Comparative Study of Potential Habitats for Simulium qinghaiense (Diptera: Simuliidae) in the Huangshui River Basin, Qinghai–Tibet Plateau: An Analysis Using Four Ecological Niche Models and Optimized Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Acquisition of Geographic Distribution Data
2.2. Software for ENMs and Related Tools
2.3. Selection and Filtering of Environmental Variables
2.4. Optimizing the MaxEnt Model
2.5. Constructing the Four Models: MaxEnt, GARP, BIOCLIM, and DOMAIN
2.6. Accuracy Evaluation of the Four Models
2.7. Prediction of Potential Distribution and Identification of Suitable Habitat
2.8. Changes in Suitable Habitats and Centroid Migration in Different Periods
3. Results
3.1. Optimization Results of the MaxEnt Model
3.2. Evaluation of Model Accuracy
3.3. Analysis of Dominant Environmental Factors
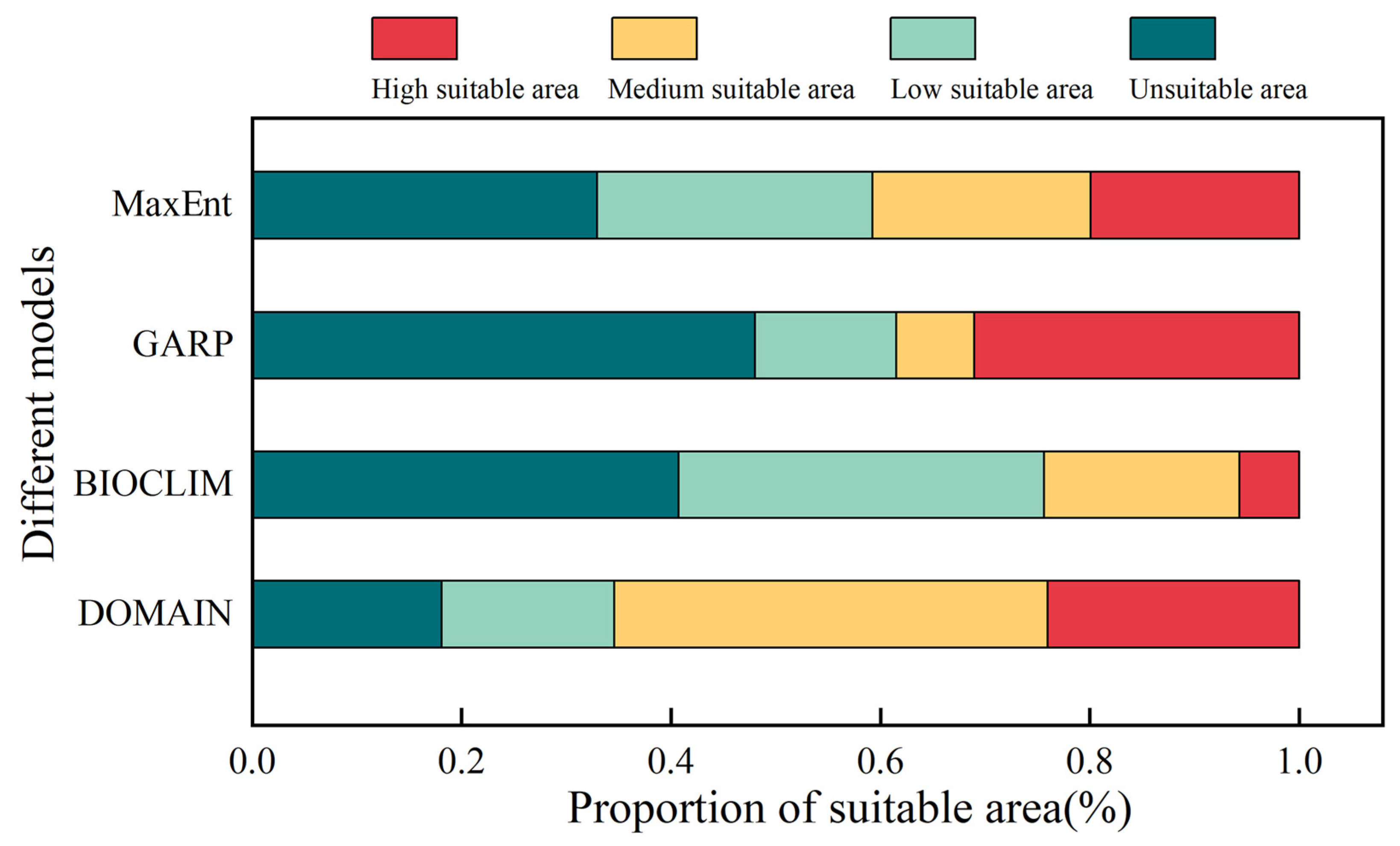
3.4. Predicted Potential Distribution of S. qinghaiense by Four Models
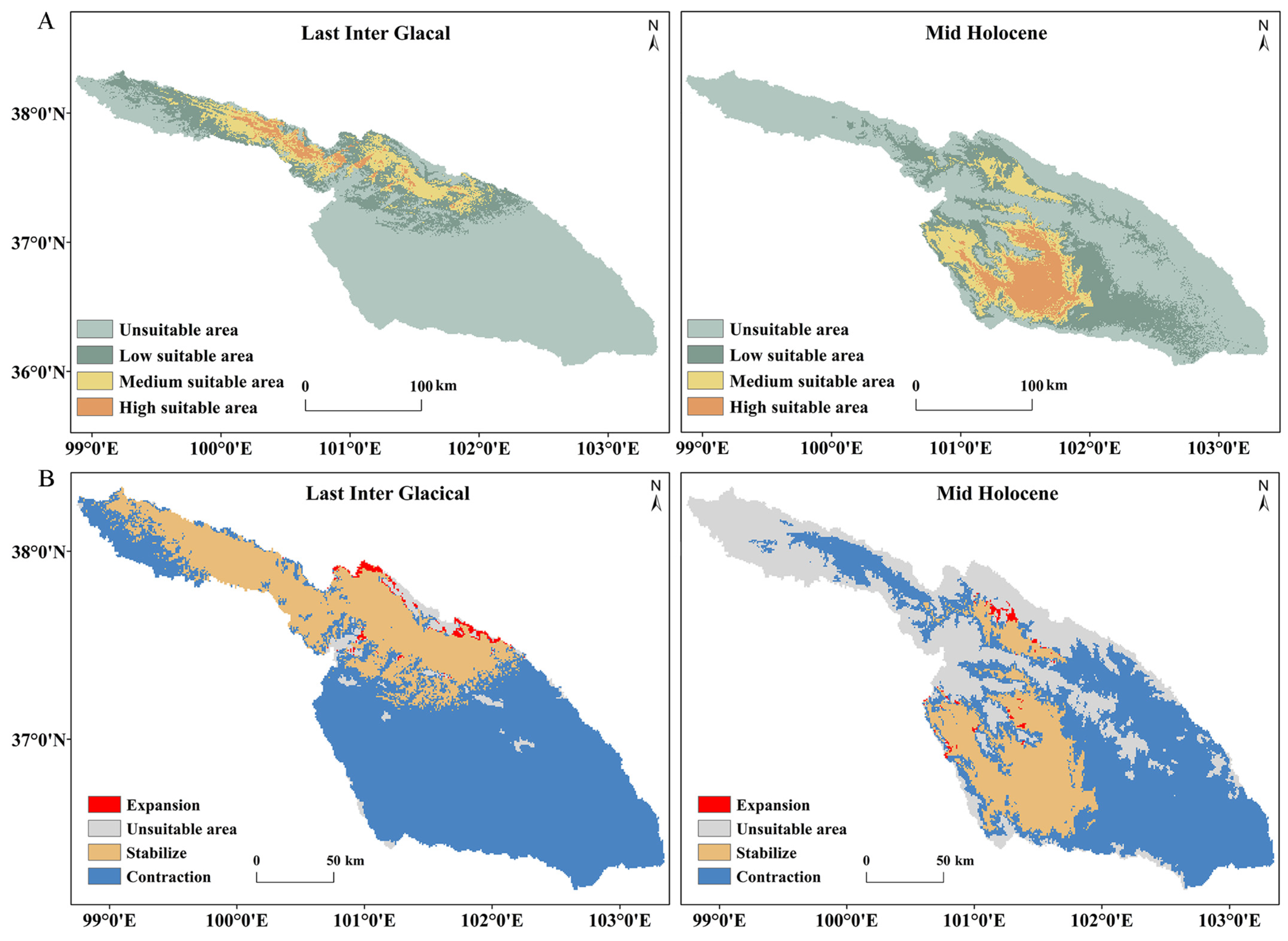
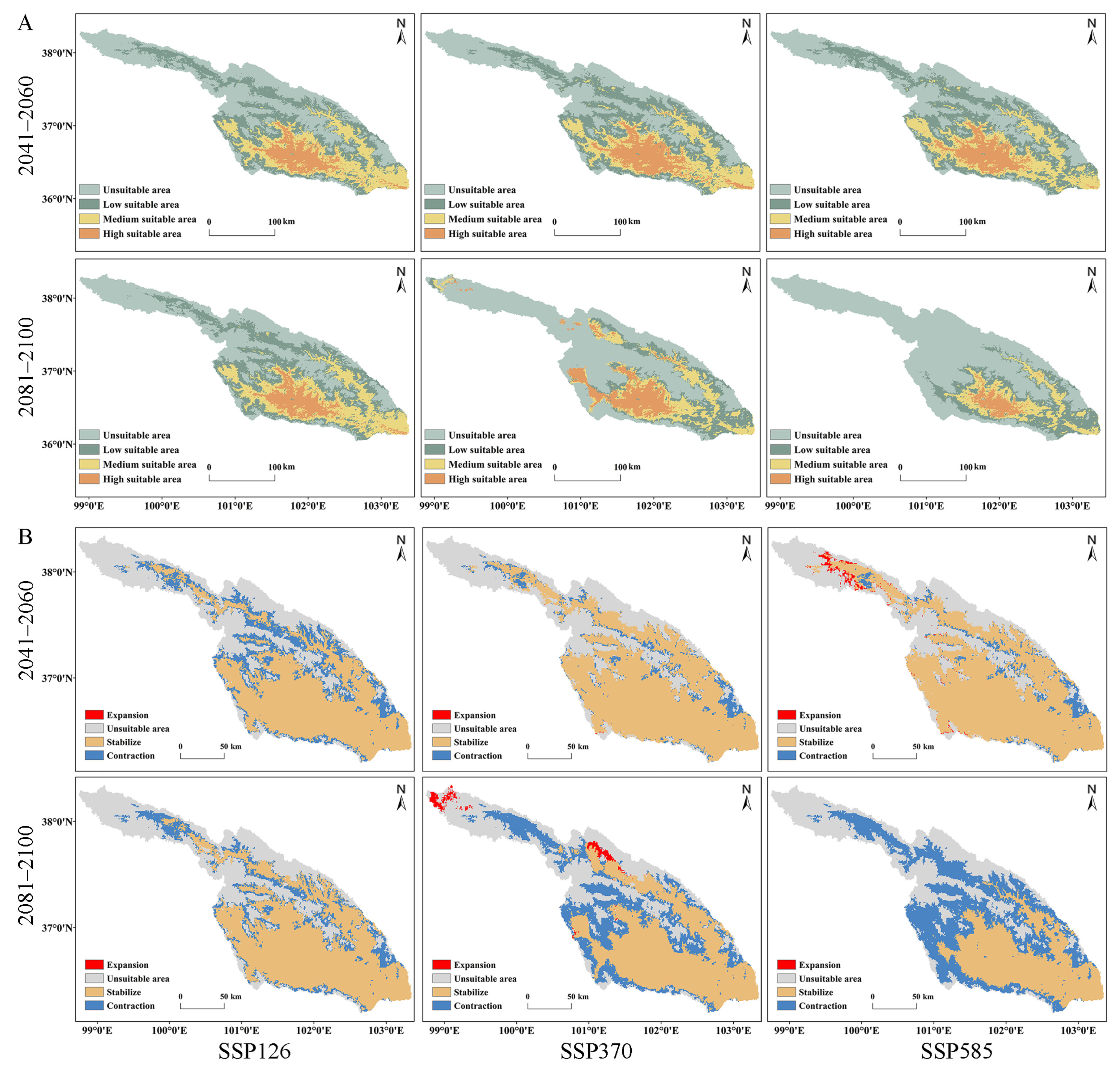
3.5. Predicted Suitable Areas and Their Changes over Different Periods and Climate Scenarios
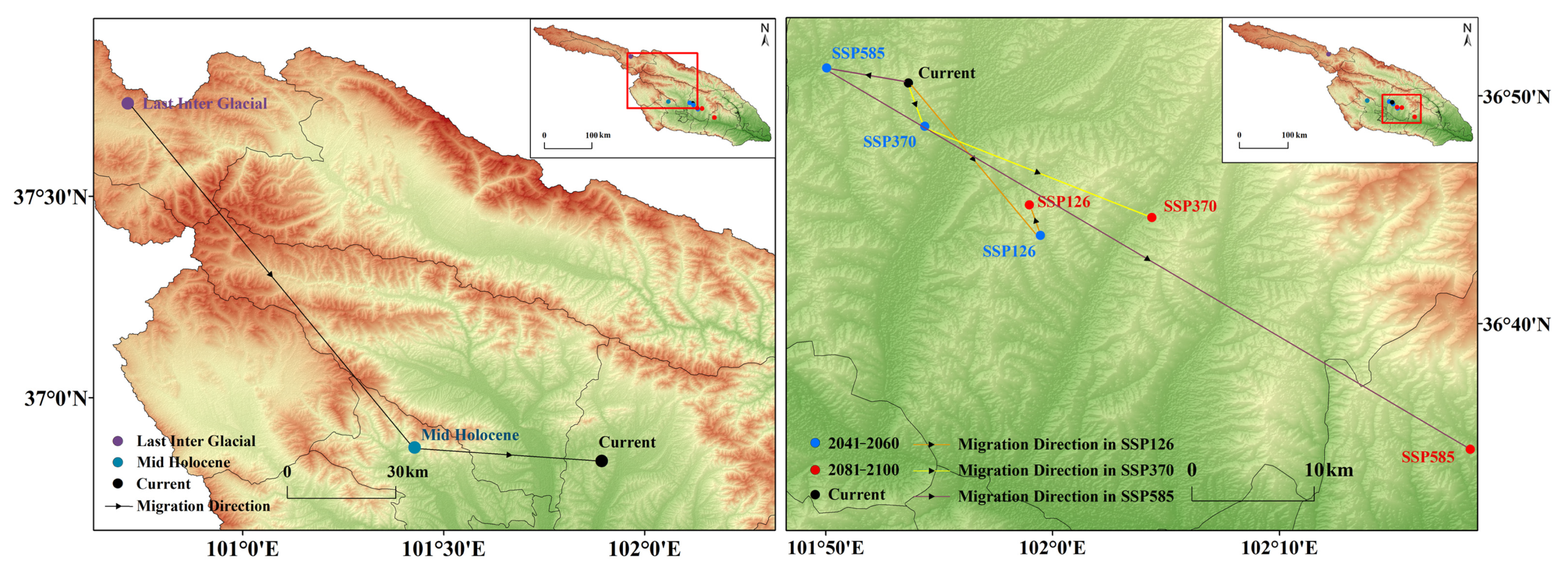
3.6. Distribution Center Changes over Different Periods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.S.; Gu, J.D. Ecological responses, adaptation and mechanisms of Mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeter. Biodegr. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Waldvogel, A.M.; Feldmeyer, B.; Rolshausen, G.; Exposito-Alonso, M.; Rellstab, C.; Kofler, R.; Mock, T.; Schmid, K.; Schmitt, I.; Bataillon, T.; et al. Evolutionary genomics can improve prediction of species’ responses to climate change. Evol. Lett. 2020, 4, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Liguori, J.A.; Ramírez-Barahona, S.; Gaut, B.S. The evolutionary genomics of species’ responses to climate change. Nat. Ecol. Evol. 2021, 5, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Zablocki, J.; Newsock, A.; Krolopp, A.; Tabas, P.; Salama, M. Durable Freshwater Protection: A framework for establishing and maintaining long-term protection for freshwater ecosystems and the values they sustain. Sustainability 2021, 13, 1950. [Google Scholar] [CrossRef]
- Erős, T.; Hermoso, V.; Langhans, S.D. Leading the path toward sustainable freshwater management: Reconciling challenges and opportunities in historical, hybrid, and novel ecosystem types. WIREs. Water 2023, 10, e1645. [Google Scholar] [CrossRef]
- John, A.; Horne, A.; Nathan, R.; Stewardson, M.; Webb, J.A.; Wang, J.; Poff, N.L. Climate change and freshwater ecology: Hydrological and ecological methods of comparable complexity are needed to predict risk. WIREs. Clim. Chang. 2021, 12, e692. [Google Scholar] [CrossRef]
