Odonata Assemblages in Urban Semi-Natural Wetlands
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
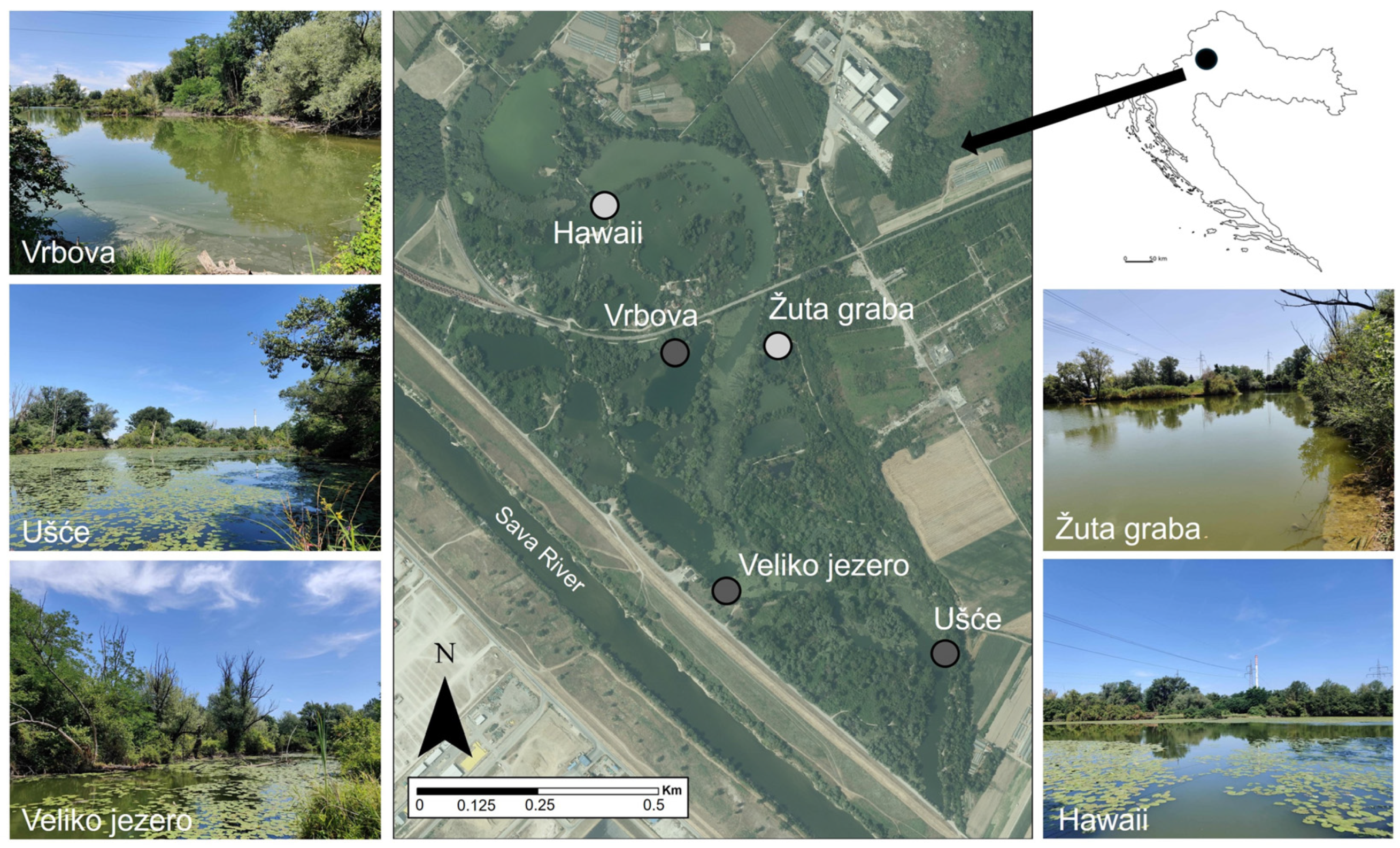
2.1. Study Area
2.2. Study Sites and Odonata Sampling
2.3. Environmental Variables
2.4. Data Analysis
3. Results
3.1. Environmental Variables
3.2. Odonata Species and Their Threat Level
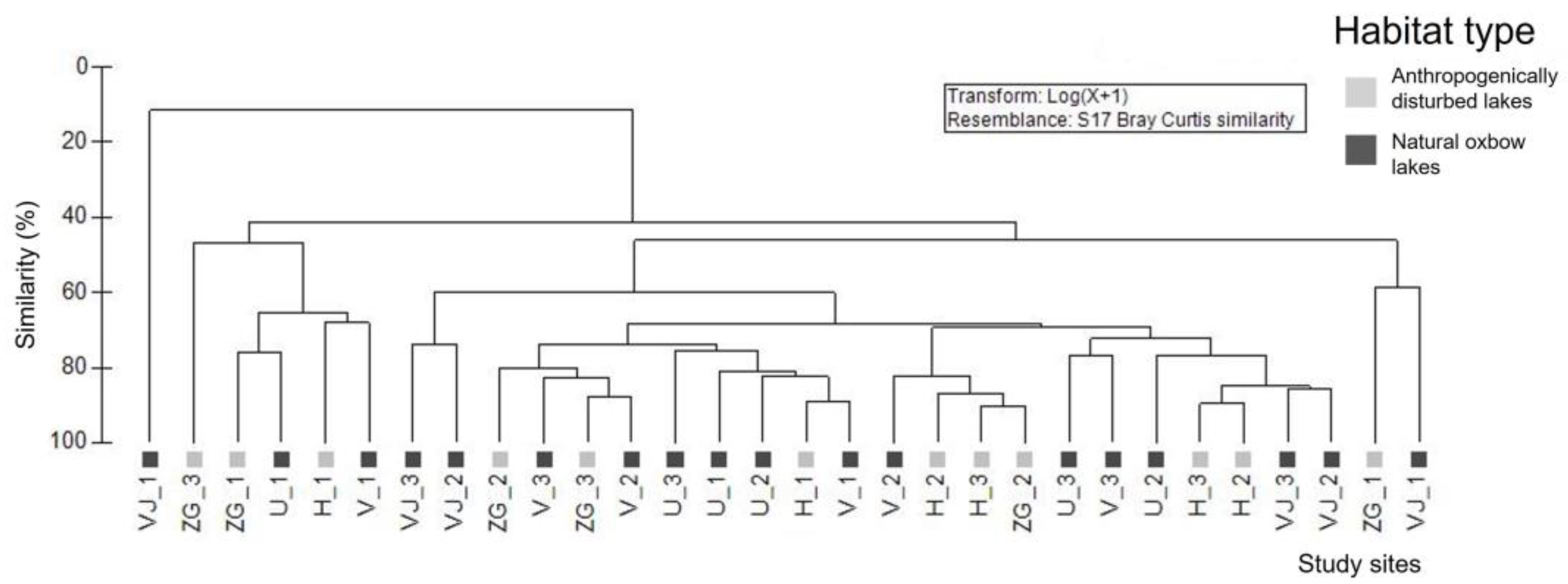
3.3. Odonata Assemblages and Their Conservation Value
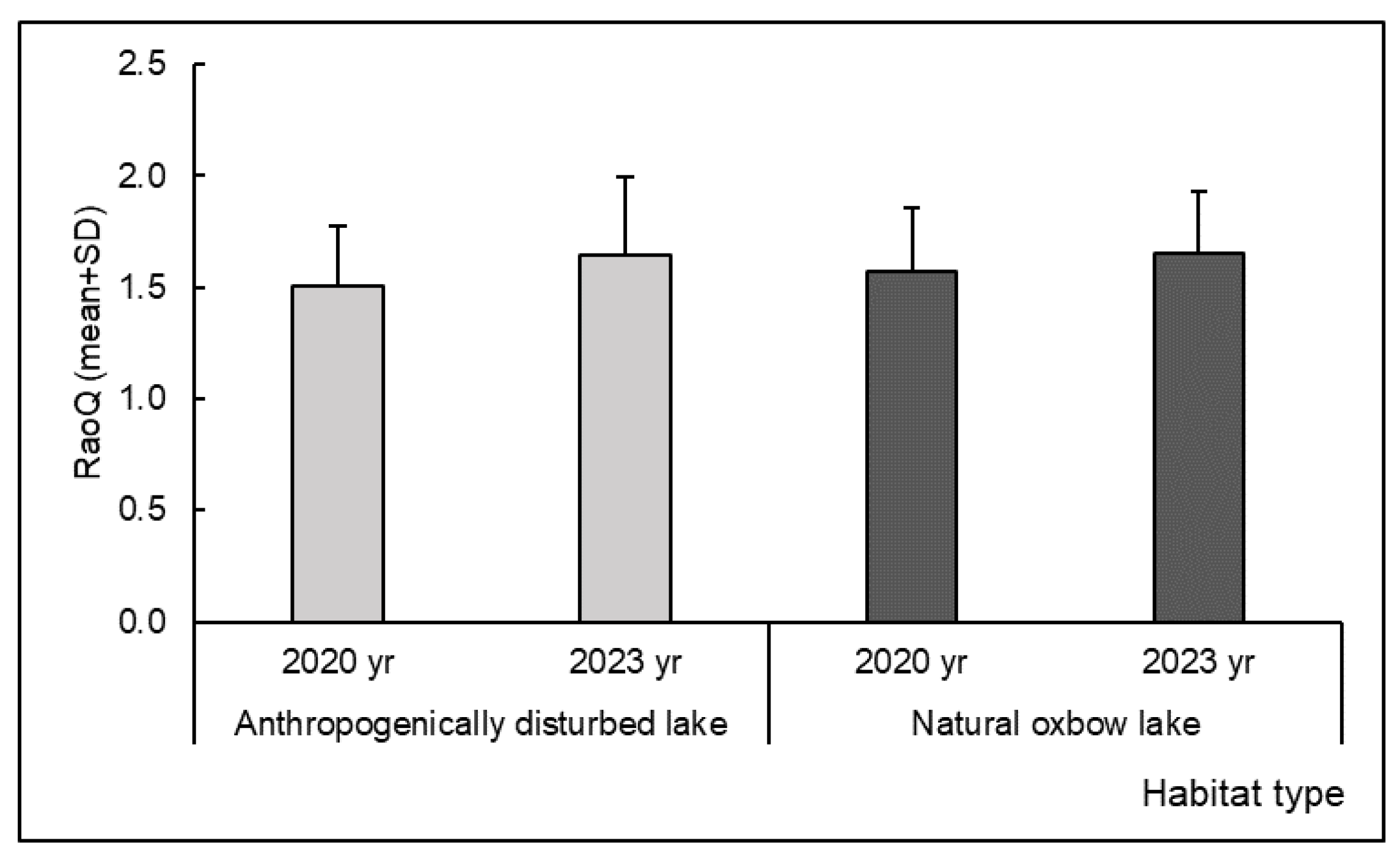
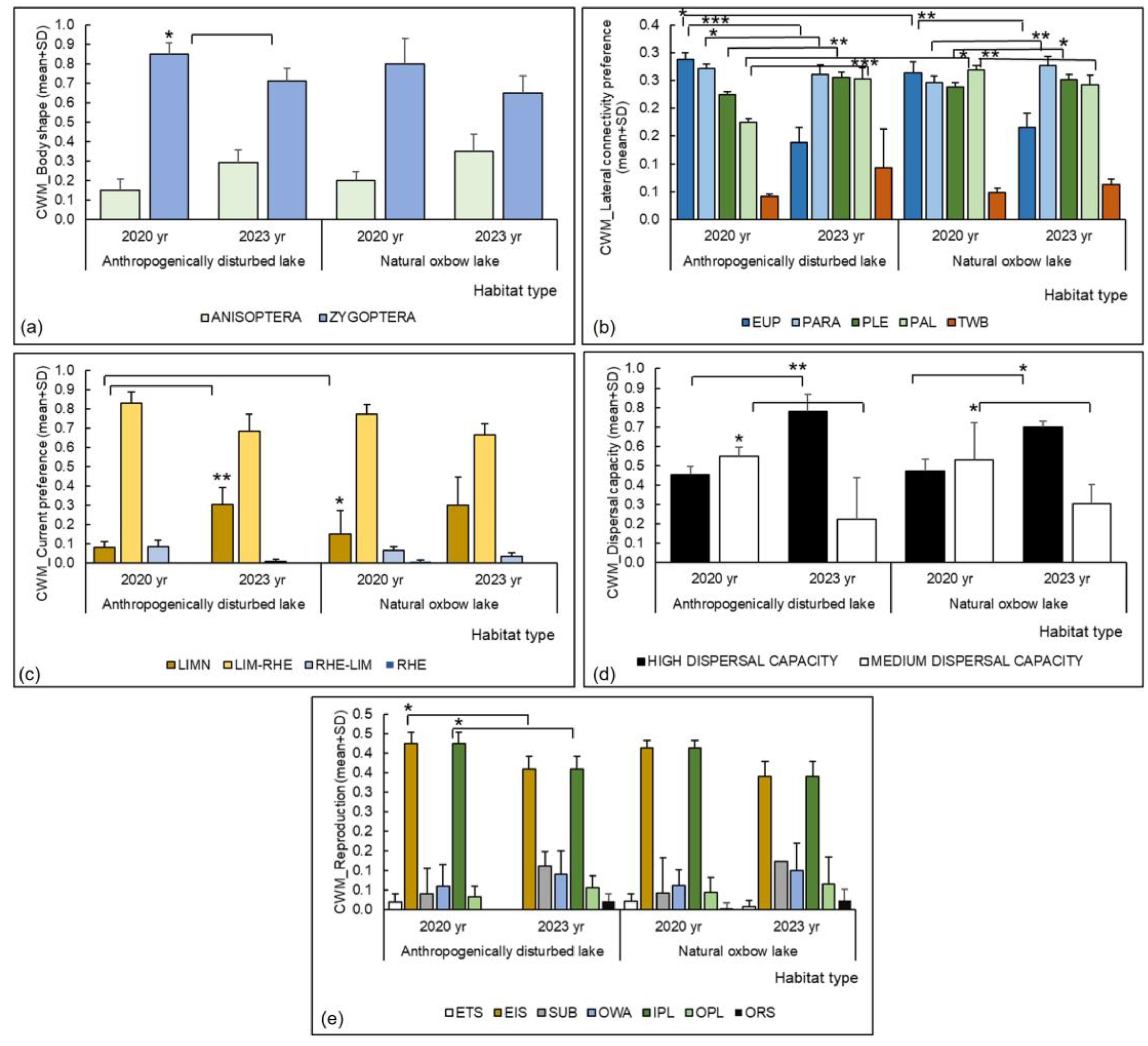
3.4. Functional Diversity of Odonata Assemblages
4. Discussion
4.1. Blue Zones in a City Have Great Potential to Function as Good Habitats for Odonata, but Time Combined with Climate Extremes Affects Their Functional Trait Representation
4.2. Implications for Conservation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Seto, K.C.; Guneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef]
- Ellis, E.C.; Klein Goldewijk, K.; Siebert, S.; Lightman, D.; Ramankutty, N. Anthropogenic transformation of the biomes, 1700 to 2000. Global Ecol. Biogeogr. 2010, 19, 589–606. [Google Scholar] [CrossRef]
- McDonald, R.I.; Kareiva, P.; Forman, R.T.T. The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol. Conserv. 2008, 141, 1695–1703. [Google Scholar] [CrossRef]
- Oertli, B.; Parris, K.M. Review: Toward management of urban ponds for freshwater biodiversity. Ecosphere 2019, 10, e02810. [Google Scholar] [CrossRef]
- Johnson, R.C.; Jin, H.; Carreiro, M.M.; Jack, J.D. Macroinvertebrate community structure, secondary production and trophic-level dynamics in urban streams affected by non-point-source pollution. Freshw. Biol. 2013, 58, 843–857. [Google Scholar] [CrossRef]
- Kim, H.H. Urban heat island. Int. J. Remote Sens. 1992, 13, 2319–2336. [Google Scholar] [CrossRef]
- McDonald, R.I. Global urbanization: Can ecologists identify a sustainable way forward? Front. Ecol. Environ. 2008, 6, 99–104. [Google Scholar] [CrossRef]
- Wilson, J.N.; Bekessy, S.; Parris, K.M.; Gordon, A.; Heard, G.W.; Wintle, B.A. Impacts of climate change and urban development on the spotted marsh frog (Limnodynastes tasmaniensis). Austral Ecol. 2013, 38, 11–22. [Google Scholar] [CrossRef]
- Collins, K.A.; Lawrence, T.J.; Stander, E.K.; Jontos, R.J.; Kaushal, S.S.; Newcomer, T.A.; Grimm, N.B.; Ekberg, M.L.C. Opportunities and challenges for managing nitrogen in urban stormwater: A review and synthesis. Ecol. Eng. 2010, 36, 1507–1519. [Google Scholar] [CrossRef]
- Vincent, J.; Kirkwood, A.E. Variability of water quality, metals and phytoplankton community structure in urban stormwater ponds along a vegetation gradient. Urban Ecosyst. 2014, 17, 839–853. [Google Scholar] [CrossRef]
- Peretyatko, A.; Teissier, S.; De Backer, S.; Triest, L. Assessment of the risk of cyanobacterial bloom occurrence in urban ponds: Probabilistic approach. Ann. Limnol. 2010, 46, 121–133. [Google Scholar] [CrossRef]
