Six Steps towards a Spatial Design for Large-Scale Pollinator Surveillance Monitoring
Abstract
Simple Summary
Abstract
1. Introduction
- How can spatial sampling units be defined to be suitable for monitoring pollinator assemblages at the landscape level, i.e., the scale at which environmental factors affect pollinators?;
- How many sampling units are needed to achieve sufficient statistical power for the analysis of pollinator trends based on assumptions about species abundance and richness?;
- Where should those sampling units be placed to represent the gradient of landscapes across the monitoring area?;
- What are suitable temporal intervals between sampling periods to meet the monitoring requirements?
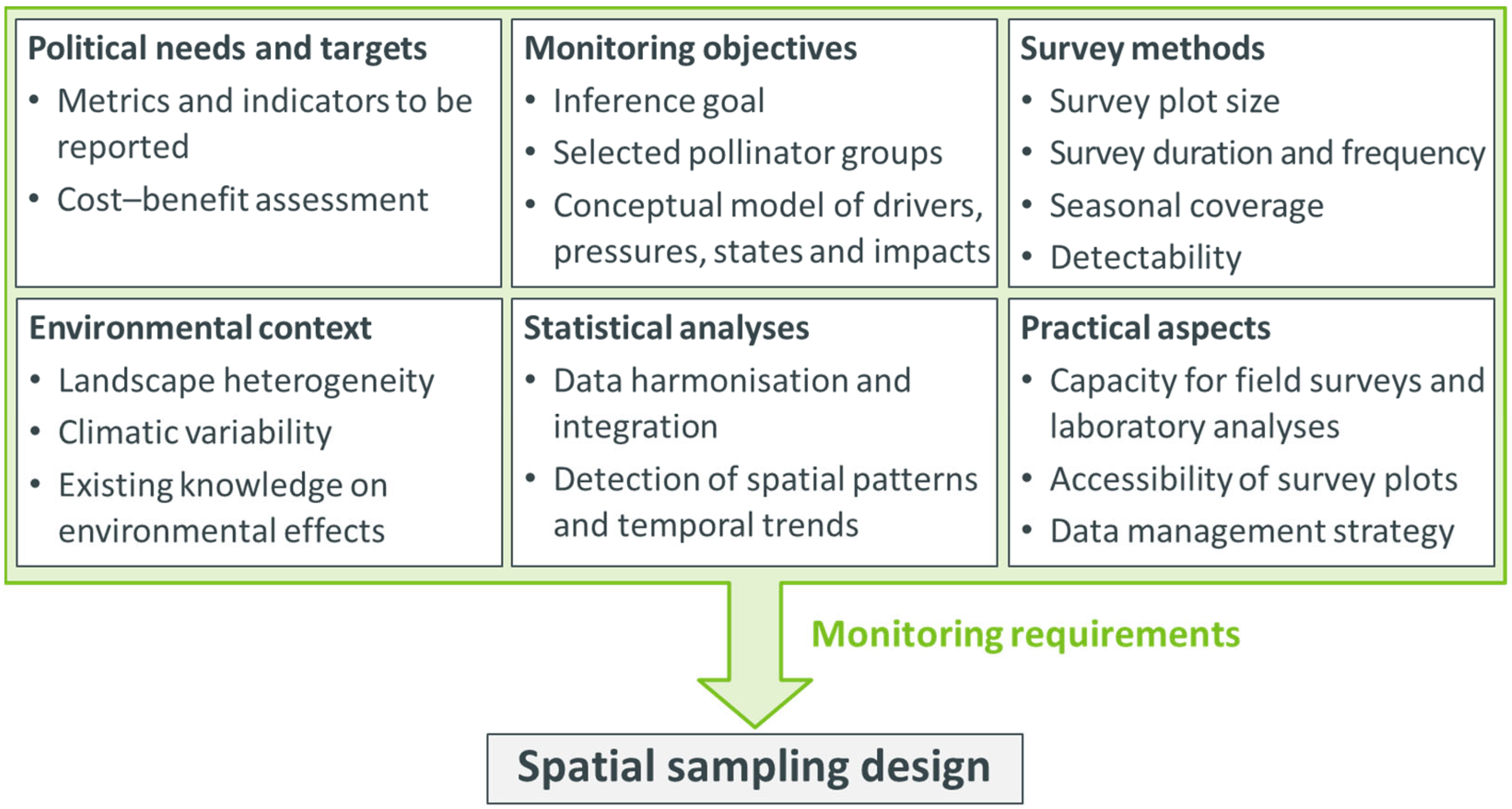