- Woodward, G.; Bonada, N.; Brown, L.E.; Death, R.G.; Durance, I.; Gray, C.; Hladyz, S.; Ledger, M.E.; Milner, A.M.; Ormerod, S.J.; et al. The effects of climatic fluctuations and extreme events on running water ecosystems. Phil. Trans. R. Soc. B 2016, 371, 20150274. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, F.; Rojas, A.; Buckland, P.I. Late holocene anthropogenic landscape change in northwestern europe impacted insect biodiversity as much as climate change did after the last ice age. Proc. R. Soc. B 2022, 289, 20212734. [Google Scholar] [CrossRef]
- Elias, S.A. 1 The history of quaternary insect studies. In Developments in Quaternary Sciences; Elias, S.A., Ed.; Advances in Quaternary Entomology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–8. [Google Scholar]
- Brooks, S.J. Fossil midges (Diptera: Chironomidae) as palaeoclimatic indicators for the Eurasian region. Quat. Sci. Rev. 2006, 25, 1894–1910. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, X.; Chen, H.; Liu, Y.; Cui, Z. Effects of agriculture and animal husbandry on heavy metal contamination in the aquatic environment and human health in HuangShui River Basin. Water 2022, 14, 549. [Google Scholar] [CrossRef]
- Kietzka, G.J.; Pryke, J.S.; Gaigher, R.; Samways, M.J. Applying the umbrella index across aquatic insect taxon sets for freshwater assessment. Ecol. Indic. 2019, 107, 105655. [Google Scholar] [CrossRef]
- Parikh, G.; Rawtani, D.; Khatri, N. Insects as an indicator for environmental pollution. Environmen. Claims J. 2021, 33, 161–181. [Google Scholar] [CrossRef]
- Cuadrado, L.A.; Moncada, L.I.; Pinilla, G.A.; Larrañaga, A.; Sotelo, A.I.; Adler, P.H. Black fly (Diptera: Simuliidae) assemblages of high Andean Rivers respond to environmental and pollution gradients. Environ. Entomol. 2019, 48, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Ciadamidaro, S.; Mancini, L.; Rivosecchi, L. Black flies (Diptera, Simuliidae) as ecological indicators of stream ecosystem health in an urbanizing area (Rome, Italy). Ann. Ist. Super. Sanita. 2016, 52, 269–276. [Google Scholar] [PubMed]
- Malmqvist, B.; Adler, P.H.; Kuusela, K.; Merritt, R.W.; Wotton, R.S. Black flies in the boreal biome, key organisms in both terrestrial and aquatic environments: A review. Écoscience 2004, 11, 187–200. [Google Scholar] [CrossRef]
- Currie, D.; Adler, P. Global diversity of Black flies (Diptera: Simuliidae) in freshwater. Dev. Hydrobiol. 2008, 198, 469–475. [Google Scholar]
- Timm, H.; Haldna, M. Do abundance and percentage of dipteran larvae and Oligochaeta indicate low water quality in streams and lake littoral? Oceanol. Hydrobiol. St. 2019, 48, 415–429. [Google Scholar] [CrossRef]
- Adler, P.H.; McCreadie, J.W. Chapter 14-Black flies (Simuliidae). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 237–259. [Google Scholar]
- McCreadie, J.W.; Williams, R.H.; Stutsman, S.; Finn, D.S.; Adler, P.H. The influence of habitat heterogeneity and latitude on gamma diversity of the Nearctic Simuliidae, a ubiquitous group of stream-dwelling insects. Insect Sci. 2018, 25, 712–720. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Chaverri, L.G.; Rodríguez- Pérez, M.A.; Prosser, S.W.J.; Hebert, P.D.N.; Gregory, T.R.; Johnson, N. DNA barcoding of neotropical black flies (Diptera: Simuliidae): Species identification and discovery of cryptic diversity in Mesoamerica. Zootaxa 2015, 3936, 93–114. [Google Scholar] [CrossRef]
