Ecological Speciation without Morphological Differentiation? A New Cryptic Species of Diodontus Curtis (Hymenoptera, Pemphredonidae) from the Centre of Europe †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Morphometric Measurements
2.3. Selection of Molecular Characters
| Abbreviation | Description | Length (bp) | Source |
|---|---|---|---|
| Mitochondrial protein-coding genes: | |||
| CO1–5’ | Cytochrome c oxidase, subunit 1, from 5′ end to 715 bp | 715 | [28] |
| CO1–3’ | Cytochrome c oxidase, subunit 1, from 710 bp to the 3′ end | 825–828 | [28] |
| CO2 | Cytochrome c oxidase, subunit 2, complete sequence | 678 | [28] |
| ATP8 | ATP synthase, subunit 8, complete sequence | 149 | new |
| ND6 | NADH dehydrogenase, subunit 6, complete sequence | 502–535 | [28] |
| CytB–5’ | Cytochrome b, from 105 to 859 bp | 754 | [28] |
| ND1 | Cytochrome b, 3′ end from 885 bp, and NADH dehydrogenase, subunit 1, 335 bp long 3′ end (considering reverse translation comparing to CytB) | 665 | [28] |
| Nuclear ribosomal DNA operon: | |||
| 18S | 18S rDNA partial sequence, including V2–V4 variable regions | 797–798 | [28] |
| 28S | 28S rDNA partial sequence, including D2–D3 variable regions | 683–685 | [28] |
| ITS1 | Internal transcribed spacer 1, partial sequence | 491–538 | [28] |
| Nuclear protein-coding genes: | |||
| PB | Proboscipedia gene, partial sequence | 500–513 | [28] |
| AbdB | Abdominal B gene, partial sequence | 648 | [28] |
| ArgK | Arginine kinase gene, partial sequence | 599–605 | [28] |
| MRPP3 | Mitochondrial ribonuclease P protein 3 gene, partial sequence | 678–691 | [28] |
| Ube2g1 | Ubiquitin-conjugating enzyme E2 G1 gene, partial sequence | 597–614 | [28] |