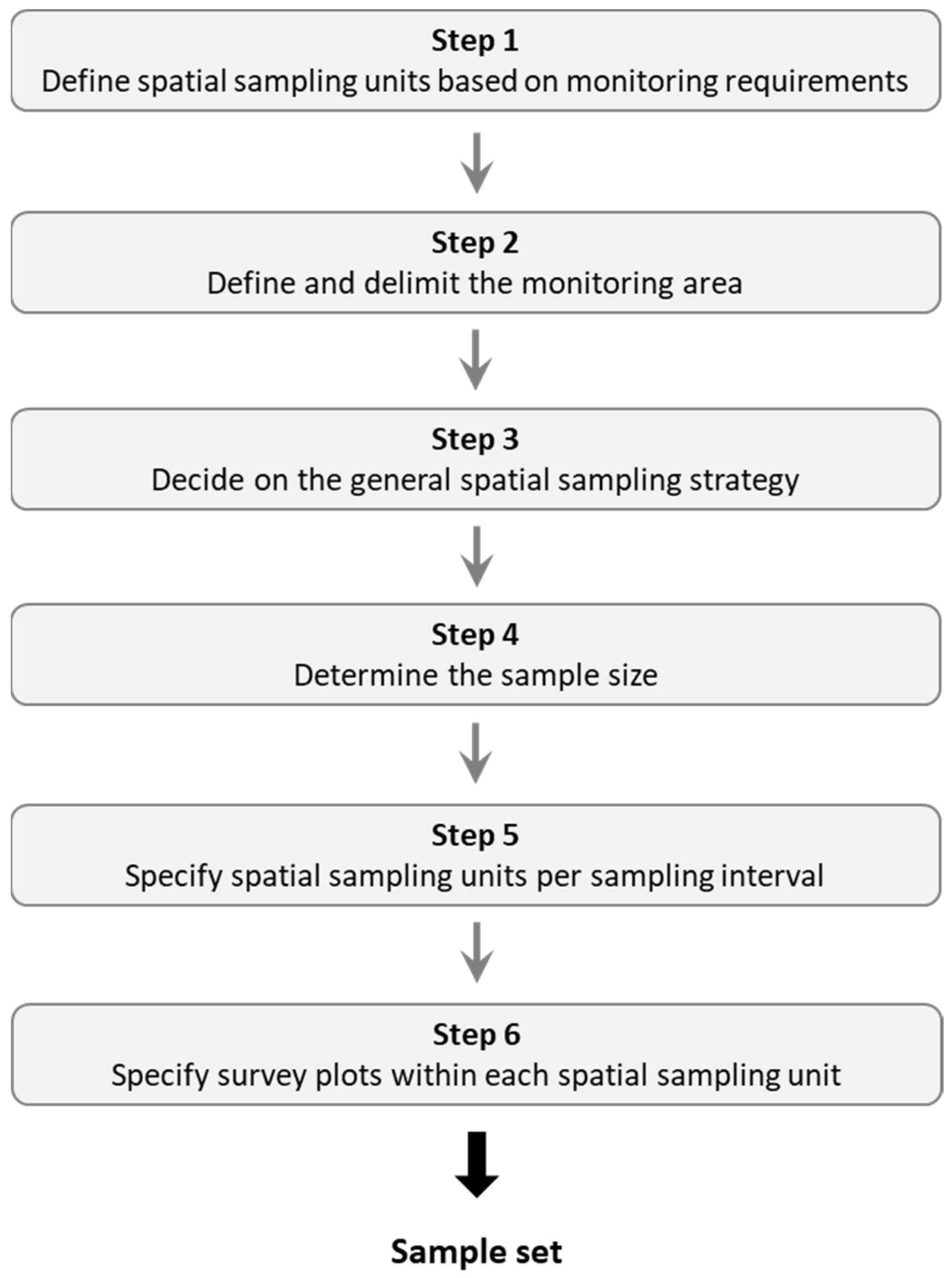
2. Conceptual Framework for the Spatial Sampling Design
2.1. Step 1: Define Spatial Sampling Units Based on Monitoring Requirements
2.2. Step 2: Define and Delimit the Monitoring Area
2.3. Step 3: Decide on the General Spatial Sampling Strategy
2.4. Step 4: Determine the Sample Size
2.5. Step 5: Specify Spatial Sampling Units per Sampling Interval