- Adler, P.H.; Kúdelová, T.; Kúdela, M.; Seitz, G.; Ignjatović-Ćupina, A. Cryptic biodiversity and the origins of pest status revealed in the macrogenome of Simulium colombaschense (Diptera: Simuliidae), history’s most destructive black fly. PLoS ONE 2016, 11, e0147673. [Google Scholar] [CrossRef]
- Ilmonen, J.; Adler, P.H.; Malmqvist, B.; Cywinska, A. The Simulium vernum group (Diptera: Simuliidae) in Europe: Multiple character sets for assessing species status. Zool. J. Linn. Soc.-Lond. 2009, 156, 847–863. [Google Scholar] [CrossRef]
- Kamtsap, P.; Ngoufo, F.N.; Ekale, D.; Yilak, A.; Schnell, R.; Akafyi, D.E.; Garbi, M.; Kamgno, J.; Djeunga, H.C.N.; Fouamno, H.L.K.; et al. Taxonomy and molecular phylogeny of black flies (Diptera: Simuliidae) in Africa and Europe by using mitochondrial Cox1 and nuclear ITS2 markers. Res. Quare 2023. [Google Scholar] [CrossRef]
- Low, V.L.; Adler, P.H.; Sofian-Azirun, M.; Srisuka, W.; Saeung, A.; Huang, Y.T.; Hadi, U.K.; Da Pham, X.; Takaoka, H. Tests of conspecificity for allopatric vectors: Simulium nodosum and Simulium shirakii (Diptera: Simuliidae) in Asia. Parasite. Vector. 2015, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B. Faunistic distribution and geographical divisions of blackflies in China (Diptera: Simuliidae). J. Zool. Syst. 2002, 27, 624–630. [Google Scholar]
- Ma, Y.L.; Wen, X.J.; Chen, H.B. A primar report on blackflies categorization (Diptera: Simuliidae) in Qinghai, China. J. Guizhou Norm. Univ. Nat. Sci. 2008, 26, 5–8. [Google Scholar]
- Ruiz-Arrondo, I.; Hernández-Triana, L.M.; Ignjatović-Ćupina, A.; Nikolova, N.; Garza-Hernández, J.A.; Rodríguez-Pérez, M.A.; Oteo, J.A.; Fooks, A.R.; Lucientes Curdi, J. DNA barcoding of blackflies (Diptera: Simuliidae) as a tool for species identification and detection of hidden diversity in the eastern regions of Spain. Parasite. Vector. 2018, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Sun, Q.; Xiu, J.F.; Yang, M. Comparisons of respiratory pupal gill development in black flies (Diptera: Simuliidae) shed light on the origin of dipteran prothoracic dorsal appendages. J. Med. Entomol. 2021, 58, 588–598. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, C.; Li, J.; Wang, Y. The complete mitochondrial genome of Simulium jisigouense (Diptera: Simuliidae) and phylogenetic analysis of Simuliidae. Front. Ecol. Evol. 2022, 10, 932601. [Google Scholar] [CrossRef]
- Rivera, J.; Currie, D.C. Identification of nearctic black flies using DNA barcodes (Diptera: Simuliidae). Mol. Ecol. Resour. 2009, 9, 224–236. [Google Scholar] [CrossRef]
- Lourdes, E.Y.; Ya’cob, Z.; Low, V.L.; Izwan-Anas, N.; Mansor, M.S.; Dawood, M.M.; Takaoka, H.; Adler, P.H. Natural infections and distributions of parasitic Mermithids (Nematoda: Mermithidae) infecting larval black flies (Diptera: Simuliidae) in tropical streams of Malaysia. Acta. Trop. 2022, 230, 106386. [Google Scholar] [CrossRef]