| PTCD2 | Pentatricopeptide repeat domain 2 gene, partial sequence | 664–668 | [28] |
| 16S (Bact.) | 16S rDNA of endosymbiotic bacteria, partial sequence | 745–785 | [28] |
| wsp | Wolbachia outer surface protein, partial sequence | 577–589 | [49] |
2.4. DNA Extraction, Polymerase Chain Reaction, and Sequencing
2.5. Phylogeny Reconstruction and Species Delimitation
3. Results
3.1. Identification
3.2. Phylogenetic Relationships and Species Delimitation
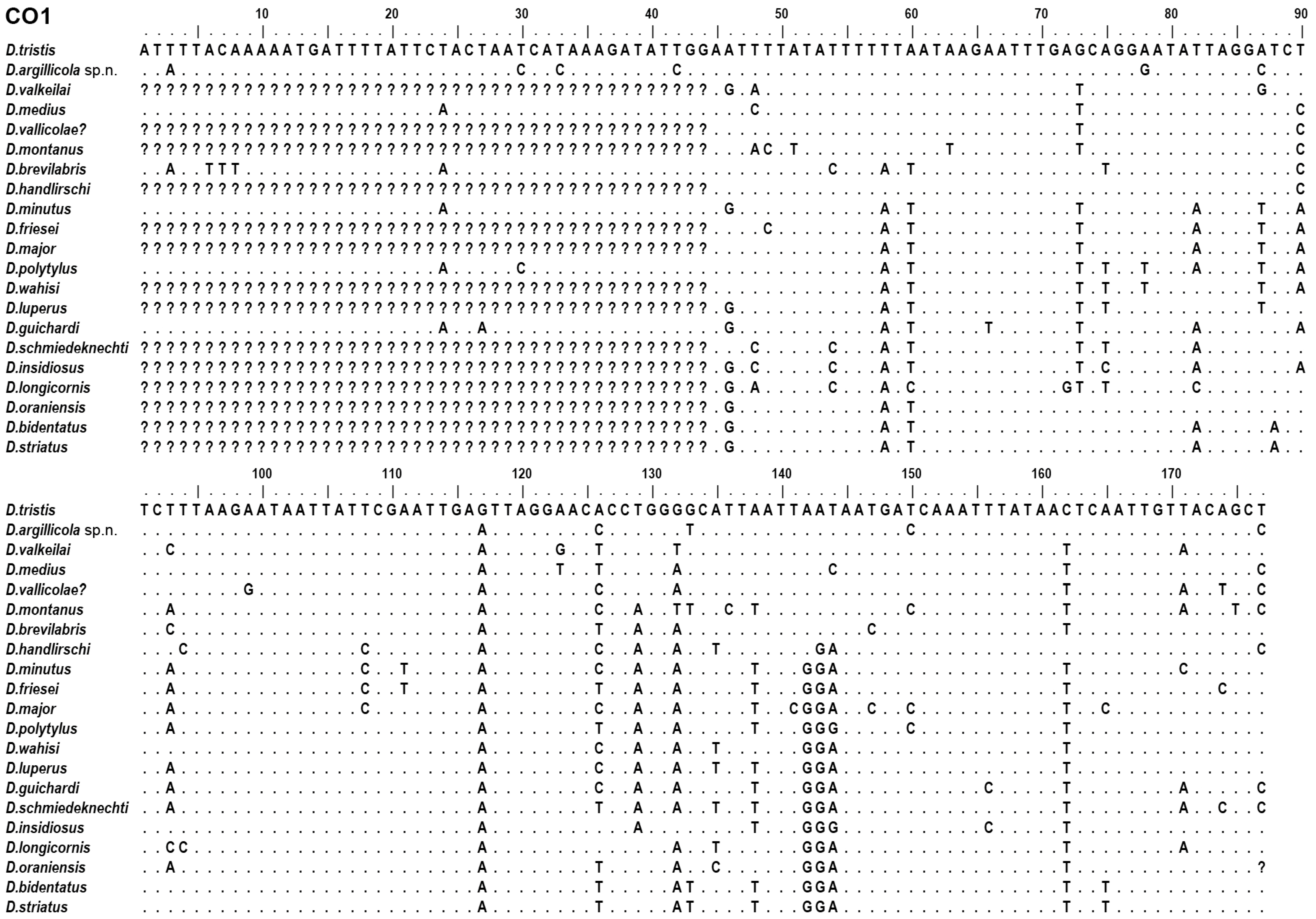

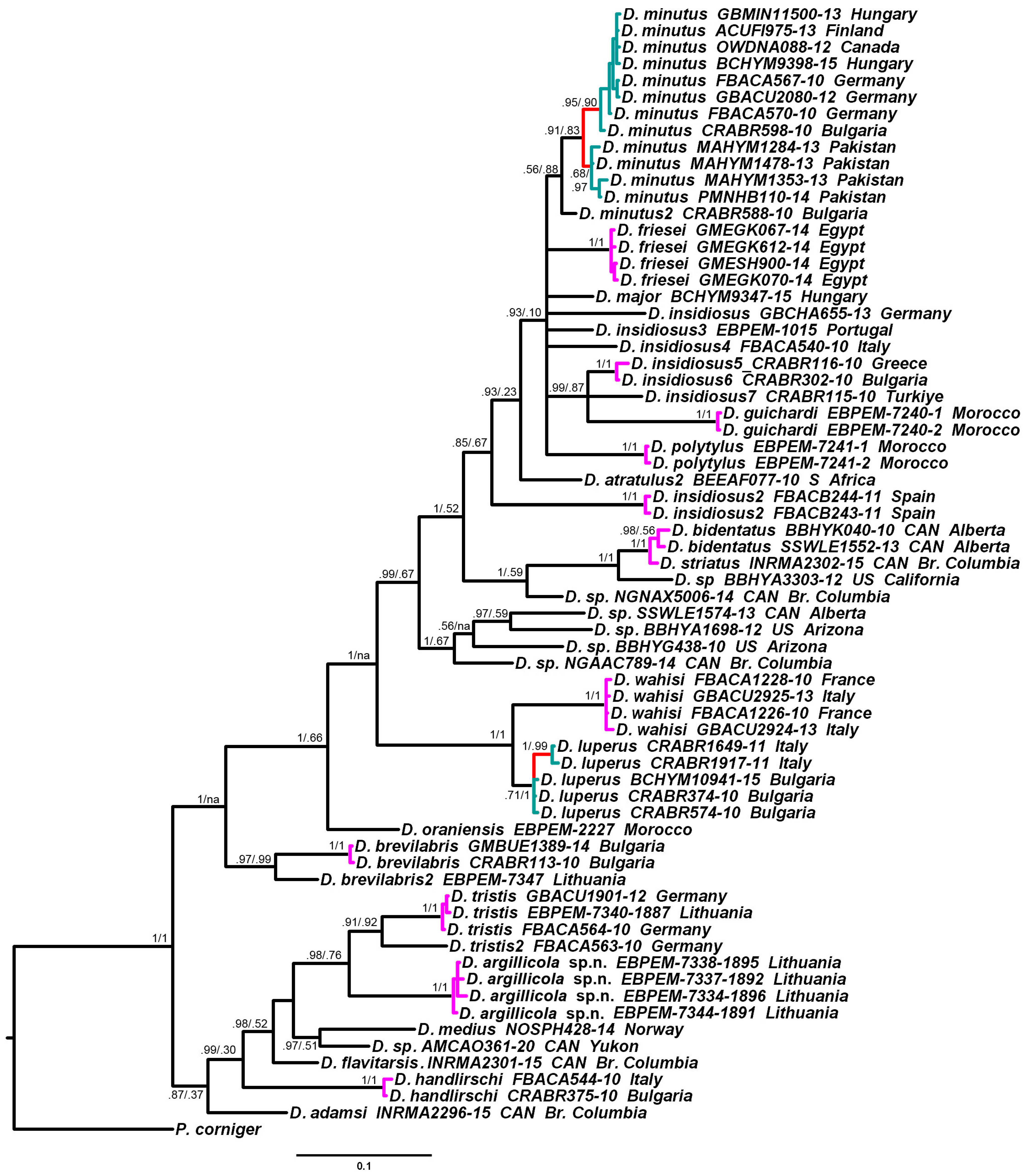
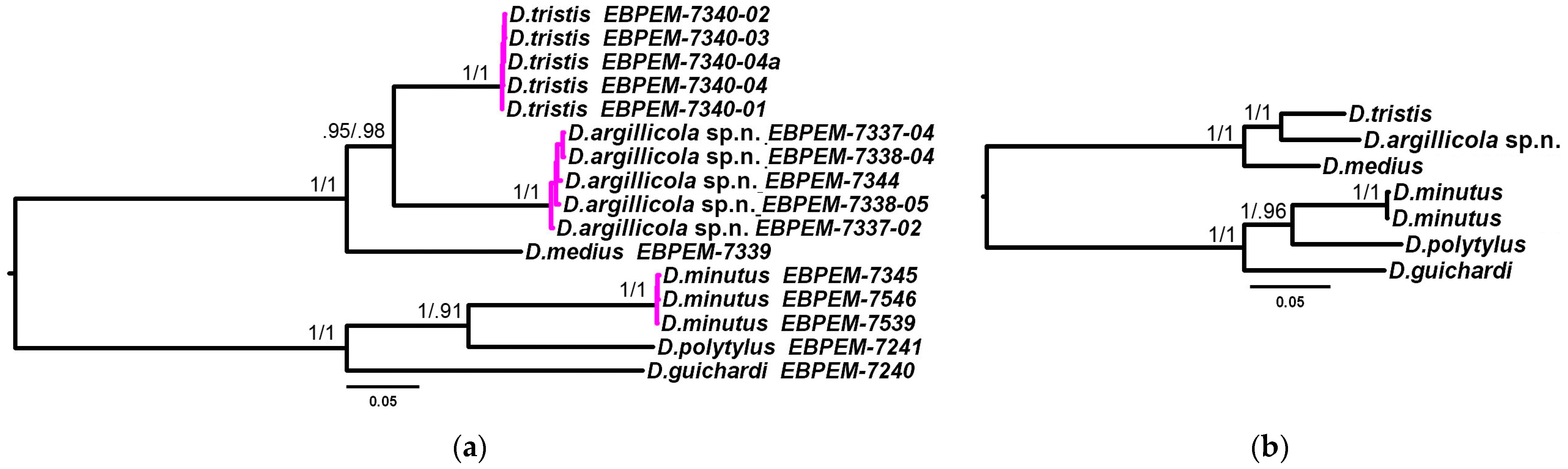
3.3. Endosymbiotic Bacteria Infestation
3.4. Diodontus argillicola Budrys, Orlovskytė & Budrienė, New Species
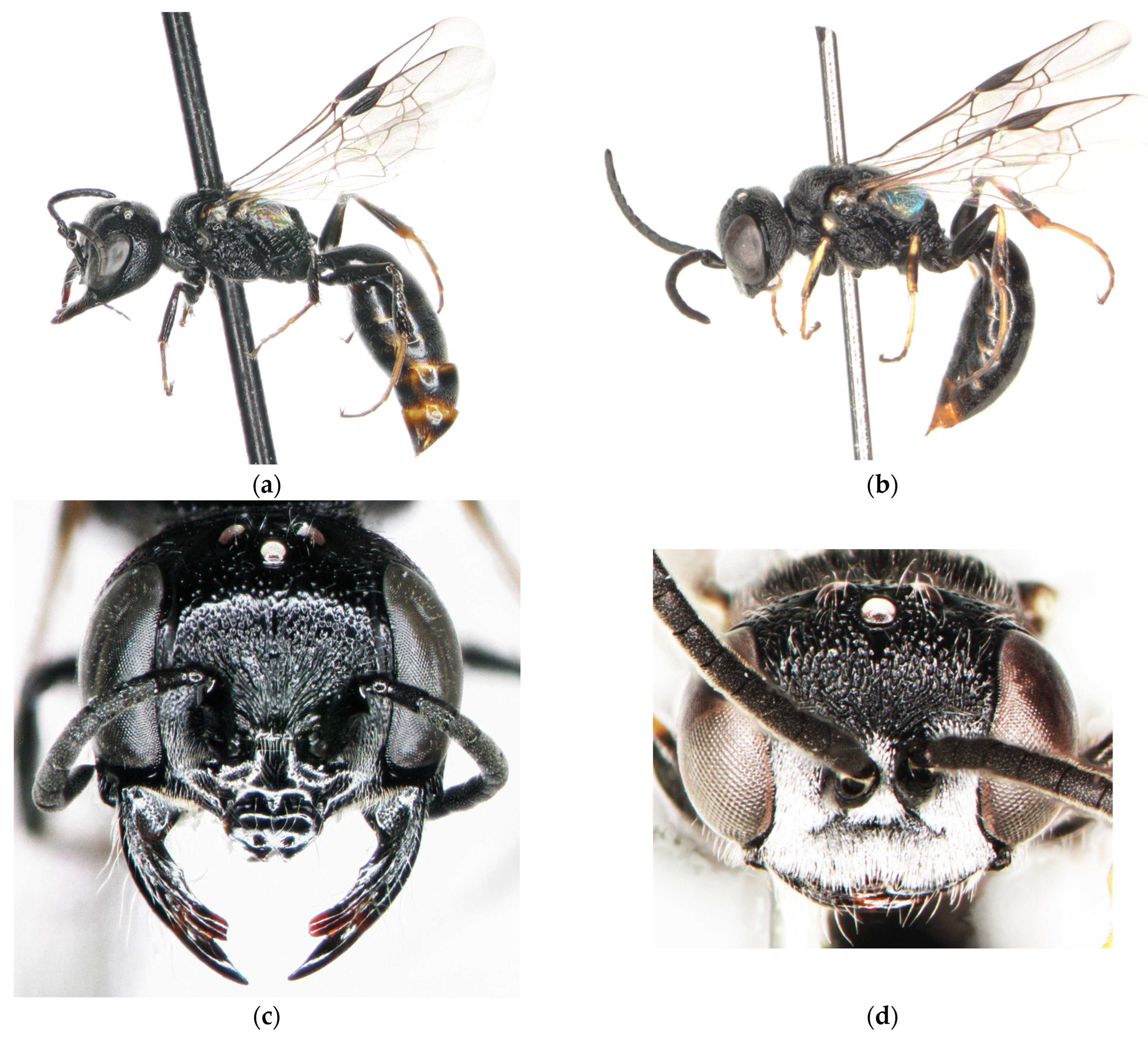
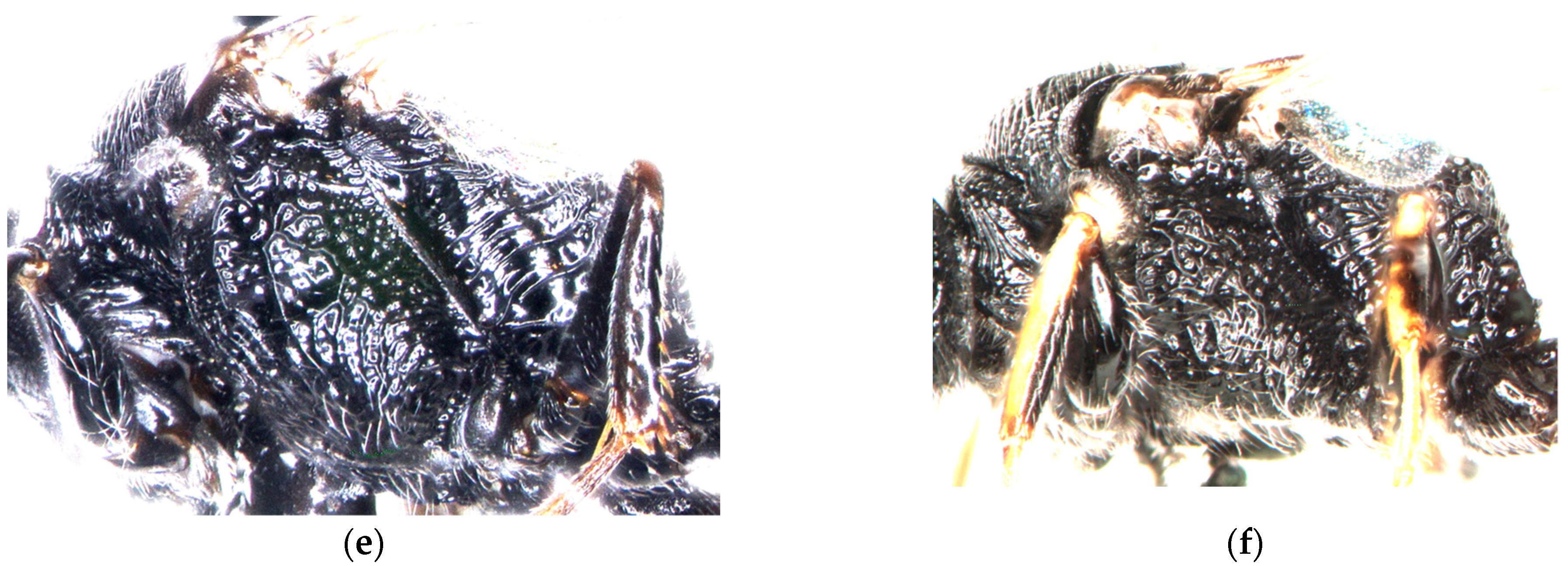
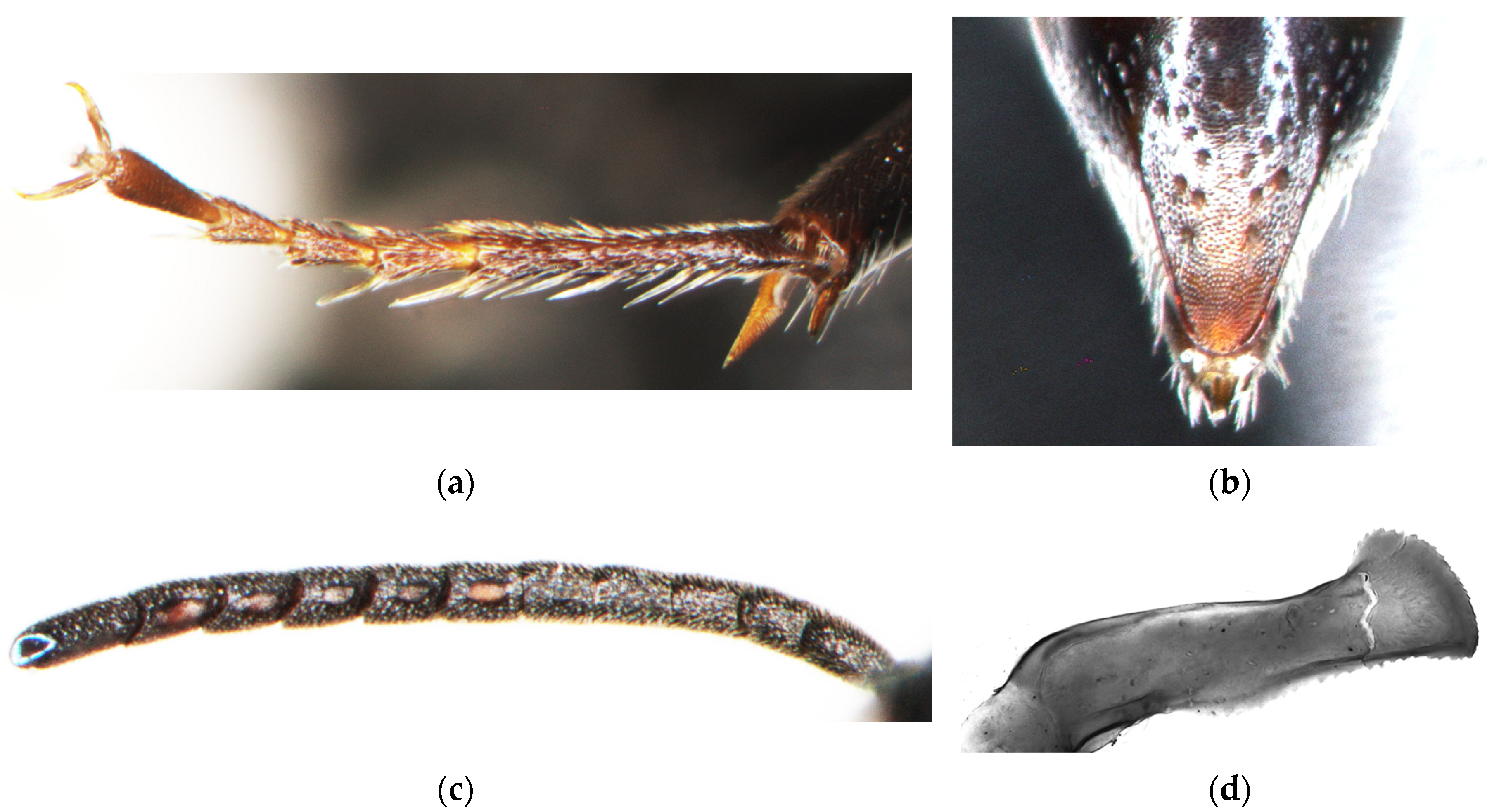

3.5. Key to the Palearctic Species of Diodontus tristis Species Group
- 1.
- In the male, tergum 6 has uniform pilosity and several longer preapical setae, without two distinct groups of thick spines. In the female, antenna, mandible, tegula, mid and hind tibiae are black or brown, without distinct yellow spots. In doubtful cases (in D. montanus, the tibiae are entirely pale brown), the face is very wide: the smallest distance between the eyes at the level of the antennal sockets is equal to the distance between the fore ocellus and the tip of the mid tooth of the clypeus; the distance between the lateral teeth of the clypeal apex is 1.4–2 times the length of the clypeus, and clearly larger than the distance between the lateral tooth and eye; the upper end of the frontal glands is flat or concave (D. tristis species group).........................................................................................................................................................................................................................2