2.6. Step 6: Specify Survey Plots within Each Spatial Sampling Unit
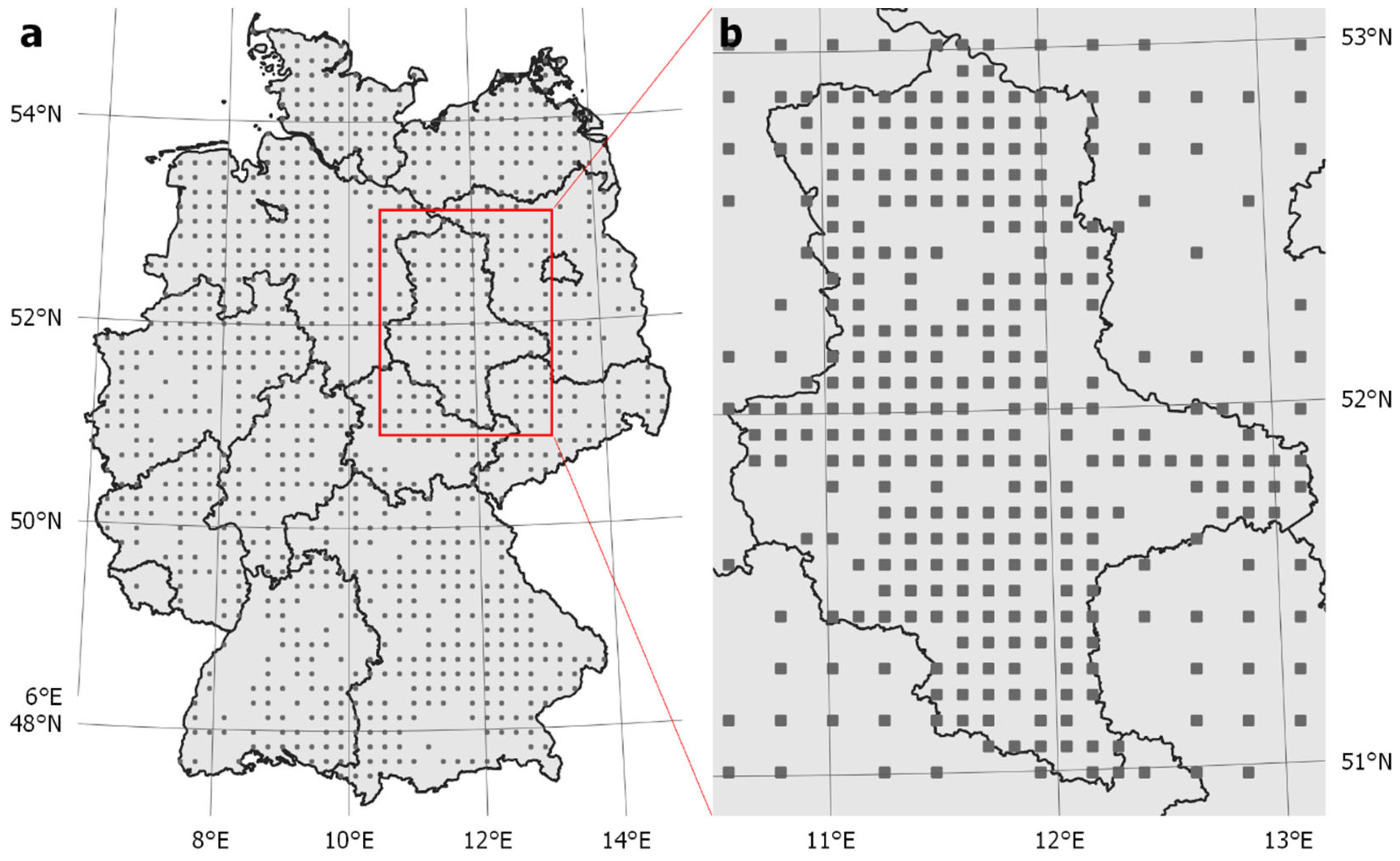
3. Case study: Monitoring Cavity-Nesting Wild Bees in Agricultural Landscapes in Germany
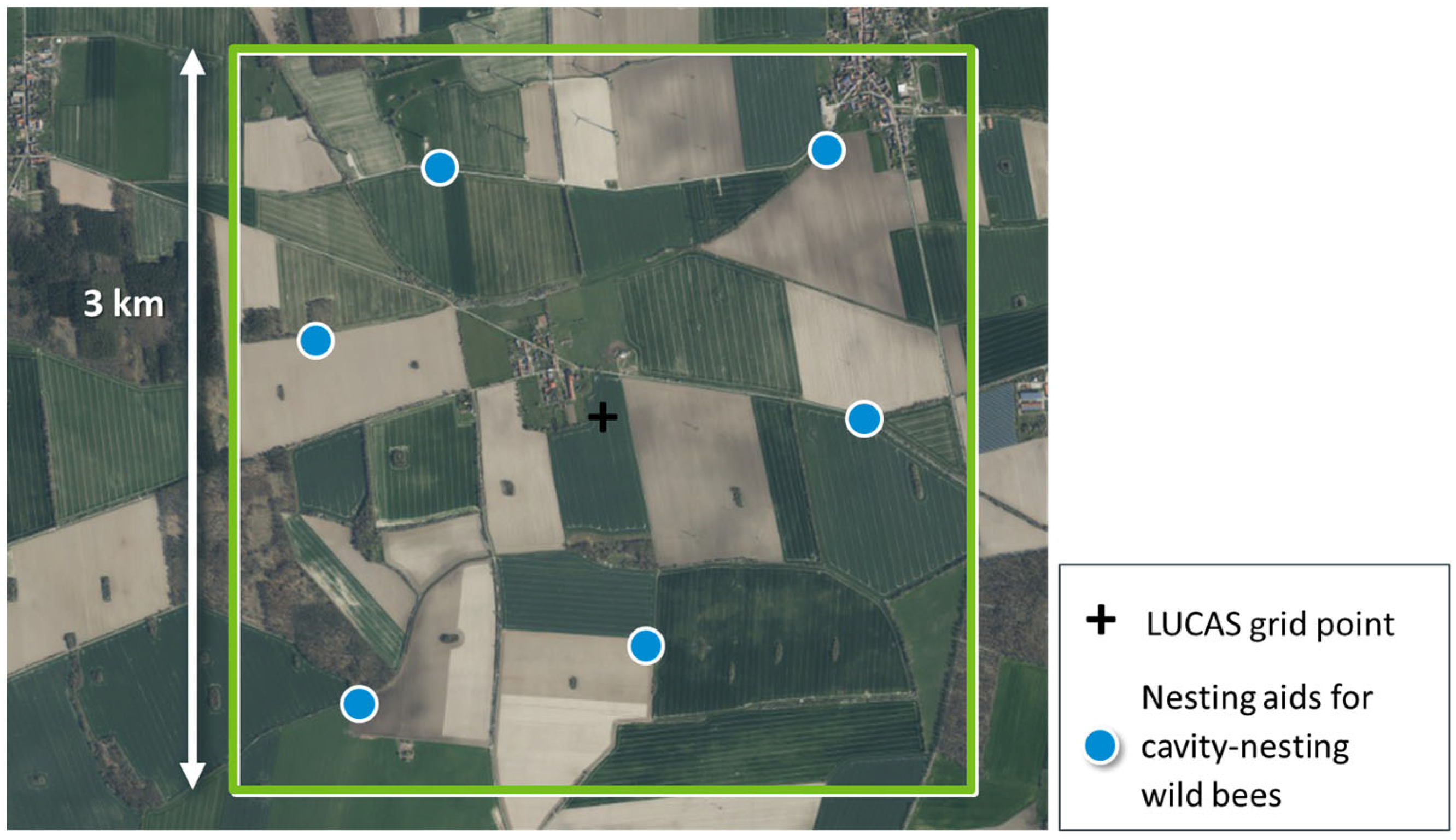
3.1. Step 1: Define Spatial Sampling Units Based on Monitoring Requirements
3.2. Step 2: Define and Delimit the Monitoring Area