- Pramual, P.; Adler, P.H. DNA barcoding of tropical black flies (Diptera: Simuliidae) of Thailand. Mol. Ecol. Resour. 2014, 14, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Lee-Yaw, J.A.; McCune, J.L.; Pironon, S.; Sheth, S.N. Species distribution models rarely predict the biology of real populations. Ecography 2022, 2022, e05877. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; Pennino, M.G.; Conesa, D.; Kikuchi, E.; Kinas, P.G.; Barbosa, F.G.; Cardoso, L.G. Modelling the distribution of marine fishery resources: Where are we? Fish Fish. 2023, 24, 159–175. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hannah, L.; Midgley, G.; Andelman, S.; Araújo, M.; Hughes, G.; Martinez-Meyer, E.; Pearson, R.; Williams, P. Protected area needs in a changing climate. Front. Ecol. Environ. 2007, 5, 131–138. [Google Scholar] [CrossRef]
- Dvořák, T.; Hadrava, J.; Knapp, M. The ecological niche and conservation value of central European grassland orthopterans: A quantitative approach. Biol. Conserv. 2022, 265, 109406. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; He, D.; Wang, X.; Bai, M. The modeling and forecasting of carabid beetle distribution in northwestern China. Insects 2021, 12, 168. [Google Scholar] [CrossRef]
- Qi, M.L.; Li, N.; Bai, L.C.; Ma, G.L.; Wang, T. Community structure of macrobenthos and its relationship with environmental factors in the upper reaches of Huangshui River. J. Nanjing Agric. Univ. 2020, 43, 72–79. [Google Scholar]
- Miao, X.; Lindsey, D.A.; Lai, Z.; Liu, X. Contingency table analysis of pebble lithology and roundness: A case study of Huangshui River, China and comparison to rivers in the Rocky Mountains, USA. Sediment. Geol. 2010, 224, 49–53. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, R.; Hou, X.K. Analysis and countermeasures of the typical watersheds environment problems in upper Yellow River: Taking Huangshui River as an example. J. Environ. Prot. 2021, 49, 17–19. [Google Scholar]
- Wu, T.; Yu, R.; Lu, Y.; Jie, W.; Fang, Y.; Zhang, J.; Zhang, L.; Xin, X.; Li, L.; Wang, Z.; et al. BCC-CSM2-HR: A high-resolution version of the Beijing climate center climate system model. Geosci. Model. Dev. 2021, 14, 2977–3006. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the coupled model intercomparison project phase 6 (CMIP6) experimental design and organization. Geosci. Model. Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model. Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized maxent model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Jiang, X.; Chen, H.; Liu, M.; Wang, R. Potential geographical distribution of the edangred plant isoetes under human activities using maxent and garp. Glob. Ecol. Conserv. 2022, 38, e02186. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Radosavljevic, A.; Anderson, R.P. Making better maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R package for detailed development of ecological niche models using maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Global. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Sun, L.Y.; Jiang, Z.; Liu, C.N.; Yin, Z.F. Analysis of the adaptive and geographical distribution of Yulania liliiflora based on DIVA-GIS. Plant Sci. J. 2018, 36, 804–811. [Google Scholar]