- -
- In the male, tergum 6 has two preapical groups of small spines, which are clearly thicker than the surrounding pilosity. In the female, the combination of the listed characters is otherwise. If the mandibles and tibiae are dark, then at least one of the following is true: (a) the face is more rounded: the smallest distance between the eyes at the level of the antennal sockets is less than 0.85 times as wide as the distance between the fore ocellus and the tip of the mid tooth of the clypeus; and/or (b) distance between the lateral teeth of the clypeal apex is not larger than the distance between the lateral tooth and eye; and/or (c) the frontal gland is convex at the upper end.…………………………………………………………………………………………..........................……...…..……...…(other species groups)
- 2.
- The frons and posterolateral part of the mesopleuron are smooth, they have sparse weak punctation and coriaceous interspaces. In the female, the pygidial plate is typically widely triangular, covering all the dorsal surface of tergum 6, without a distinct marginal rim; the frontal glands are narrow, with their upper part deeply impressed in relation to the oculo-ocellar area; the tibiae are brown to pale brown. In the male, the pilosity of the lower frons and clypeus is sparse and weakly shining, and it does not hide the surface of cuticle. (Mountains of Central Asia from SE Kazakhstan to Tajikistan) ................................................................................D. montanus Kazenas, 1992
- -
- The central part of the frons is strongly and densely punctate. The posterolateral part of the mesopleuron is obliquely or irregularly rugose or areolate. In the female, the pygidial plate is narrower, it is subelliptic with a distinct marginal rim; the upper part of the frontal gland is flat or weakly concave; the mid and hind tibiae are black or dark brown. In the male, the pilosity of the lower frons and clypeus is dense, silvery, and it hides the surface of the cuticle.……………………………………………….......………………………………….....……………...…………........…………………………....3
- 3.
- The scutum is finely and very densely punctate; the interspaces are mostly narrower than the punctures. In the female, the frontal gland is twice as wide as the space between the gland and the eye; all tibiae are laterally black and medially brown, without a yellow pattern. In the male, the tegula and spiracular lobe have ivory spots; the interspaces between the punctures on the oculo-ocellar area are coarsely granulose, matt. (Pan-palearctic boreal).....……………………………...............…….....…....…………..D. medius Dahlbom, 1844
- -
- The scutum has more or less scattered punctation, particularly in the female; at least medially, the interspaces are wider than the punctures. In the female, the frontal gland is as wide as the space between the gland and eye. In the male, the tegula and spiracular lobe are black (D. valkeilai and D. asiaticus); if they are with ivory spots (most of D. tristis and D. argillicola), then the interspaces between the punctures on the oculo-ocellar area are alutaceous and shiny......………………………………….....……………..............................................................................................................................................4
- 4.