3.3. Step 3: Decide on the General Spatial Sampling Strategy
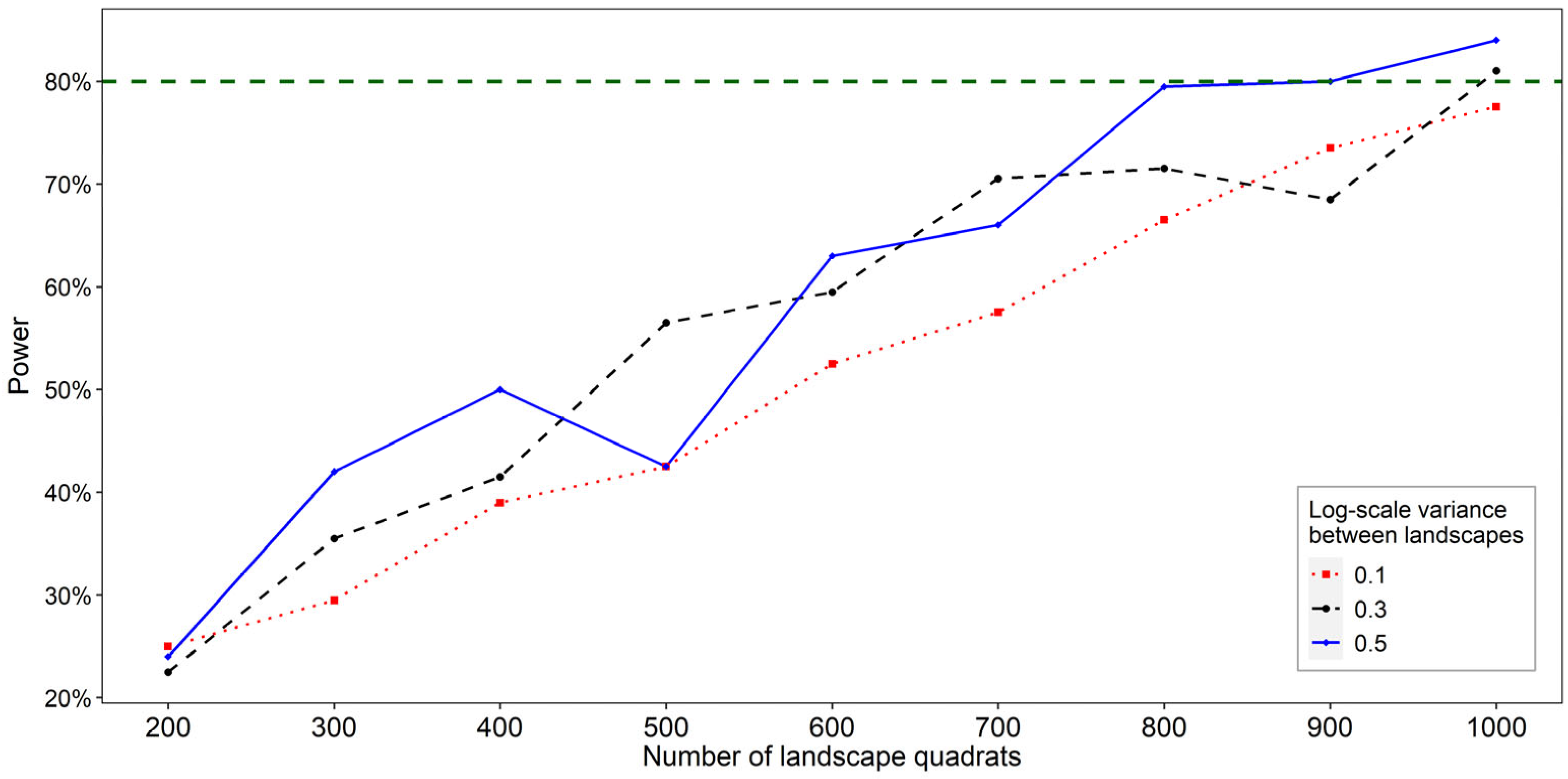
3.4. Step 4: Determine the Sample Size
3.5. Step 5: Specify Spatial Sampling Units per Sampling Interval
3.6. Step 6: Specify Survey Plots within Each Spatial Sampling Unit
- Extract boundaries of areas under agricultural land use per 3 km × 3 km landscape quadrat (Basic DLM, [67]).