- Zhang, Q.; Zhang, D.F.; Wu, M.L.; Guo, J.; Sun, C.Z.; Xie, C.X. Predicting the global areas for potential distribution of Gastrodia elata based on ecological niche models. J. Plant Ecol. 2017, 41, 770. [Google Scholar]
- Karami, P.; Shayesteh, K.; Rastegar Pouyani, N. Evaluation the distribution of effective factors on habitat diversity in kermanshah protected areas. GeoSus 2020, 10, 105–123. [Google Scholar]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Tao, J. Predicting the potential distribution of Paeonia veitchii (Paeoniaceae) in China by incorporating climate change into a maxent model. Forests 2019, 10, 190. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Stockwell, D.R.B.; Peterson, A.T. Effects of sample size on accuracy of species distribution models. Ecol. Model. 2002, 148, 1–13. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. Bioclim: The first species distribution modelling package, its early applications and relevance to most current maxent studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Márcia Barbosa, A.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [PubMed]
- Yates, K.L.; Bouchet, P.J.; Caley, M.J.; Mengersen, K.; Randin, C.F.; Parnell, S.; Fielding, A.H.; Bamford, A.J.; Ban, S.; Barbosa, A.M.; et al. Outstanding challenges in the transferability of ecological models. Trends Ecol. Evol. 2018, 33, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Oforka, L.C.; Adeleke, M.A.; Anikwe, J.C.; Makanjuola, W.A. Population fluctuations and effect of climatic factors on the relative abundance of Simulium damnosum complex (Diptera: Simuliidae). Environ. Entomol. 2019, 48, 284–290. [Google Scholar] [CrossRef]
- Figueiró, R.; Nascimento, É.S.D.; Gil-Azevedo, L.; Maia-Herzog, M.; Monteiro, R.F. Local distribution of blackfly (Diptera, Simuliidae) larvae in two adjacent streams: The role of water current velocity in the diversity of blackfly larvae. Rev. Bras. Entomol. 2008, 52, 452–454. [Google Scholar] [CrossRef]
- Brannin, M.T.; O’donnell, M.K.; Fingerut, J. Effects of larval size and hydrodynamics on the growth rates of the black fly Simulium tribulatum. Integr. Zool. 2014, 9, 61–69. [Google Scholar] [CrossRef]
- Senapathi, D.; Fründ, J.; Albrecht, M.; Garratt, M.P.D.; Kleijn, D.; Pickles, B.J.; Potts, S.G.; An, J.; Andersson, G.K.S.; Bänsch, S.; et al. Wild insect diversity increases inter-annual stability in global crop pollinator communities. Process. Roy. Soc. B-Biol. Sci. 2021, 288, 20210212. [Google Scholar] [CrossRef]