- The upper frons has dense erect setosity; the setae are longer than the width of the ocellus. In the female, the fore tibia anteriorly has a yellow strip. The face is very wide: the smallest distance between the eyes at the level of the antennal sockets is equal to the distance between the fore ocellus and the tip of the mid tooth of the clypeus. The body length is 8 mm in females and 6.5 mm in males. (Siberia: Chita oblast)......………………………………….....……………..............................................................................................D. valkeilai Budrys, 1992
- -
- The upper frons has sparser subappressed setosity; the setae are shorter than the width of the ocellus. In the female, the fore tibia is without a yellow strip; the face is narrower: the smallest distance between the eyes at the level of the antennal sockets is 0.8–0.95 as long as distance between the fore ocellus and the tip of the mid tooth of the clypeus. The body length is typically up to 7 mm in females and up to 6 mm in males.......………………………………….....……………............................................................................................................................................5
- 5.
- The oculo-ocellar area has scattered punctation: the interspaces are twice as wide as the punctures in females and 1.5 times as wide as the punctures in males. The posterolateral part of the mesopleuron is more or less regularly obliquely striate. In the female, the upper frons has moderately dense punctation, the interspaces are approximately as wide as the punctures and smooth. In the male, the tegula and spiracular lobe are black, while the hind tibia is black with a yellow base. (Mongolia)........................D. asiaticus Tsuneki, 1972
- -
- The oculo-ocellar area has dense punctation: the interspaces are as wide as the punctures or smaller. The posterolateral part of the mesopleuron is more irregularly rugose-areolate. In the female, the upper frons is very densely rugulose-punctate: the interspaces are smaller than the punctures. In the male, the tegula and spiracular lobe are usually (but not always) with ivory spots, and the hind tibia is either black with a yellow base or with more or less extended pale brown colouration antero-medially. (The following two species may be securely separated only using molecular characters)......………………………………….....……………...................................................................................................................................6
- 6.
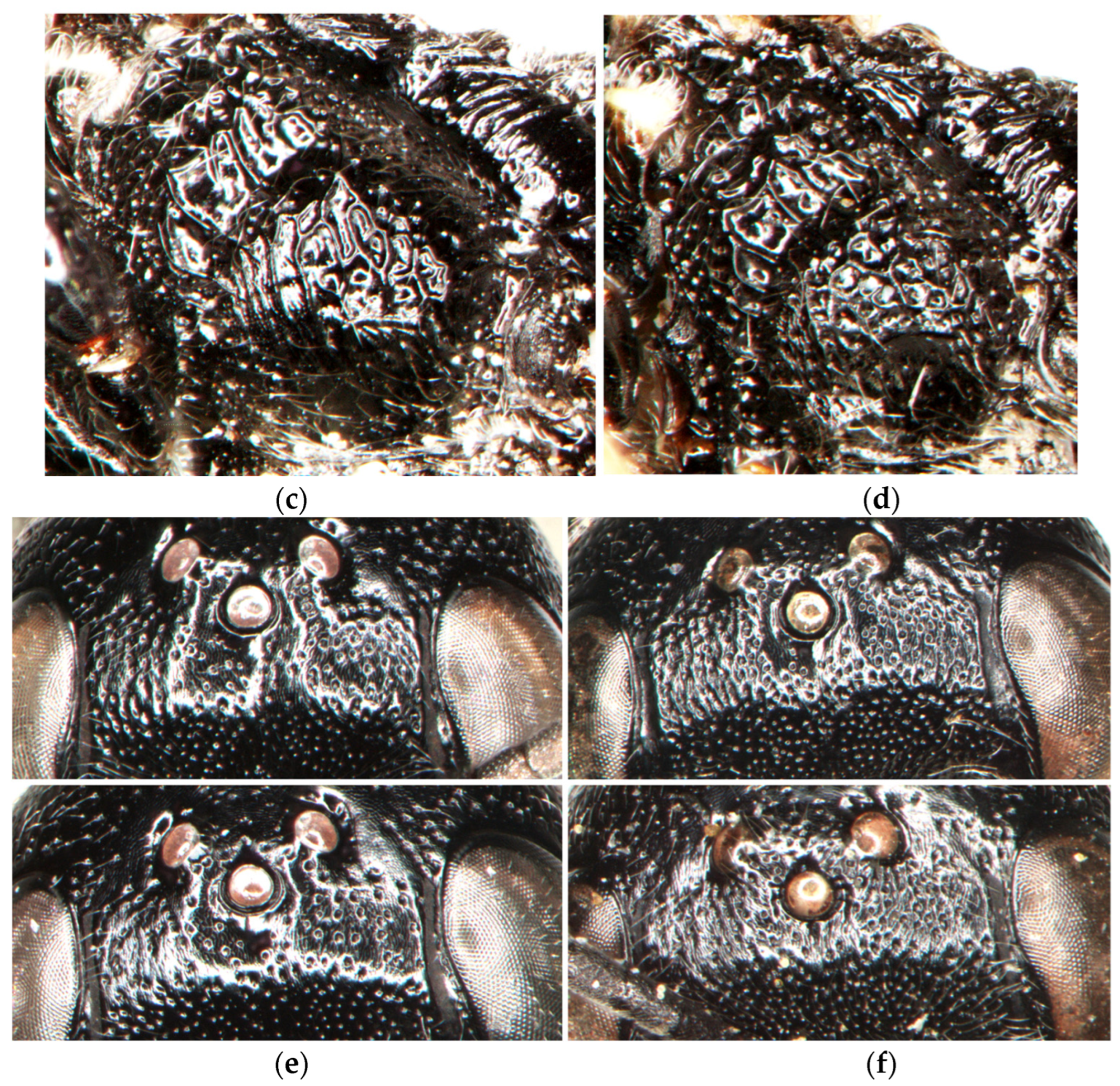
- The posterolateral part of the mesopleuron is more regularly rugose (Figure 8a,b). In the female, the dorsal declivous part of the upper frons between the fore ocellus and anterior vertical surface is slightly longer than the width of the fore ocellus; the area between the fore ocellus and frontal gland is smoother, shinier, and more sparsely punctate; its interspaces are mostly wider than or equal to the punctures (Figure 8e). …………………………......…………………………......………………….......................………......D. argillicola, sp. nov.