- Randomly sample six sites across all boundaries from Step (A) with a minimum distance of 250 m to the margin of the landscape quadrat.
- Calculate all pairwise distances between the six sites for nesting aids.
- Repeat Steps (B) and (C) 10,000 times.
- Select that option (of all 10,000 options) that maximises the smallest of all distances calculated in Step (C).
4. Discussion
4.1. Learning and Revising the Sampling Design
4.2. Implications for Large-Scale Pollinator Monitoring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winfree, R. The conservation and restoration of wild bees. Ann. N. Y. Acad. Sci. 2010, 1195, 169–197. [Google Scholar] [CrossRef] [PubMed]
- Senapathi, D.; Biesmeijer, J.C.; Breeze, T.D.; Kleijn, D.; Potts, S.G.; Carvalheiro, L.G. Pollinator conservation—The difference between managing for pollination services and preserving pollinator diversity. Curr. Opin. Insect Sci. 2015, 12, 93–101. [Google Scholar] [CrossRef]
- Tscharntke, T.; Gathmann, A.; Steffan-Dewenter, I. Bioindication using trap-nesting bees and wasps and their natural enemies: Community structure and interactions. J. Appl. Ecol. 1998, 35, 708–719. [Google Scholar] [CrossRef]
- Sepp, K.; Mikk, M.; Mänd, M.; Truu, J. Bumblebee communities as an indicator for landscape monitoring in the agri-environmental programme. Landsc. Urban Plan. 2004, 67, 173–183. [Google Scholar] [CrossRef]
- Schindler, M.; Diestelhorst, O.; Haertel, S.; Saure, C.; Scharnowski, A.; Schwenninger, H.R. Monitoring agricultural ecosystems by using wild bees as environmental indicators. BioRisk 2013, 8, 53–71. [Google Scholar] [CrossRef]
- Bartholomée, O.; Lavorel, S. Disentangling the diversity of definitions for the pollination ecosystem service and associated estimation methods. Ecol. Indic. 2019, 107, 105576. [Google Scholar] [CrossRef]
- Carvell, C.; Harvey, M.; Mitschunas, N.; Beckmann, B.; Isaac, N.J.B.; Powney, G.D.; Hatfield, J.; Mancini, F.; Garbutt, A.; Fitos, E.; et al. Establishing a UK Pollinator Monitoring and Research Partnership (PMRP); Final report to the Department for Environment, Food and Rural Affairs (Defra), Scottish Government, Welsh Government and JNCC: Project BE0125; UKCEH: Gwynedd, UK, 2020. [Google Scholar]
- Van Swaay, C.A.M.; Dennis, E.B.; Schmucki, R.; Sevilleja, C.G.; Aghababyan, K.; Åström, S.; Balalaikins, M.; Bonelli, S.; Botham, M.; Bourn, N.; et al. Assessing Butterflies in Europe-Butterfly Indicators 1990–2018; Technical report; Butterfly Conservation Europe & ABLE/eBMS: Wageningen, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Woodard, S.H.; Federman, S.; James, R.R.; Danforth, B.N.; Griswold, T.L.; Inouye, D.; McFrederick, Q.S.; Morandin, L.; Paul, D.L.; Sellers, E.; et al. Towards a U.S. national program for monitoring native bees. Biol. Conserv. 2020, 252, 108821. [Google Scholar] [CrossRef]
- Potts, S.G.; Dauber, J.; Hochkirch, A.; Oteman, B.; Roy, D.B.; Ahmé, K.; Biesmeijer, K.; Breeze, T.D.; Carvell, C.; Ferreira, C.; et al. Proposal for EU Pollinator Monitoring Scheme; JRC Technical Report; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- IPBES. The Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Potts, S.G., Imperatriz-Fonseca, V., Ngo, H.T., Eds.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Muller, A.; Sumser, H.; Horren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed]