- Kellermann, V.; van Heerwaarden, B. Terrestrial insects and climate change: Adaptive responses in key traits. Physiol. Entomol. 2019, 44, 99–115. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Bryson, R.W.; Prendini, L.; Savary, W.E.; Pearman, P.B. Caves as microrefugia: Pleistocene phylogeography of the troglophilic North American scorpion Pseudouroctonus reddelli. BMC Evol. Biol. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M. Evidence for multiple refugia at different time scales during pleistocene climatic oscillations in southern australia inferred from phylogeography. Quat. Sci. Rev. 2008, 27, 2576–2585. [Google Scholar] [CrossRef]
- Shepard, D.B.; Burbrink, F.T. Phylogeographic and demographic effects of pleistocene climatic fluctuations in a montane salamander, plethodon fourchensis. Mol. Ecol. 2009, 18, 2243–2262. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sgrò, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across central Asia under climate change in the 21st century. Agr. Forest. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Ostad-Ali-Askari, K.; Ghorbanizadeh Kharazi, H.; Shayannejad, M.; Zareian, M.J. Effect of climate change on precipitation patterns in an arid region using GCM models: Case study of Isfahan-Borkhar Plain. Nat. Hazards Rev. 2020, 21, 04020006. [Google Scholar] [CrossRef]
- Urban, M.C.; Bocedi, G.; Hendry, A.P.; Mihoub, J.-B.; Pe’er, G.; Singer, A.; Bridle, J.R.; Crozier, L.G.; De Meester, L.; Godsoe, W.; et al. Improving the forecast for biodiversity under climate change. Science 2016, 353, aad8466. [Google Scholar] [CrossRef]
- Elbrecht, V.; Beermann, A.J.; Goessler, G.; Neumann, J.; Tollrian, R.; Wagner, R.; Wlecklik, A.; Piggott, J.; Matthaei, C.; Leese, F. Multiple–stressor effects on stream invertebrates: A mesocosm experiment manipulating nutrients, fine sediment and flow velocity. Freshwater Biol. 2016, 61, 362–375. [Google Scholar] [CrossRef]
- Bishop, T.R.; Parr, C.L.; Gibb, H.; van Rensburg, B.J.; Braschler, B.; Chown, S.L.; Foord, S.H.; Lamy, K.; Munyai, T.C.; Okey, I.; et al. Thermoregulatory traits combine with range shifts to alter the future of montane ant assemblages. Global Chang. Biol. 2019, 25, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, G.; Wang, N.; Feng, G.; Yang, F.; Svenning, J.C.; Yang, J. Taxonomic, phylogenetic and functional homogenization of bird communities due to land use change. Biol. Conserv. 2019, 236, 37–43. [Google Scholar] [CrossRef]
- Piacenza, S.E.; Thurman, L.L.; Barner, A.K.; Benkwitt, C.E.; Boersma, K.S.; Cerny-Chipman, E.B.; Ingeman, K.E.; Kindinger, T.L.; Lindsley, A.J.; Nelson, J.; et al. Evaluating temporal consistency in marine biodiversity hotspots. PLoS ONE 2015, 10, e0133301. [Google Scholar] [CrossRef] [PubMed]