- -
- The posterolateral part of the mesopleuron is more irregularly areolate (Figure 8c,d). In the female, the dorsal declivous part of the upper frons between the fore ocellus and anterior vertical surface is not longer than the width of the fore ocellus; the area between the fore ocellus and frontal gland is more distinctly undose, less shiny, and more densely punctate; its interspaces are mostly narrower than the punctures (Figure 8f). (Western Palearctic, southern Siberia, Mongolia).........................................D. tristis (Vander Linden, 1829)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loxdale, H.D.; Davis, B.J.; Davis, R.A. Known knowns and unknowns in biology. Biol. J. Linn. Soc. 2016, 117, 386–398. [Google Scholar] [CrossRef]
- Gokhman, V.E. Integrative taxonomy and its implications for species-level systematics of parasitoid Hymenoptera. Entomol. Rev. 2018, 98, 834–864. [Google Scholar] [CrossRef]
- Fišer, C.; Robinson, C.T.; Malard, F. Cryptic species as a window into the paradigm shift of the species concept. Mol. Ecol. 2018, 7, 613–635. [Google Scholar] [CrossRef]
- Struck, T.H.; Feder, J.L.; Bendiksby, M.; Birkeland, S.; Cerca, J.; Gusarov, V.I.; Kistenich, S.; Larsson, K.-H.; Liow, L.H.; Nowak, M.D.; et al. Finding evolutionary processes hidden in cryptic species. Trends Ecol. Evol. 2018, 33, 153–163. [Google Scholar] [CrossRef]
- Kress, W.J.; Garcia-Robledo, C.; Uriarte, M.; Erickson, D.L. DNA barcodes for ecology, evolution, and conservation. Trends Ecol. Evol. 2015, 30, 25–35. [Google Scholar] [CrossRef]
- DeSalle, R.; Goldstein, P. Review and interpretation of trends in DNA barcoding. Front. Ecol. Evol. 2019, 7, 302. [Google Scholar] [CrossRef]
- Lukhtanov, V.A. Species delimitation and analysis of cryptic species diversity in the XXI century. Entomol. Rev. 2019, 99, 463–472. [Google Scholar] [CrossRef]
- Schlick-Steiner, B.C.; Steiner, F.M.; Seifert, B.; Stauffer, C.; Christian, E.; Crozier, R.H. Integrative taxonomy: A multisource approach to exploring biodiversity. Annu. Rev. Entomol. 2010, 55, 421–438. [Google Scholar] [CrossRef]
- Lecocq, T.; Dellicour, S.; Michez, D.; Dehon, M.; Dewulf, A.; De Meulemeester, T.; Brasero, N.; Valterová, I.; Rasplus, J.-Y.; Rasmont, P. Methods for species delimitation in bumblebees (Hymenoptera, Apidae, Bombus): Towards an integrative approach. Zool. Scr. 2015, 44, 281–297. [Google Scholar] [CrossRef]
- Zamani, A.; Dal Pos, D.; Fric, Z.F.; Orfinger, A.B.; Scherz, M.D.; Bartoňová, A.S.; Gante, H.F. The future of zoological taxonomy is integrative, not minimalist. Syst. Biodivers. 2022, 20, 2063964. [Google Scholar] [CrossRef]
- Wilson, J.S.; Clark, S.L.; Williams, K.A.; Pitts, J.P. Historical biogeography of the arid-adapted velvet ant Sphaeropthalma arota (Hymenoptera: Mutillidae) reveals cryptic species. J. Biogeogr. 2012, 39, 336–352. [Google Scholar] [CrossRef]
- Kurushima, H.; Yoshimura, J.; Kim, J.-K.; Kim, J.-K.; Nishimoto, Y.N.; Sayama, K.; Kato, M.; Watanabe, K.; Hasegawa, E.; Roff, D.A.; et al. Co-occurrence of ecologically equivalent cryptic species of spider wasps. R. Soc. Open Sci. 2016, 3, 160119. [Google Scholar] [CrossRef]
- Orlovskytė, S.; Budrys, E.; Budrienė, A.; Radzevičiūtė, R.; Soon, V. Sibling species in the Chrysis ignita complex: Molecular, morphological and trophic differentiation of Baltic species, with a description of two new cryptic species (Hymenoptera: Chrysididae). Syst. Entomol. 2016, 41, 771–793. [Google Scholar] [CrossRef]
- Darwell, C.T.; Cook, J.M. Cryptic diversity in a fig wasp community—Morphologically differentiated species are sympatric but cryptic species are parapatric. Mol. Ecol. 2017, 26, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Somavilla, A.; Barroso, P.C.S.; Aragão, M.; Mateus, S.; Menezes, R.S.T. An integrative taxonomic and phylogenetic approach reveals a new Neotropical swarm-founding social wasp, Pseudopolybia cryptica sp. n. (Vespidae: Polistinae: Epiponini). Arthropod Syst. Phylogeny 2021, 79, 25–35. [Google Scholar] [CrossRef]
- Rudoy, A.; Zhu, C.-D.; Ferrari, R.R.; Zhang, Y.-Z. Integrative taxonomy based on morphometric and molecular data supports recognition of the three cryptic species within the Encyrtus sasakii complex (Hymenoptera, Encyrtidae). J. Hymenopt. Res. 2022, 90, 129–152. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Sheikh, S.I.; Ward, A.K.G.; Forbes, A.A.; Prior, K.M.; Stone, G.N.; Gates, M.W.; Egan, S.P.; Zhang, L.; Davis, C.; et al. Delimiting the cryptic diversity and host preferences of Sycophila parasitoid wasps associated with oak galls using phylogenomic data. Mol. Ecol. 2022, 31, 4417–4433. [Google Scholar] [CrossRef]
- Sann, M.; Niehuis, O.; Peters, R.S.; Mayer, C.; Kozlov, A.; Podsiadlowski, L.; Bank, S.; Meusemann, K.; Misof, B.; Bleidorn, C.; et al. Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evol. Biol. 2018, 18, 71. [Google Scholar] [CrossRef]
- Pulawski, W.J. Catalog of Sphecidae sensu lato (= Apoidea Excluding Apidae). 2018. Available online: https://www.calacademy.org/scientists/projects/catalog-of-sphecidae (accessed on 10 November 2023).
