; Smyth, T. Why Artificial light at night should be a focus for global change research in the 21st century. Glob. Chang. Biol. 2018, 24, 872–882. [Google Scholar] [CrossRef]
- Owens, A.C.S.; Lewis, S.M. The impact of artificial light at night on nocturnal Insects: A review and synthesis. Ecol. Evol. 2018, 8, 11337–11358. [Google Scholar] [CrossRef]
- Ganguly, A.; Candolin, U. Impact of light pollution on aquatic invertebrates: Behavioral responses and ecological consequences. Behav. Ecol. Sociobiol. 2023, 77, 104. [Google Scholar] [CrossRef]
- Dacke, M.; Nilsson, D.-E.; Scholtz, C.H.; Byrne, M.; Warrant, E.J. Insect orientation to polarized moonlight. Nature 2003, 424, 33. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.J.; Smolka, J.; Nilsson, D.-E.; Dacke, M. How animals follow the stars. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172322. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef] [PubMed]



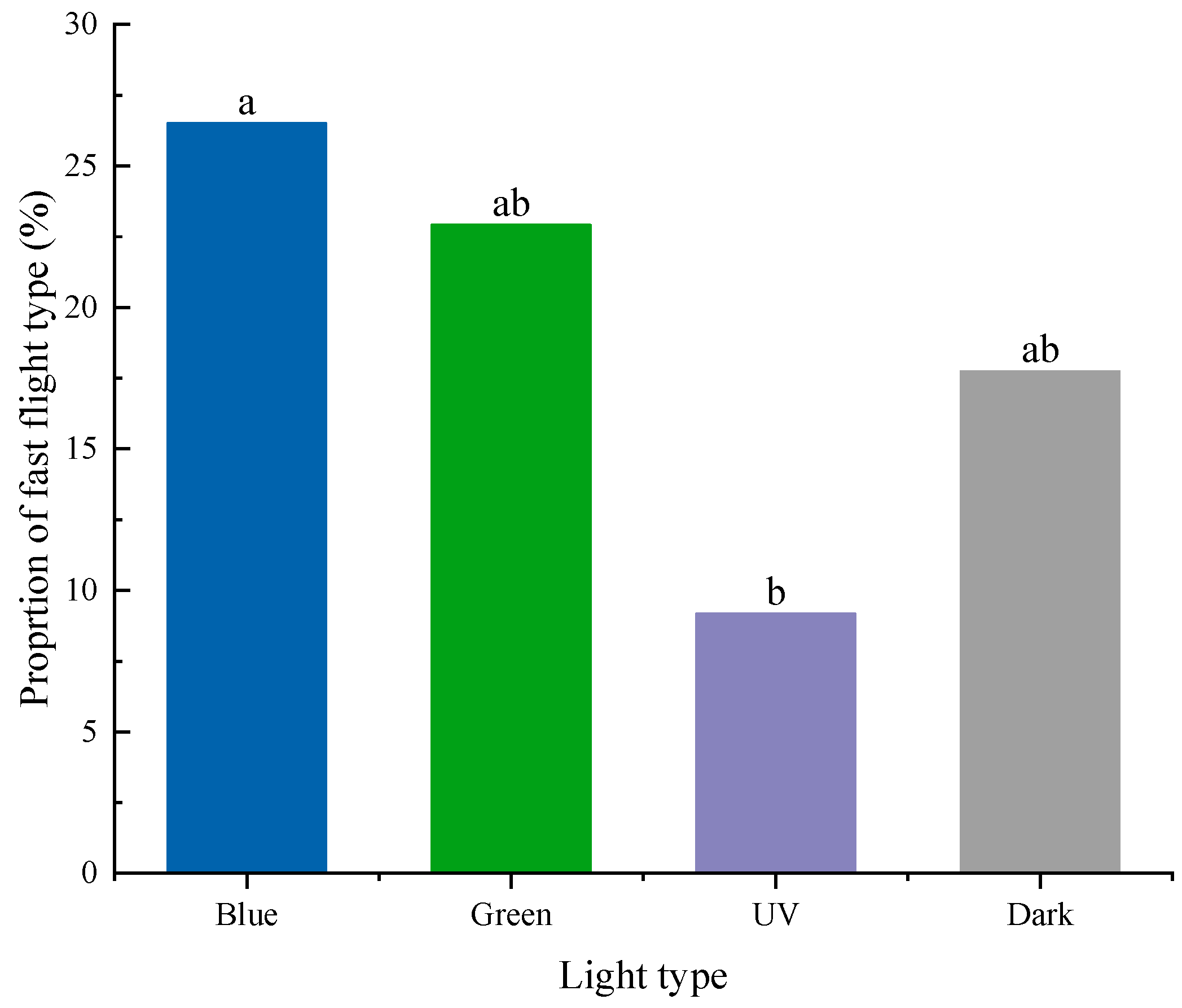
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Lu, J.; Zhu, P.; Sun, Y.; Hu, Z.; Li, D.; Huang, J. Blue Light Attracts More Spodoptera frugiperda Moths and Promotes Their Flight Speed. Insects 2024, 15, 129. https://doi.org/10.3390/insects15020129
Yang H, Lu J, Zhu P, Sun Y, Hu Z, Li D, Huang J. Blue Light Attracts More Spodoptera frugiperda Moths and Promotes Their Flight Speed. Insects. 2024; 15(2):129. https://doi.org/10.3390/insects15020129
Chicago/Turabian StyleYang, Haibo, Jing Lu, Pinhong Zhu, Yalan Sun, Zhenjie Hu, Dingxu Li, and Jianrong Huang. 2024. "Blue Light Attracts More Spodoptera frugiperda Moths and Promotes Their Flight Speed" Insects 15, no. 2: 129. https://doi.org/10.3390/insects15020129