- Marquard, E.; Dauber, J.; Doerpinghaus, A.; Dröschmeister, R.; Frommer, J.; Frommolt, K.-H.; Gemeinholzer, B.; Henle, K.; Hillebrand, H.; Kleinschmit, B.; et al. Biodiversitätsmonitoring in Deutschland: Herausforderungen für Politik, Forschung und Umsetzung. Nat. Landsch. 2013, 88, 337–341. [Google Scholar] [CrossRef]
- Geschke, J.; Vohland, K.; Bonn, A.; Dauber, J.; Gessner, M.O.; Henle, K.; Nieschulze, J.; Schmeller, D.; Settele, J.; Sommerwerk, N.; et al. Biodiversitätsmonitoring in Deutschland: Wie Wissenschaft, Politik und Zivilgesellschaft ein nationales Monitoring unterstützen können. GAIA 2019, 28, 265–270. [Google Scholar] [CrossRef]
- European Commission. Proposal for a Nature Restoration Law. 2022. Available online: https://environment.ec.europa.eu/publications/nature-restoration-law_en (accessed on 16 September 2022).
- Lindenmayer, D.B.; Likens, G.E. The science and application of ecological monitoring. Biol. Conserv. 2010, 143, 1317–1328. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Likens, G.E. Effective Ecological Monitoring, 2nd ed.; CSIRO publishing: Clayton South, VIC, Australia, 2018. [Google Scholar] [CrossRef]
- Aldercotte, A.H.; Simpson, D.T.; Winfree, R. Crop visitation by wild bees declines over an 8-year time series: A dramatic trend, or just dramatic between-year variation? Insect Conserv. Divers. 2022, 15, 522–533. [Google Scholar] [CrossRef]
- de Gruijter, J.; Brus, D.; Bierkens, M.; Knotters, M. Sampling for Natural Resource Monitoring; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Woinarski, J.; Legge, S.; Southwell, D.; Lavery, T.; Robinson, N.; Scheele, B.; Wintle, B. A checklist of attributes for effective monitoring of threatened species and threatened ecosystems. J. Environ. Manag. 2020, 262, 110312. [Google Scholar] [CrossRef]
- Reynolds, J.H.; Knutson, M.G.; Newman, K.B.; Silverman, E.D.; Thompson, W.L. A road map for designing and implementing a biological monitoring program. Environ. Monit. Assess. 2016, 188, 399. [Google Scholar] [CrossRef]
- Herzog, F.; Franklin, J. State-of-the-art practices in farmland biodiversity monitoring for North America and Europe. Ambio 2016, 45, 857–871. [Google Scholar] [CrossRef]
- Breeze, T.D.; Bailey, A.P.; Balcombe, K.G.; Brereton, T.; Comont, R.; Edwards, M.; Garratt, M.P.; Harvey, M.; Hawes, C.; Isaac, N.; et al. Pollinator monitoring more than pays for itself. J. Appl. Ecol. 2021, 58, 44–57. [Google Scholar] [CrossRef]
- O’Connor, R.S.; Kunin, W.E.; Garratt, M.P.D.; Potts, S.G.; Roy, H.E.; Andrews, C.; Jones, C.M.; Peyton, J.M.; Savage, J.; Harvey, M.C.; et al. Monitoring insect pollinators and flower visitation: The effectiveness and feasibility of different survey methods. Methods Ecol. Evol. 2019, 10, 2129–2140. [Google Scholar] [CrossRef]
- Schuch, S.; Ludwig, H.; Wesche, K. Erfassungsmethoden für ein Insektenmonitoring; Eine Materialsammlung; BfN (=BfN Skripten 565); Deutschland/Bundesamt für Naturschutz: Bonn, Germany, 2020. [Google Scholar] [CrossRef]