- Naselli-Flores, L. Urban Lakes: Ecosystems at Risk, Worthy of the Best Care. In Proceedings of the Taal 2007: The 12th World Lake Conference, Jaipur, India, 28 October–2 November 2007; pp. 1333–1337. [Google Scholar]
- Mahler, B.J.; Van Metre, P.C.; Callender, E. Trends in metals in urban and reference lake sediments across the United States, 1970–2001. Environ. Toxicol. Chem. 2006, 25, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Urbanization, biodiversity, and conservation. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Noble, A.; Hassal, C. Poor ecological quality of urban ponds in northern England: Causes and consequences. Urban Ecosyst. 2014, 18, 649–662. [Google Scholar] [CrossRef]
- Thornhill, I.; Batty, L.; Death, R.G.; Friberg, N.R.; Ledger, M.E. Local and landscape scale determinants of macroinvertebrate assemblages and their conservation value in ponds across an urban land-use gradient. Biodivers. Conserv. 2017, 26, 1065–1086. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Villalobos-Jiménez, G.; Perron, M.A.C.; Sahlén, G.; Assandri, G.; Vilenica, M.; Calvão Batista, L.; Juen, L.; Cerini, F.; Bried, J.T. Odonata assemblages in human-modified landscapes. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research, 2nd ed.; Cordoba-Aguilar, A., Beatty, C., Bried, J., Eds.; Oxford Academic: Oxford, UK, 2023; pp. 247–260. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.F.; Alexander, L.C.; Lamp, W.O. Dispersal by terrestrial stages of stream insects in urban watersheds: A synthesis of current knowledge. J. N. Am. Benthol. Soc. 2009, 28, 1022–1037. [Google Scholar] [CrossRef]
- Mackintosh, T.J.; Davis, J.A.; Thompson, R.M. The influence of urbanisation on macroinvertebrate biodiversity in constructed stormwater wetlands. Sci. Total Environ. 2015, 536, 527–537. [Google Scholar] [CrossRef]
- Hill, M.J.; Biggs, J.; Thornhill, I.; Briers, R.A.; Gledhill, D.G.; White, J.C.; Wood, P.J.; Hassall, C. Urban ponds as an aquatic biodiversity resource in modified landscapes. Glob. Chang. Biol. 2017, 23, 986–999. [Google Scholar] [CrossRef]
- Hannon, E.R.; Hafernik, J.E. Reintroduction of the rare damselfly Ischnura gemina (Odonata: Coenagrionidae) into an urban California park. J. Insect Conserv. 2007, 11, 141–149. [Google Scholar] [CrossRef]
- Goertzen, D.; Suhling, F. Central European cities maintain substantial dragonfly species richness—A chance for biodiversity conservation? Insect Conserv. Diver. 2015, 8, 238–246. [Google Scholar] [CrossRef]
- Hale, R.; Coleman, R.; Pettigrove, V.; Swearer, S.E. Review: Identifying, preventing and mitigating ecological traps to improve the management of urban aquatic ecosystems. J. Appl. Ecol. 2015, 52, 928–939. [Google Scholar] [CrossRef]