- Kazenas, V.L. Fauna and Biology of Sphecid Wasps (Hymenoptera, Sphecidae) of Kazakhstan and Central Asia; Kazgos INTI: Almaty, Kazakhstan, 2001; Available online: https://zool.kz/wp-content/uploads/2020/10/kazenas_2001-1.pdf (accessed on 10 November 2023). (In Russian)
- O’Neill, K. Solitary Wasps: Behavior and Natural History; Cornell University Press: Ithaca, NY, USA, 2001; ISBN 0-8014-3721-0. [Google Scholar]
- Biddinger, D.J.; Surcică, A.; Joshi, N.K. A native predator utilizing the invasive brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae) as a food source. Biocontrol Sci. Technol. 2017, 27, 903–907. [Google Scholar] [CrossRef]
- Bashir, N.H.; Yue, D.; Jiang, H.; Ma, L.; Li, Q. Taxonomic study of the subtribe Pemphredonina Dahlbom, 1835 (Hymenoptera: Crabronidae) with a new species and six new records from China. J. Asia Pac. Entomol. 2021, 24, 1055–1065. [Google Scholar] [CrossRef]
- Noël, G.; Van Keymeulen, V.; Barbier, Y.; Smets, S.; Van Damme, O.; Colinet, G.; Ruelle, J.; Francis, F. Nest aggregations of wild bees and apoid wasps in urban pavements: A “street life” to be promoted in urban planning. bioRxiv 2021. [Google Scholar] [CrossRef]
- Vanoye-Eligio, M.; Horta-Vega, J.V.; Vanoye-Eligio, V.; Estrada-Ramírez, L.J.; Martí-Canché, B.R. Crabronidae (Hymenoptera: Apoidea) in Mexico: Occurrence and potential uses in biological control. Rev. Colomb. Entomol. 2022, 48, e11654. [Google Scholar] [CrossRef]
- Poláček, R. The occurrence of Hymenoptera: Aculeata on mine spoil heaps after shale extraction (Jakartovice, Nízký Jeseník Mts). Acta Mus. Sil. Sci. Nat. 2020, 69, 55–63. [Google Scholar] [CrossRef]
- Olszewski, P.; Ljubomirov, T.; Wiśniowski, B.; Kowalczyk, J.K.; Krzyżyński, M. New records of the genus Diodontus Curtis, 1834 (Hymenoptera: Crabronidae) from Bulgaria, Montenegro and Poland, with a key to central and eastern European species. Zootaxa 2016, 4061, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Budrys, E.; Budrienė, A.; Orlovskytė, S.; Soon, V. Two new species of Diodontus (Hymenoptera: Pemphredonidae) from the western Mediterranean and their phylogenetic relationships. Can. Entomol. 2019, 151, 558–583. [Google Scholar] [CrossRef]
- Park, E.; Poulin, R. Extremely divergent COI sequences within an amphipod species complex: A possible role for endosymbionts? Ecol. Evol. 2022, 12, e9448. [Google Scholar] [CrossRef] [PubMed]
- Hardin, G. The competitive exclusion principle. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef]
- Pigot, A.L.; Tobias, J.A. Species interactions constrain geographic range expansion over evolutionary time. Ecol. Lett. 2013, 16, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Vodă, R.; Dapporto, L.; Dincă, V.; Vila, R. Cryptic matters: Overlooked species generate most butterfly beta-diversity. Ecography 2015, 38, 405–409. [Google Scholar] [CrossRef]
- Leibold, M.A. Similarity and local co-existence of species in regional biotas. Evol. Ecol. 1998, 12, 95–110. [Google Scholar] [CrossRef]
- Wellborn, G.A.; Cothran, R.D. Niche diversity in crustacean cryptic species: Complementarity in spatial distribution and predation risk. Oecologia 2007, 154, 175–183. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150249. [Google Scholar] [CrossRef]
- Feldhaar, H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Engelstädter, J.; Hurst, G.D.D. The ecology and evolution of microbes that manipulate host reproduction. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 127–149. [Google Scholar] [CrossRef]
- Brucker, R.M.; Bordenstein, S.R. Speciation by symbiosis. Trends Ecol. Evol. 2012, 27, 443–451. [Google Scholar] [CrossRef]
- Cariou, M.; Duret, L.; Charlat, S. The global impact of Wolbachia on mitochondrial diversity and evolution. J. Evol. Biol. 2017, 30, 2204–2210. [Google Scholar] [CrossRef]
- Kaur, R.; Shropshire, J.D.; Cross, K.L.; Leigh, B.; Mansueto, A.J.; Stewart, V.; Bordenstein, S.R.; Bordenstein, S.R. Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host Microbe 2020, 20, 879–893. [Google Scholar] [CrossRef]
- Ramalho, M.D.O.; Kim, Z.; Wang, S.; Moreau, C.S. Wolbachia across social insects: Patterns and implications. Ann. Entomol. Soc. Am. 2021, 114, 206–218. [Google Scholar] [CrossRef]
- Budrys, E. Morphometric similarity and summary of measurements of Palearctic species of the genus Diodontus Curtis (Hymenoptera, Sphecidae). In Lietuvos Entomologų Darbai (Lietuvos Entomologų Draugijos 30-Mečiui); Jonaitis, V., Ed.; Lithuanian Entomological Society; Institute of Ecology: Vilnius, Lithuania, 1996; pp. 35–47. [Google Scholar]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Niehuis, O. Evolutionary history of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Wheeler, D. GenBank. Nucleic Acids Res. 2005, 33, D34–D38. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The barcode of life data system (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed]
- Budrys, E.; Orlovskytė, S.; Lazauskaitė, M.; Budrienė, A. Ancistrocerus wasps (Hymenoptera, Vespidae) from the Centre of Europe: Phylogeny, cryptic species, neutral and non-neutral markers. Zool. Scr. 2023, 52, 454–474. [Google Scholar] [CrossRef]
- Stunžėnas, V.; Petkevičiūtė, R.; Stanevičiūtė, G. Phylogeny of Sphaerium solidum (Bivalvia) based on karyotype and sequences of 16S and ITS1 rDNA. Cent. Eur. J. Biol. 2011, 6, 105–117. [Google Scholar] [CrossRef]
- Tusnády, G.E.; Simon, I.; Váradi, A.; Arányi, T. BiSearch: Primer-design and search tool for PCR on bisulfite-treated genomes. Nucleic Acids Res. 2005, 33, e9. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 5–98. [Google Scholar]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.04. 2015. Available online: https://mesquiteproject.org (accessed on 10 November 2022).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Pons, J.; Barraclough, T.G.; Gomez-Zurita, J.; Cardoso, A.; Duran, D.P.; Hazell, S.; Kamoun, S.; Sumlin, W.D.; Vogler, A.P. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 2006, 55, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2020, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, M.J.; Janzen, D.; Hallwachs, W.; Chapman, E.G.; Smith, M.A.; Dapkey, T.; Brown, A.; Ratnasingham, S.; Naik, S.; Manjunath, R.; et al. Minimalist revision and description of 403 new species in 11 subfamilies of Costa Rican braconid parasitoid wasps, including host records for 219 species. ZooKeys 2021, 1013, 1–665. [Google Scholar] [CrossRef] [PubMed]