- Amburgey, S.M.; Miller, D.A.W.; Campbell Grant, E.H.; Rittenhouse, T.A.G.; Benard, M.F.; Richardson, J.L.; Urban, M.C.; Hughson, W.; Brand, A.B.; Davis, C.J.; et al. Range position and climate sensitivity: The structure of among-population demographic responses to climatic variation. Glob. Chang. Biol. 2018, 24, 439–454. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; De Palma, A.; Ferrier, S.; Hill, S.L.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.P.; et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, M.J.; Grytnes, J.A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.A. Designing agricultural landscapes for biodiversity-based ecosystem services. Basic. Appl. Ecol. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Kerby, J.T.; Phoenix, G.K.; Bjerke, J.W.; Epstein, H.E.; Assmann, J.J.; John, C.; Andreu-Hayles, L.; Angers-Blondin, S.; Beck, P.S.A.; et al. Complexity revealed in the greening of the arctic. Nat. Clim. Chang. 2020, 10, 106–117. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhang, S.Y.; Dong, S.F.; Zhang, G.C.; Yang, J.; Li, R.; Wang, H.Q. Pest24: A large-scale very small object data set of agricultural pests for multi-target detection. Comput. Electron. Agric. 2020, 175, 105585. [Google Scholar] [CrossRef]
- Getzin, S.; Wiegand, K.; Schöning, I. Assessing biodiversity in forests using very high-resolution images and unmanned aerial vehicles. Methods Ecol. Evol. 2012, 3, 397–404. [Google Scholar] [CrossRef]

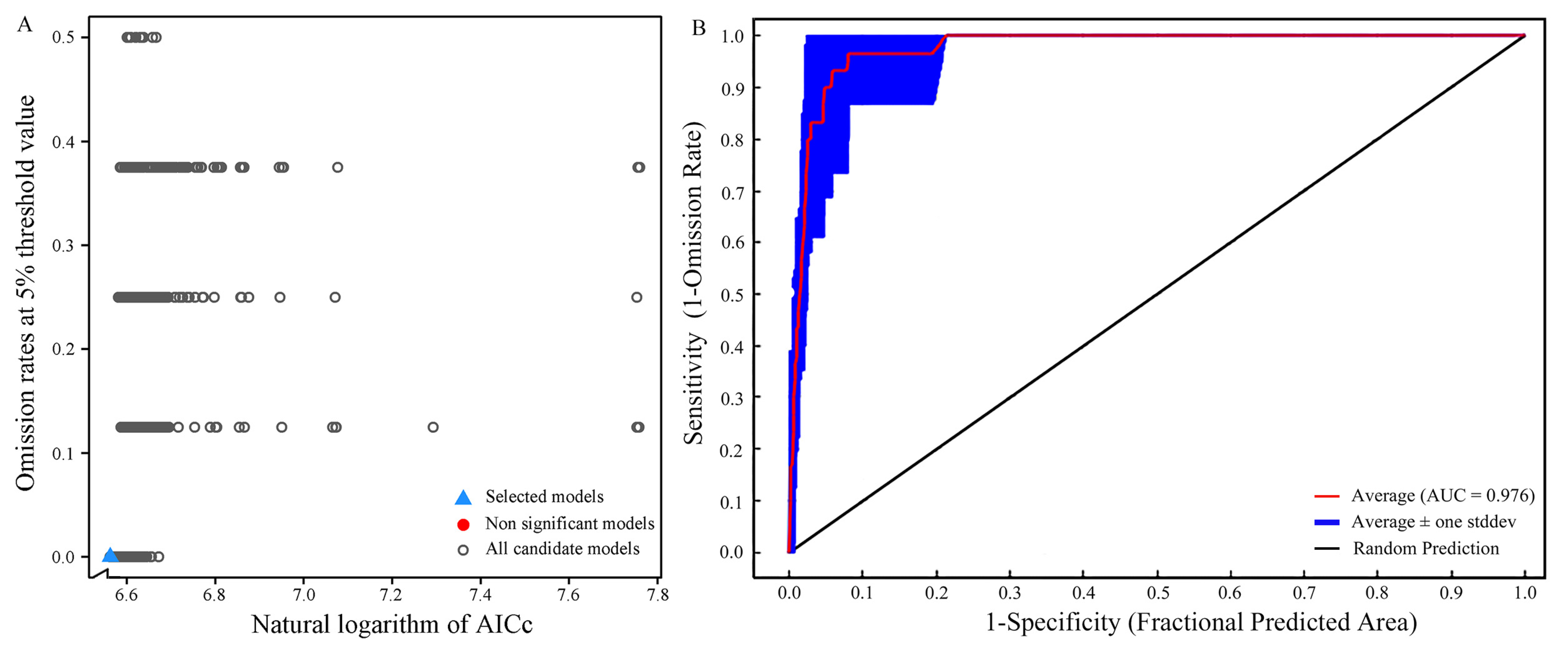

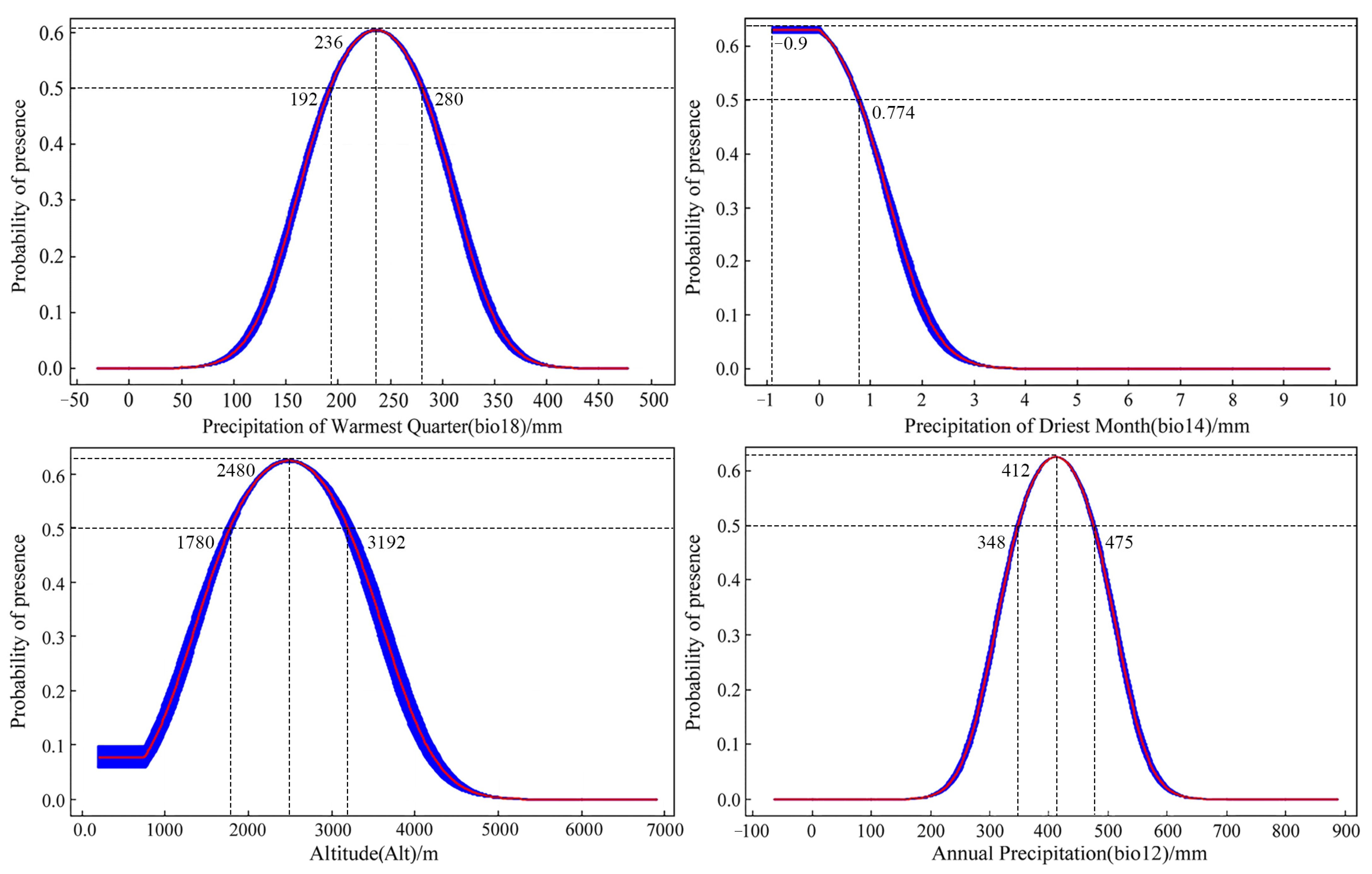
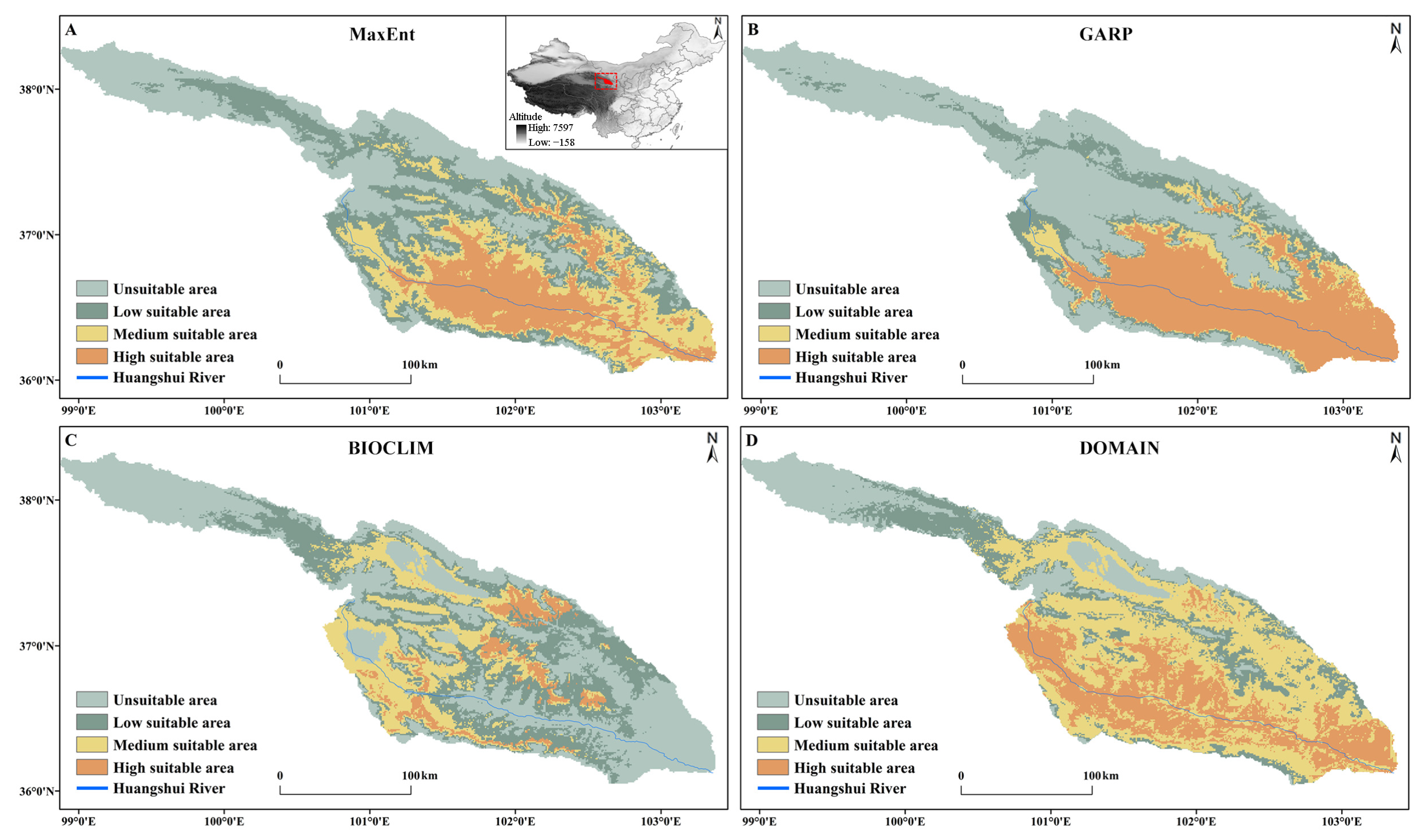
| Criteria | Number of Models | |
|---|---|---|
| All candidate models | 1160 | |
| Statistically significant models | 1156 | |
| Models meeting omission rate criteria | 150 | |
| Models meeting AICc criteria | 1 | |
| Statistically significant models meeting omission rate criteria | 146 | |
| Statistically significant models meeting AICc criteria | 1 | |
| Selected model | RM(0.6) FC(LQ) | |
| Mean AUC ratio | 1.9267 | |
| Omission rate | 0 | |
| AICc | 708.0172 | |
| Delta AICc | 0 | |
| Group | MaxEnt | GARP | BIOCLIM | DOMAIN | ||||
|---|---|---|---|---|---|---|---|---|
| AUC | Kappa | AUC | Kappa | AUC | Kappa | AUC | Kappa | |
| 1 | 0.9054 | 0.7735 | 0.8996 | 0.6877 | 0.9425 | 0.7625 | 0.8667 | 0.6426 |
| 2 | 0.9830 | 0.8314 | 0.8775 | 0.6938 | 0.8845 | 0.6933 | 0.8613 | 0.6536 |
| 3 | 0.9911 | 0.8532 | 0.9210 | 0.7324 | 0.8678 | 0.6867 | 0.9325 | 0.7352 |
| 4 | 0.9878 | 0.8325 | 0.9168 | 0.7417 | 0.9311 | 0.7236 | 0.9434 | 0.7124 |
| 5 | 0.9768 | 0.7843 | 0.9567 | 0.7359 | 0.9242 | 0.7124 | 0.9145 | 0.7243 |
| 6 | 0.9811 | 0.7893 | 0.8439 | 0.6426 | 0.9432 | 0.7686 | 0.8750 | 0.6207 |
| 7 | 0.9841 | 0.8424 | 0.9519 | 0.7512 | 0.8767 | 0.6893 | 0.8857 | 0.6550 |
| 8 | 0.9830 | 0.7982 | 0.9479 | 0.7627 | 0.8582 | 0.6521 | 0.8929 | 0.6750 |
| 9 | 0.9888 | 0.8423 | 0.9433 | 0.7310 | 0.9314 | 0.7333 | 0.8932 | 0.6883 |
| 10 | 0.9753 | 0.7711 | 0.8950 | 0.6988 | 0.8894 | 0.6993 | 0.8438 | 0.6723 |
| Average | 0.9756 | 0.8118 | 0.9154 | 0.7178 | 0.9049 | 0.7121 | 0.8909 | 0.6780 |
| Standard deviation | 0.0252 | 0.0316 | 0.0367 | 0.0364 | 0.0327 | 0.0359 | 0.0317 | 0.0371 |
| Different Models | Area of Different Suitable Habitats (km2) | |||
|---|---|---|---|---|