- Samways, M.J.; Steytler, N.S. Dragonfly (Odonata) distribution patterns in urban and forest landscapes, and recommendations for riparian management. Biol. Conserv. 1996, 78, 279–288. [Google Scholar] [CrossRef]
- Oertli, B. The use of dragonflies in the assessment and monitoring of aquatic habitats. In Dragonflies and Damselflies; Cordoba-Aguilar, A., Ed.; Oxford University Press: Oxford, UK, 1990; pp. 79–95. [Google Scholar]
- Smith, J.; Samways, M.J.; Taylor, S. Assessing riparian quality using two complementary sets of bioindicators. Biodivers. Conserv. 2007, 16, 2695–2713. [Google Scholar] [CrossRef]
- Boudot, J.P.; Kalkman, V.J. Atlas of the European Dragonflies and Damselflies; KNNV Uitgeverij: Zeist, The Netherlands, 2015. [Google Scholar]
- Bried, J.T.; Samways, M.J. A review of odonatology in freshwater applied ecology and conservation science. Freshw. Sci. 2015, 34, 1023–1031. [Google Scholar] [CrossRef]
- Dolný, A.; Harabiš, F.; Bárta, D.; Lhota, S.; Drozd, P. Aquatic insects indicate terrestrial habitat degradation: Changes in taxonomical structure and functional diversity of dragonflies in tropical rainforest of East Kalimantan. Trop. Zool. 2012, 25, 141–157. [Google Scholar] [CrossRef]
- Kutcher, T.E.; Bried, J.T. Adult Odonata conservatism as an indicator of freshwater wetland condition. Ecol. Indic. 2014, 38, 31–39. [Google Scholar] [CrossRef]
- Jeanmougin, M.; Leprieur, F.; Lois, G.; Clergeau, P. Fine-scale urbanization affects Odonata species diversity in ponds of a megacity (Paris, France). Acta Oecol. 2014, 59, 26–34. [Google Scholar] [CrossRef]
- Villalobos-Jiménez, G.; Hassall, C. Effects of the urban heat island on the phenology of Odonata in London, UK. Int. J. Biometeorol. 2017, 61, 1337–1346. [Google Scholar] [CrossRef]
- Perron, M.A.C.; Richmond, I.C.; Pick, F.R. Plants, water quality and land cover as drivers of Odonata assemblages in urban ponds. Sci. Total Environ. 2021, 773, 145467. [Google Scholar] [CrossRef]
- Alegro, A.; Bogdanović, S.; Rešetnik, I.; Boršić, I.; Cigić, P.; Nikolić, T. Flora of the seminatural marshland Savica, part of the (sub)urban flora of the city of Zagreb (Croatia). Nat. Croat. 2013, 22, 111–134. [Google Scholar]
- Bišćan, M.; Kos, R.; Jerman Vranić, M.; Kovačić, G.; Horvatić Viduka, E.; Jelavić, V.; Abrashi, A.; Marković, K.; Stanec, D.; Malbaša, H.; et al. Elaborat Zaštite Okoliša. Zahvat: Mala Hidroelektrana te-to Zagreb; EKONERG—Institut za Energetiku i Zaštitu Okolišad.o.o: Zagreb, Croatia, 2019. [Google Scholar]
- Fanjek, I.; Grabundžija, M.; Kelemen Pepeonik, V.; Šiško, D.; Šterk, R.; Vojnić Rogić, I.; Dulčić, A.; Ninić, V. Prostorni Plan Područja Posebnih Obilježja, Priobalje Save (Krajobraz uz Savu—Savski Park); Gradski Zavod za Prostorno Uređenje; Odsjek za Zaštitu Okoliša: Zagreb, Croatia, 2006. [Google Scholar]