- Amit, L.; Ben-Shlomo, R.; Chiel, E. Are microbial symbionts involved in the speciation of the gall-inducing aphid, Slavum wertheimae? Arthropod Plant Interact. 2017, 11, 475–484. [Google Scholar] [CrossRef]
- Devescovi, F.; Conte, C.A.; Augustinos, A.; Martinez, E.I.C.; Segura, D.F.; Caceres, C.; Lanzavecchia, S.B.; Bourtzis, K. Symbionts do not affect the mating incompatibility between the Brazilian-1 and Peruvian morphotypes of the Anastrepha fraterculus cryptic species complex. Sci. Rep. 2019, 9, 18319. [Google Scholar] [CrossRef] [PubMed]
- Andreason, S.A.; Shelby, E.A.; Moss, J.B.; Moore, P.J.; Moore, A.J.; Simmons, A.M. Whitefly endosymbionts: Biology, evolution, and plant virus interactions. Insects 2020, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Andrianto, E.; Kasai, A. Wolbachia in black spiny whiteflies and their new parasitoid wasp in Japan: Evidence of the distinct infection status on Aleurocanthus camelliae cryptic species complex. Insects 2022, 13, 788. [Google Scholar] [CrossRef]
- Lin, N. Sequential hypermalaxation in the digger wasp Diodontus franclemonti, Krombein (Hymenoptera: Sphecidae). J. Kans. Entomol. Soc. 1978, 51, 235–238. [Google Scholar]
- Guo, J.; Hatt, S.; He, K.; Chen, J.; Francis, F.; Wang, Z. Nine facultative endosymbionts in aphids. A review. J. Asia-Pac. Entomol. 2017, 20, 794–801. [Google Scholar] [CrossRef]
- Srba, M.; Heneberg, P. Nesting habitat segregation between closely related terricolous sphecid species (Hymenoptera: Spheciformes): Key role of soil physical characteristics. J. Insect Conserv. 2012, 16, 557–570. [Google Scholar] [CrossRef]
- Waters, J.; Darvill, B.; Lye, G.; Goulson, D. Niche differentiation of a cryptic bumblebee complex in the Western Isles of Scotland. Insect Conserv. Divers. 2011, 4, 46–52. [Google Scholar] [CrossRef]
- Scriven, J.J.; Whitehorn, P.R.; Goulson, D.; Tinsley, M.C. Niche partitioning in a sympatric cryptic species complex. Ecol. Evol. 2016, 6, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Leys, M.; Keller, I.; Robinson, C.T.; Räsänen, K. Cryptic lineages of a common alpine mayfly show strong life-history divergence. Mol. Ecol. 2017, 26, 1670–1686. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, G.; Gueuning, M.; Genoud, D.; Frey, J.E.; Praz, C. Why are there so many species of mining bees (Hymenoptera, Andrenidae)? The possible roles of phenology and Wolbachia incompatibility in maintaining species boundaries in the Andrena proxima-complex. Syst. Entomol. 2023, 48, 127–141. [Google Scholar] [CrossRef]
- Heneberg, P. Flagship bird species habitat management supports the presence of ground-nesting aculeate hymenopterans. J. Insect Conserv. 2012, 16, 899–908. [Google Scholar] [CrossRef]
- Twerd, L.; Szefer, P.; Sobieraj-Betlińska, A.; Olszewski, P. The conservation value of Aculeata communities in sand quarries changes during ecological succession. Glob. Ecol. Conserv. 2021, 28, e01693. [Google Scholar] [CrossRef]

| Species | D. argillicola sp. nov. | D. tristis | D. medius | D. minutus |
|---|---|---|---|---|
| Collection place | 54°13′09″ N 23°24′13″ E | 55°52′39″ N 23°28′16″ E | 55°52′41″ N 23°28′08″ E | 55°35′32″ N 24°02′58″ E |
| Collection date | 13.vi.2015 | 12.vii.2015 | 12.vii.2015 | 03.vi.2017 |
| Voucher number | EBPEM-7338 | EBPEM-7340 | EBPEM-7339 | EBPEM-7546 |
| DNA markers: | ||||
| CO1–CO2–ATP8 | PP025829–35 | MK625008 | PP025835 | MK625005 |
| ND6–CytB–ND1 | PP025836–40 | MK757260 | PP025841 | MK757257 |
| 18S | PP059061 | MK640428 | PP059062 | MK640425 |
| 28S | PP059063 | MK640440 | PP059064 | MK640437 |
| ITS1 | PP059065 | MK640432 | PP059066 | MK640429 |
| PB | PP025842–43 | MK628914 | PP025844 | MK628911 |
| AbdB | PP025845 | MK628918 | PP025846 | MK628915 |
| ArgK | PP025847 | MK628922 | PP025848 | MK628919 |
| Ube2g1 | PP025849 | MK628926 | PP025850 | MK628923 |
| MRPP3 | PP025851 | MK634482 | PP025852 | MK634479 |
| PTCD2 | PP025853 | MK634478 | PP025854 | MK634475 |
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Measurement | Root 1 | Root 2 | Measurement | Root 1 | Root 2 | |
| WH | −10.85 | 22.37 | WH | −15.30 | 0.41 | |
| LID | 39.82 | 11.30 | LID | 21.09 | 39.24 | |
| UID | 6.06 | 19.43 | UID | −24.19 | −3.51 | |
| IMD | −36.18 | −7.64 | WCA | −20.32 | 11.00 | |
| WCA | 25.94 | 4.19 | POD | −27.62 | 8.22 | |
| WLA | −1.73 | 17.74 | OOD | 84.73 | −45.76 | |
| LM | −3.02 | −4.06 | WHO | 7.62 | −62.84 | |
| POD | −55.25 | −23.05 | LF | 3.19 | 16.15 | |
| OOD | 10.52 | −9.02 | LSC | −16.19 | −52.17 | |
| WHO | 32.81 | −68.44 | 3FL | 25.11 | 2.19 | |
| LV | −14.31 | −2.81 | L6F | −20.06 | 38.94 | |
| LF | 17.08 | −35.31 | W6F | 1.93 | −55.94 | |
| LCL | −33.64 | 31.40 | PRN | 7.61 | −7.89 | |
| LSC | 0.18 | −8.46 | COL | −5.92 | 5.09 | |
| 3FL | −4.39 | 23.45 | Constant | 2.83 | −1.22 | |
| PRN | 14.13 | −19.10 | ||||
| COL | −5.61 | 6.87 | ||||
| Constant | 2.73 | 6.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budrys, E.; Orlovskytė, S.; Budrienė, A. Ecological Speciation without Morphological Differentiation? A New Cryptic Species of Diodontus Curtis (Hymenoptera, Pemphredonidae) from the Centre of Europe. Insects 2024, 15, 86. https://doi.org/10.3390/insects15020086
Budrys E, Orlovskytė S, Budrienė A. Ecological Speciation without Morphological Differentiation? A New Cryptic Species of Diodontus Curtis (Hymenoptera, Pemphredonidae) from the Centre of Europe. Insects. 2024; 15(2):86. https://doi.org/10.3390/insects15020086
Chicago/Turabian StyleBudrys, Eduardas, Svetlana Orlovskytė, and Anna Budrienė. 2024. "Ecological Speciation without Morphological Differentiation? A New Cryptic Species of Diodontus Curtis (Hymenoptera, Pemphredonidae) from the Centre of Europe" Insects 15, no. 2: 86. https://doi.org/10.3390/insects15020086
APA StyleBudrys, E., Orlovskytė, S., & Budrienė, A. (2024). Ecological Speciation without Morphological Differentiation? A New Cryptic Species of Diodontus Curtis (Hymenoptera, Pemphredonidae) from the Centre of Europe. Insects, 15(2), 86. https://doi.org/10.3390/insects15020086