| Unsuitable Area | Low Suitable Area | Medium Suitable Area | High Suitable Area | |
| MaxEnt | 10,972.2 | 8766.67 | 6938.19 | 6627.83 |
| GARP | 15,990.95 | 4507.69 | 2479.17 | 10,327.08 |
| BIOCLIM | 13,571.53 | 11,615.28 | 6221.53 | 1896.55 |
| DOMAIN | 6023.23 | 5494.42 | 13,778.23 | 8009.01 |
| Different Periods and Climate Scenarios | Unsuitable Area (km2) | Change (%) | Low Suitable Area (km2) | Change (%) | Medium Suitable Area (km2) | Change (%) | High Suitable Area (km2) | Change (%) | Expansion (km2) | Stabilize (km2) | Contraction (km2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Current | 10,972.2 | - | 8766.67 | - | 6938.19 | - | 6627.83 | - | - | - | - |
| Last Inter Glacial | 24,245.1 | −54.74% | 5362.53 | 63.48% | 2863.19 | 142.32% | 834.07 | 694.64% | 315.15 | 8529.21 | 23,011.56 |
| Mid Holocene | 19,085.4 | −42.51% | 7984.03 | 9.80% | 3622.27 | 91.54% | 2613.19 | 153.63% | 187.7 | 6180.24 | 15,902.86 |
| SSP126 (2041–2060) | 13,628.5 | 24.21% | 9582.64 | 9.31% | 7015.28 | 1.11% | 3078.47 | −53.55% | 0 | 16,144.63 | 5938.47 |
| SSP370 (2041–2060) | 12,807.6 | 16.73% | 9856.29 | 12.43% | 6612.51 | −4.69% | 4028.49 | −39.22% | 9.27 | 19,832.23 | 2250.87 |
| SSP585 (2041–2060) | 12,602.8 | 14.86% | 10,666.7 | 21.67% | 6696.53 | −3.48% | 3338.86 | −49.62% | 454.19 | 20,133.48 | 1949.62 |
| SSP126 (2081–2100) | 13,704.9 | 24.91% | 9475.69 | 8.09% | 7148.61 | 3.03% | 2975.69 | −55.10% | 0 | 17,294.01 | 4789.09 |
| SSP370 (2081–2100) | 18,834 | 71.65% | 5488.19 | −37.40% | 5035.45 | −27.42% | 3947.25 | −40.44% | 624.13 | 14,028.94 | 8054.16 |
| SSP585 (2081–2100) | 22,191.7 | 102.25% | 5801.39 | −33.82% | 3856.25 | −44.42% | 1455.55 | −78.04% | 0 | 10,266.41 | 11,816.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, C.; Shao, H. Comparative Study of Potential Habitats for Simulium qinghaiense (Diptera: Simuliidae) in the Huangshui River Basin, Qinghai–Tibet Plateau: An Analysis Using Four Ecological Niche Models and Optimized Approaches. Insects 2024, 15, 81. https://doi.org/10.3390/insects15020081
Liu Y, Li C, Shao H. Comparative Study of Potential Habitats for Simulium qinghaiense (Diptera: Simuliidae) in the Huangshui River Basin, Qinghai–Tibet Plateau: An Analysis Using Four Ecological Niche Models and Optimized Approaches. Insects. 2024; 15(2):81. https://doi.org/10.3390/insects15020081
Chicago/Turabian StyleLiu, Yunxiang, Chuanji Li, and Hainan Shao. 2024. "Comparative Study of Potential Habitats for Simulium qinghaiense (Diptera: Simuliidae) in the Huangshui River Basin, Qinghai–Tibet Plateau: An Analysis Using Four Ecological Niche Models and Optimized Approaches" Insects 15, no. 2: 81. https://doi.org/10.3390/insects15020081
APA StyleLiu, Y., Li, C., & Shao, H. (2024). Comparative Study of Potential Habitats for Simulium qinghaiense (Diptera: Simuliidae) in the Huangshui River Basin, Qinghai–Tibet Plateau: An Analysis Using Four Ecological Niche Models and Optimized Approaches. Insects, 15(2), 81. https://doi.org/10.3390/insects15020081