- Tvrtković, N.; Vuković, M.; Pavlinić, I.; Šašić, M.; Mihoci, I.; Grbac, I.; Godec, Z. Inventarizacija Odabrane Faune Savice sa Zonacijom Prostora. Prilog za Izradu Prostornog Plana Područja Posebnih Obilježja Priobalje Save (Krajobraz uz Savu–Savski Park). I. Etapa–Savica. Zoološki Odjel Hrvatskog Prirodoslovnog Muzeja: Zagreb, Croatia, 2007. [Google Scholar]
- APHA. Standard Method for the Examination of Water and Wastewater, 16th ed.; American Public Health Association: Washington, DC, USA, 1985. [Google Scholar]
- Deutsches Institut für Normung. Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung, 16th ed.; Verlag Chemie: Weinheim, Germany, 1986; Volume II. [Google Scholar]
- Sertić Perić, M.; Matoničkin Kepčija, R.; Miliša, M.; Gottstein, S.; Lajtner, J.; Dragun, Z.; Filipović Marijić, V.; Krasnići, N.; Ivanković, D.; Erk, M. Benthos-drift relationships as proxies for the detection of the most suitable bioindicator taxa in flowing waters—A pilot-study within a Mediterranean karst river. Ecotoxicol. Environ. Saf. 2018, 163, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual/Tutorial; Plymouth: Auckland, New Zealand, 2006. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 27.0; IBM: Armonk, NY, USA, 2020. [Google Scholar]
- Samways, M.J.; Taylor, S. Impacts of Invasive Alien Plants on Red-Listed South African Dragonflies (Odonata). S. Afr. J. Sci. 2004, 100, 78–81. [Google Scholar]
- Kotarac, M.; Šalamun, A.; Vilenica, M. EU Natura 2000 Integration Project: Field Research and Laboratory Processing for Collecting New Inventory Data for Taxonomic Groups: Actinopterygii and Cephalaspidomorphi, Amphibia and Reptilia, Aves, Chiroptera, Decapoda, Lepidoptera, Odonata, Plecoptera–Final Report for the Taxonomic Group Odonata; Ministry of Environmental and Nature Protection: Zagreb, Croatia, 2016.
- Belančić, A.; Bogdanović, T.; Franković, M.; Ljuština, M.; Mihoković, N.; Vitas, B. Red Data Book of Dragonflies of Croatia; Ministry of Culture, State Institute for Nature Protection: Zagreb, Croatia, 2008.
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Kloiber, A.; Hering, D. www.freshwaterecology.info—An online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecol. Indic. 2015, 53, 271–282. [Google Scholar] [CrossRef]
- Dijkstra, K.-D.B.; Wildermuth, H.; Martens, A. Dataset “Odonata” the Taxa and Autecology Database for Freshwater Organisms, Version 8.0. 2024. Available online: www.freshwaterecology.info (accessed on 20 January 2024).
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Micro-Computer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; SAGE Publications: London, UK, 2009. [Google Scholar]
- Oram, B. Nitrates and Nitrites in Drinking Water; Groundwater and Surface Waters; Water Research Center 2014. Available online: https://water-research.net/index.php/nitrate (accessed on 20 December 2023).
- Wetzel, R.G.; Likens, G.E. Limnological Analysis, 2nd ed.; Springer Verlag: New York, NY, USA, 1991. [Google Scholar]
- Perron, M.A.C.; Pick, F.R. Stormwater ponds as habitat for Odonata in urban areas: The importance of obligate wetland plant species. Biodivers. Conserv. 2019, 29, 913–931. [Google Scholar] [CrossRef]
- Askew, R.R. The Dragonflies of Europe, 2nd ed.; Harley Books: Essex, UK, 2004. [Google Scholar]
- Dolný, A.; Ožana, S.; Burda, M.; Harabiš, F. Effects of landscape patterns and their changes to species richness, species composition, and the conservation value of Odonates (Insecta). Insects 2021, 12, 478. [Google Scholar] [CrossRef]
- Garrido-Perez, J.M.; Sergio, M.; Vicente-Serrano, S.M.; Barriopedro, D.; García-Herrera, R.; Trigo, R.; Beguería, S. Examining the outstanding Euro-Mediterranean drought of 2021–2022 and its historical context. J. Hydrol. 2024, 630, 130653. [Google Scholar] [CrossRef]
- Battaglioli, F.; Groenemeijer, P.; Púčik, T.; Taszarek, M.; Ulbrich, U.; Rust, H. Modelled multidecadal trends of lightning and (very) large hail in Europe and North America (1950–2021). J. Appl. Meteorol. Climatol. 2023, 62, 1627–1653. [Google Scholar] [CrossRef]
- Jakob, C.; Suhling, F. Risky Times? Mortality During Emergence in Two Species of Dragonflies (Odonata: Gomphidae, Libellulidae). Aquat. Insects 1999, 21, 1–10. [Google Scholar] [CrossRef]
- Hassall, C.; Thompson, D.J. The effects of environmental warming on Odonata: A review. Int. J. Odonatol. 2008, 11, 131–153. [Google Scholar] [CrossRef]
- Silva, L.; Diego, F.R.; Castro, M.P.; Juen, L.; Callisto, M.; Hughes, R.M.; Hermes, M.G. Functional responses of Odonata larvae to human disturbances in neotropical savanna headwater streams. Ecol. Indic. 2021, 133, 108367. [Google Scholar] [CrossRef]
- Willigalla, C.; Fartmann, T. Patterns in the diversity of dragonflies (Odonata) in cities across Central Europe. Eur. J. Entomol. 2012, 109, 235–245. [Google Scholar] [CrossRef]
- Crabot, J.; Mauchamp, A.; Bergerot, B.; Bonis, A.; Gore, O.; Rossignol, N.; Paillisson, J.M. How hydrology and landscape shape Odonata assemblages in marshlands crossed by ditches. Freshw. Biol. 2022, 67, 1228–1241. [Google Scholar] [CrossRef]
- Holtmann, L.; Juchem, M.; Brüggeshemke, J.; Möhlmeyer, A.; Fartmann, T. Stormwater ponds promote dragonfly (Odonata) species richness and density in urban areas. Ecol. Eng. 2018, 118, 1–11. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behavior and Ecology of Odonata; Harley Books: Colchester, UK, 2004. [Google Scholar]
- Harabiš, F.; Dolný, A. The effects of ecological determinants on the dispersal abilities of central European dragonflies (Odonata). Odonatologica 2011, 40, 17–26. [Google Scholar]
- Hof, C.; Brändle, M.; Brandl, R. Lentic odonates have larger and more northern ranges than lotic species. J. Biogeogr. 2006, 33, 63–70. [Google Scholar] [CrossRef]
- Bota-Sierra, C.A.; García-Robledo, C.; Escobar, F.; Novelo-Gutiérrez, R.; Londoño, G.A. Environment, taxonomy and mor-phology constrain insect thermal physiology along tropical mountains. Funct. Ecol. 2022, 36, 1924–1935. [Google Scholar] [CrossRef]
- Olsen, K.; Svenning, J.-C.; Balslev, H. Climate change is driving shifts in dragonfly species richness across Europe via differential dynamics of taxonomic and biogeographic groups. Diversity 2022, 14, 1066. [Google Scholar] [CrossRef]
- Vilenica, M.; Mičetić Stanković, V.; Franković, M. Dragonfly fauna (Insecta, Odonata) in the Turopolje region (Croatia). Nat. Croat. 2011, 20, 141–158. [Google Scholar]
- Wildermuth, H. Visual and tactile stimuli in choice of oviposition substrates by the dragonfy Perithemis mooma Kirby (Anisoptera: Libellulidae). Odonatologica 1992, 21, 309–321. [Google Scholar]
- Dijkstra, K.-D.B.; Lewington, R. Field Guide to the Dragonflies of Britain and Europe, 1st ed.; British Wildlife Publishing: Gillingham, UK, 2006. [Google Scholar]
| Physico-Chemical Water Parameter | Anthropogenically Disturbed Lake | Natural Oxbow Lake | F | p | d.f. |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Water temperature (°C) | 29.79 ± 1.87 | 27.40 ± 2.66 | 0.683 | 0.413 | 1 |
| Oxygen saturation (%) | 124.94 ± 16.13 | 97.24 ± 22.99 | 4.566 | 0.038 | 1 |
| pH | 7.81 ± 0.12 | 7.65 ± 0.27 | 0.976 | 0.329 | 1 |
| Conductivity (µS/cm) | 344 ± 19 | 315 ± 16 | 0.757 | 0.389 | 1 |
| Water hardness (mg CaCO3/L) | 188.82 ± 11.13 | 198.58 ± 10.48 | 2.009 | 0.164 | 1 |
| Chemical oxygen demand (mg O2/L) | 5.06 ± 0.90 | 4.14 ± 0.41 | 0.188 | 0.667 | 1 |
| Nitrate concentration (mg N/L) | 0.379 ± 0.122 | 0.086 ± 0.031 | 1.227 | 0.274 | 1 |
| Nitrite concentration (mg N/L) | 0.014 ± 0.002 | 0.005 ± 0.0003 | 941.837 | p < 0.001 | 1 |
| Species | Anthropogenically Disturbed Lakes | Natural Oxbow Lakes |
|---|---|---|
| Calopteryx splendens (Harris, 1782) | 0.2 | 0.2 |
| Lestes sponsa (Hansemann, 1823) | 0.1 | |
| Platycnemis pennipes (Pallas, 1771) | 23.1 | 26.5 |
| Ischnura elegans (Vander Linden, 1820) | 28.3 | 34.3 |
| Ischnura pumilio (Charpentier, 1825) | 0.7 | 0.1 |
| Erythromma viridulum (Charpentier, 1840) | 0.3 | 0.7 |
| Cordulia aenea (Linnaeus, 1758) | 0.1 | |
| Onychogomphus forcipatus (Linnaeus, 1758) | 0.1 | |
| Aeshna affinis (Vander Linden, 1820) | 0.1 | |
| Aeshna isoceles (Müller, 1767) | 0.1 | |
| Anax imperator (Leach, 1815) | 0.1 | 0.4 |
| Crocothemis erythraea (Brullé, 1832) | 0.5 | 1.1 |
| Libellula depressa (Linnaeus, 1758) | 0.2 | |
| Orthetrum albistylum (Selys, 1848) | 1.4 | 1.0 |
| Orthetrum brunneum (Fonscolombe, 1837) | 0.2 | 0.3 |
| Orthetrum cancellatum (Linnaeus, 1758) | 0.3 | 0.2 |
| Orthetrum coerulescens (Fabricius, 1798) | 0.8 | 0.7 |
| Sympetrum sanguineum (Müller, 1764) | 0.6 | 0.7 |
| Sympetrum striolatum (Charpentier, 1840) | 0.2 | 0.8 |
| Species richness (S) | 13.0 | 19.0 |
| Sum of all species abundances (mean, N) | 56.5 | 67.4 |
| Group: anthropogenically disturbed lakes | ||
| Average similarity: 54.29 | ||
| Species | Mean abundance per replicate | Similarity contribution within group (%) |
| Ischnura elegans | 2.97 | 56.66 |
| Platycnemis pennipes | 2.46 | 33.87 |
| Group: natural oxbow lakes | ||
| Average similarity: 55.14 | ||
| Species | Mean abundance per replicate | Similarity contribution within group (%) |
| Ischnura elegans | 3.18 | 52.69 |
| Platycnemis pennipes | 2.72 | 38.28 |
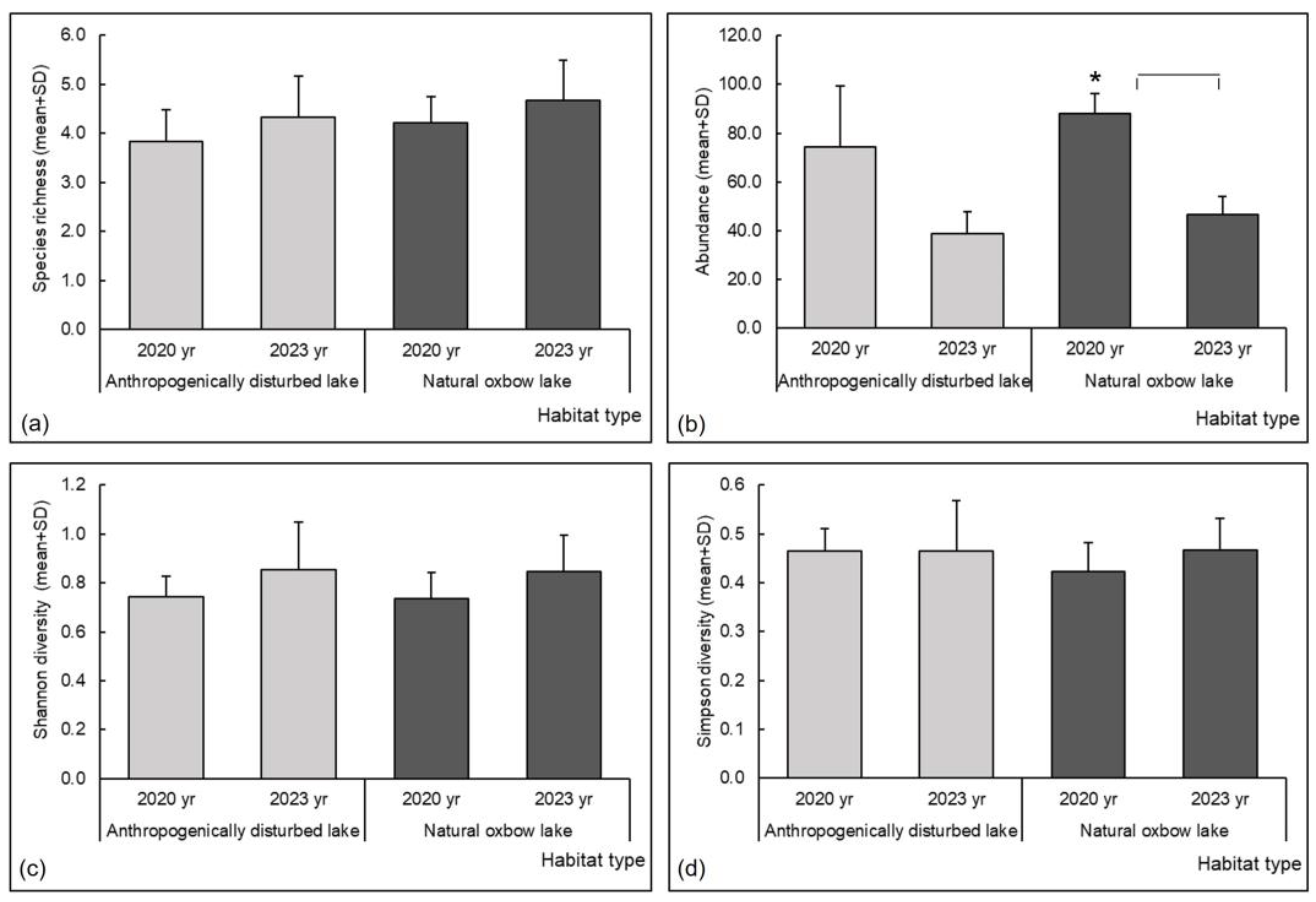
| Taxonomic Parameter | Year | Habitat Type Pairwise Contrast | F | p | d.f.1 | d.f.2 | t | p |
|---|---|---|---|---|---|---|---|---|
| Species richness (S) | 2020 | D-N | 0.259 | 0.853 | 3 | 26 | 0.000 | 1.000 |
| 2023 | D-N | 3 | 26 | 0.913 | 0.424 | |||
| 2020–2023 | D-D | 3 | 26 | 0.446 | 0.675 | |||
| 2020–2023 | N-N | 3 | 26 | 0.220 | 0.834 | |||
| Abundance (N) | 2020 | D-N | 3.317 | 0.035 | 3 | 26 | 0.417 | 0.642 |
| 2023 | D-N | 3 | 26 | 0.464 | 0.647 | |||
| 2020–2023 | D-D | 3 | 26 | 1.382 | 0.179 | |||
| 2020–2023 | N-N | 3 | 26 | 2.041 | 0.050 | |||
| Simpson diversity (1 − λ) | 2020 | D-N | 4.563 | 0.047 | 3 | 24 | 0.018 | 0.987 |
| 2023 | D-N | 3 | 24 | 0.286 | 0.803 | |||
| 2020–2023 | D-D | 3 | 24 | 2.344 | 0.108 | |||
| 2020–2023 | N-N | 3 | 24 | 2.426 | 0.095 | |||
| Shannon diversity (H′) | 2020 | D-N | 0.693 | 0.577 | 3 | 26 | 0.536 | 0.606 |
| 2023 | D-N | 3 | 26 | 0.486 | 0.639 | |||
| 2020–2023 | D-D | 3 | 24 | 1.470 | 0.155 | |||
| 2020–2023 | N-N | 3 | 24 | 0.310 | 0.155 | |||
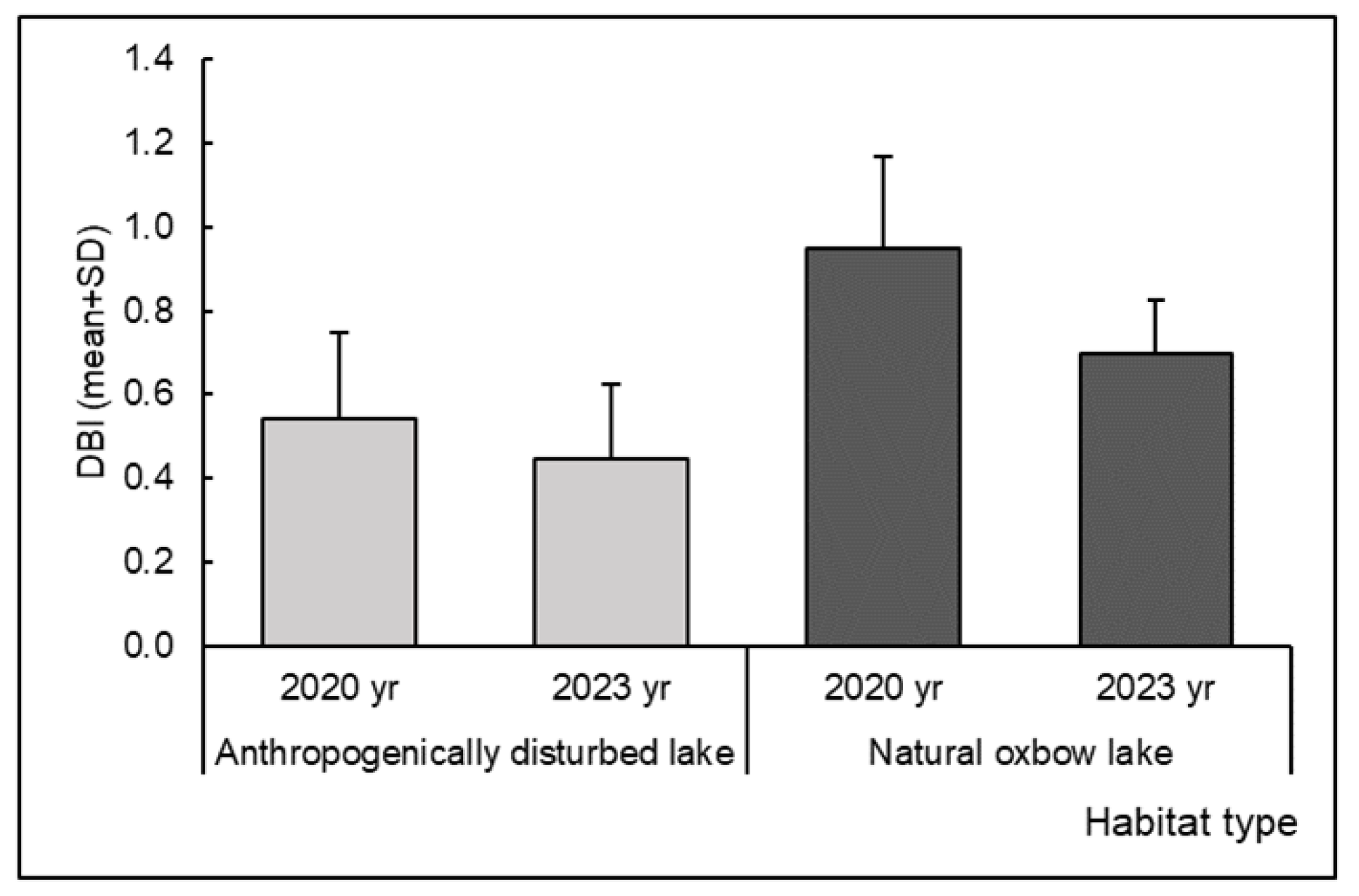
| Dragonfly Biotic Index (DBI) | 2020 | D-N | 4.385 | 0.045 | 3 | 26 | 0.903 | 0.392 |
| 2023 | D-N | 3 | 26 | 1.911 | 0.124 | |||
| 2020–2023 | D-D | 3 | 26 | 0.970 | 0.341 | |||
| 2020–2023 | N-N | 3 | 26 | 1.6699 | 0.376 |
| Functional Trait Group | Functional Trait | Year | Habitat Type Pairwise Contrast | F | p | d.f.1 | d.f.2 | t | p |
|---|---|---|---|---|---|---|---|---|---|
| Functional Diversity (RaoQ) | 2020 | D–N | 0.927 | 0.422 | 3 | 26 | 1.424 | 0.116 | |
| 2023 | D–N | 3 | 26 | 0.863 | 0.396 | ||||
| 2020–2023 | D–D | 3 | 26 | 1.078 | 0.291 | ||||
| 2020–2023 | N–N | 3 | 26 | 1.100 | 0.281 | ||||
| Body shape | Anisoptera | 2020 | D-N | 0.618 | 0.610 | 3 | 22 | 0.072 | 0.943 |
| 2023 | D-N | 3 | 22 | 0.771 | 0.449 | ||||
| 2020–2023 | D-D | 3 | 22 | 1.116 | 0.276 | ||||
| 2020–2023 | N-N | 3 | 22 | 0.497 | 0.624 | ||||
| Zygoptera | 2020 | D-N | 1.587 | 0.218 | 3 | 25 | 0.445 | 0.660 | |
| 2023 | D-N | 3 | 25 | 0.680 | 0.503 | ||||
| 2020–2023 | D-D | 3 | 25 | 2.005 | 0.050 | ||||
| 2020–2023 | N-N | 3 | 25 | 0.596 | 0.556 | ||||
| Lateral connectivity preference | eupotamon | 2020 | D-N | 7.736 | <0.001 | 3 | 26 | 2.117 | 0.044 |
| 2023 | D-N | 3 | 26 | 0.990 | 0.331 | ||||
| 2020–2023 | D-D | 3 | 26 | 5.044 | <0.001 | ||||
| 2020–2023 | N-N | 3 | 26 | 2.862 | 0.008 | ||||
| parapotamon | 2020 | D-N | 5.595 | 0.003 | 3 | 26 | 1.539 | 0.136 | |
| 2023 | D-N | 3 | 26 | 1.095 | 0.284 | ||||
| 2020–2023 | D-D | 3 | 26 | 2.088 | 0.047 | ||||
| 2020–2023 | N-N | 3 | 26 | 3.173 | 0.004 | ||||
| plesiopotamon (including lakes) | 2020 | D-N | 5.331 | 0.005 | 3 | 26 | 0.757 | 0.456 | |
| 2023 | D-N | 3 | 26 | 0.244 | 0.809 | ||||
| 2020–2023 | D-D | 3 | 26 | 3.197 | 0.004 | ||||
| 2020–2023 | N-N | 3 | 26 | 1.972 | 0.050 | ||||
| palaeopotamon (including pools, ponds) | 2020 | D-N | 16.677 | <0.001 | 3 | 26 | 2.013 | 0.050 | |
| 2023 | D-N | 3 | 26 | 0.005 | 0.996 | ||||
| 2020–2023 | D-D | 3 | 26 | 5.136 | <0.001 | ||||
| 2020–2023 | N-N | 3 | 26 | 3.486 | 0.002 | ||||
| temporary water bodies | 2020 | D-N | 2.282 | 0.104 | 3 | 25 | 1.031 | 0.313 | |
| 2023 | D-N | 3 | 25 | 1.722 | 0.097 | ||||
| 2020–2023 | D-D | 3 | 25 | 0.736 | 0.469 | ||||
| 2020–2023 | N-N | 3 | 25 | 1.574 | 0.128 | ||||
| Current preference | limnophilous | 2020 | D-N | 6.732 | 0.002 | 3 | 21 | 2.008 | 0.050 |
| 2023 | D-N | 3 | 21 | 0.815 | 0.424 | ||||
| 2020–2023 | D-D | 3 | 21 | 3.059 | 0.006 | ||||
| 2020–2023 | N-N | 3 | 21 | 1.827 | 0.082 | ||||
| limno- to rheophilous | 2020 | D-N | 1.827 | 0.167 | 3 | 26 | 1.422 | 0.167 | |
| 2023 | D-N | 3 | 26 | 0.467 | 0.644 | ||||
| 2020–2023 | D-D | 3 | 26 | 1.150 | 0.143 | ||||
| 2020–2023 | N-N | 3 | 26 | 0.426 | 0.673 | ||||
| rheo- to limnophilous | 2020 | D-N | 0.000 | 1.000 | 3 | 9 | 0.000 | 1.000 | |
| 2023 | D-N | 3 | 9 | 0.000 | 1.000 | ||||
| 2020–2023 | D-D | 3 | 9 | 0.000 | 1.000 | ||||
| 2020–2023 | N-N | 3 | 9 | 0.000 | 1.000 | ||||
| rheophilous | 2020 | D-N | 0.000 | - | - | - | - | ||
| 2023 | D-N | - | - | - | - | ||||
| 2020–2023 | D-D | - | - | - | - | ||||
| 2020–2023 | N-N | - | - | - | - | ||||
| Dispersal capacity | high | 2020 | D-N | 4.841 | 0.009 | 3 | 25 | 1.244 | 0.225 |
| 2023 | D-N | 3 | 25 | 0.149 | 0.882 | ||||
| 2020–2023 | D-D | 3 | 25 | 3.357 | 0.003 | ||||
| 2020–2023 | N-N | 3 | 25 | 2.174 | 0.039 | ||||
| medium | 2020 | D-N | 4.466 | 0.013 | 3 | 24 | 0.753 | 0.459 | |
| 2023 | D-N | 3 | 24 | 0.385 | 0.703 | ||||
| 2020–2023 | D-D | 3 | 24 | 2.426 | 0.023 | ||||
| 2020–2023 | N-N | 3 | 24 | 2.359 | 0.027 | ||||
| Reproduction | eggs attached to the substrate | 2020 | D-N | 0.000 | 1.000 | 2 | 8 | 0.001 | 0.999 |
| 2023 | D-N | 2 | 8 | - | - | ||||
| 2020–2023 | D-D | 2 | 8 | - | - | ||||
| 2020–2023 | N-N | 2 | 8 | 0.000 | 1.000 | ||||
| eggs laid in the substrate | 2020 | D-N | 1.744 | 0.183 | 3 | 26 | 0.310 | 0.759 | |
| 2023 | D-N | 3 | 26 | 1.110 | 0.277 | ||||
| 2020–2023 | D-D | 3 | 26 | 2.102 | 0.045 | ||||
| 2020–2023 | N-N | 3 | 26 | 0.635 | 0.531 | ||||
| eggs not attached to or in the substrate | 2020 | D-N | 3.180 | 0.045 | 3 | 21 | 0.088 | 0.930 | |
| 2023 | D-N | 3 | 21 | 0.509 | 0.616 | ||||
| 2020–2023 | D-D | 3 | 21 | 1.860 | 0.077 | ||||
| 2020–2023 | N-N | 3 | 21 | 1.927 | 0.068 | ||||
| eggs laid in open water | 2020 | D-N | 0.261 | 0.853 | 3 | 22 | 0.573 | 0.572 | |
| 2023 | D-N | 3 | 22 | 0.550 | 0.588 | ||||
| 2020–2023 | D-D | 3 | 22 | 0.266 | 0.793 | ||||
| 2020–2023 | N-N | 3 | 22 | 0.342 | 0.736 | ||||
| eggs laid inside plant tissue | 2020 | D-N | 1.744 | 0.183 | 3 | 26 | 0.326 | 0.747 | |
| 2023 | D-N | 3 | 26 | 1.104 | 0.280 | ||||
| 2020–2023 | D-D | 3 | 26 | 2.102 | 0.045 | ||||
| 2020–2023 | N-N | 3 | 26 | 0.635 | 0.531 | ||||
| eggs laid onto plant material | 2020 | D-N | 0.590 | 0.629 | 3 | 20 | 0.546 | 0.591 | |
| 2023 | D-N | 3 | 20 | 0.580 | 0.568 | ||||
| 2020–2023 | D-D | 3 | 20 | 0.379 | 0.709 | ||||
| 2020–2023 | N-N | 3 | 20 | 0.812 | 0.426 | ||||
| eggs on exposed soil or rock | 2020 | D-N | 0.214 | 0.812 | 2 | 7 | 0.488 | 0.641 | |
| 2023 | D-N | - | - | - | - | ||||
| 2020–2023 | D-D | - | - | - | - | ||||
| 2020–2023 | N-N | 2 | 7 | 0.318 | 0.760 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilenica, M.; Brigić, A.; Štih Koren, A.; Koren, T.; Sertić Perić, M.; Schmidt, B.; Bužan, T.; Gottstein, S. Odonata Assemblages in Urban Semi-Natural Wetlands. Insects 2024, 15, 207. https://doi.org/10.3390/insects15030207
Vilenica M, Brigić A, Štih Koren A, Koren T, Sertić Perić M, Schmidt B, Bužan T, Gottstein S. Odonata Assemblages in Urban Semi-Natural Wetlands. Insects. 2024; 15(3):207. https://doi.org/10.3390/insects15030207
Chicago/Turabian StyleVilenica, Marina, Andreja Brigić, Ana Štih Koren, Toni Koren, Mirela Sertić Perić, Bruno Schmidt, Tomislava Bužan, and Sanja Gottstein. 2024. "Odonata Assemblages in Urban Semi-Natural Wetlands" Insects 15, no. 3: 207. https://doi.org/10.3390/insects15030207
APA StyleVilenica, M., Brigić, A., Štih Koren, A., Koren, T., Sertić Perić, M., Schmidt, B., Bužan, T., & Gottstein, S. (2024). Odonata Assemblages in Urban Semi-Natural Wetlands. Insects, 15(3), 207. https://doi.org/10.3390/insects15030207