- Montgomery, G.A.; Belitz, M.W.; Guralnick, R.P.; Tingley, M.W. Standards and Best Practices for Monitoring and Benchmarking Insects. Front. Ecol. Evol. 2021, 8, 579193. [Google Scholar] [CrossRef]
- Thompson, A.; Frenzel, M.; Schweiger, O.; Musche, M.; Groth, T.; Roberts, S.P.M.; Kuhlmann, M.; Knight, T.M. Pollinator sampling methods influence community patterns assessments by capturing species with different traits and at different abundances. Ecol. Indic. 2021, 132, 108284. [Google Scholar] [CrossRef]
- Hutchinson, L.A.; Oliver, T.H.; Breeze, T.D.; O’Connor, R.S.; Potts, S.G.; Roberts, S.P.M.; Garratt, M.P.D. Inventorying and monitoring crop pollinating bees: Evaluating the effectiveness of common sampling methods. Insect Conserv. Divers. 2022, 15, 299–311. [Google Scholar] [CrossRef]
- Leclercq, N.; Marshall, L.; Weekers, T.; Anselmo, A.; Benda, D.; Bevk, D.; Bogusch, P.; Cejas, D.; Drepper, B.; Galloni, M.; et al. A comparative analysis of crop pollinator survey methods along a large-scale climatic gradient. Agric. Ecosyst. Environ. 2022, 329, 107871. [Google Scholar] [CrossRef]
- Senapathi, D.; Goddard, M.A.; Kunin, W.E.; Baldock, K.C.R. Landscape impacts on pollinator communities in temperate systems: Evidence and knowledge gaps. Funct. Ecol. 2017, 31, 26–37. [Google Scholar] [CrossRef]
- Dicks, L.V.; Breeze, T.D.; Ngo, H.T.; Senapathi, D.; An, J.; Aizen, M.A.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.A.; et al. A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat. Ecol. Evol. 2021, 5, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- LeBuhn, G.; Vargas Luna, J. Pollinator decline: What do we know about the drivers of solitary bee declines? Curr. Opin. Insect Sci. 2021, 46, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, N.; Schubert, L.F.; Kirmer, A.; Tischew, S.; Dieker, P. Effects of wildflower strips, landscape structure and agricultural practices on wild bee assemblages—A matter of data resolution and spatial scale? Agric. Ecosyst. Environ. 2022, 326, 107764. [Google Scholar] [CrossRef]
- Roulston, T.H.; Goodell, K. The role of resources and risks in regulating wild bee populations. Annu. Rev. Entomol. 2011, 56, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.J. Sampling units derived from geopolitical boundaries bias biodiversity analyses. Glob. Ecol. Biogeogr. 2021, 30, 1876–1888. [Google Scholar] [CrossRef]
- BfN. Einheitlicher Methodenleitfaden “Insektenmonitoring“. 2021. Available online: https://www.bfn.de/sites/default/files/2021-11/Methodenleitfaden_Insektenmonitoring_202104_Barrierefrei_1.pdf (accessed on 20 September 2022).
- Abrahamczyk, S.; Kessler, M.; Roth, T.; Heer, N. Temporal changes in the Swiss flora: Implications for flower-visiting insects. BMC Ecol. Evol. 2022, 22, 109. [Google Scholar] [CrossRef]
- Bretagnolle, V.; Berthet, E.; Gross, N.; Gauffre, B.; Plumejeaud, C.; Houte, S.; Badenhausser, I.; Monceau, K.; Allier, F.; Monestiez, P.; et al. Towards sustainable and multifunctional agriculture in farmland landscapes: Lessons from the integrative approach of a French LTSER platform. Sci. Total Environ. 2018, 627, 822–834. [Google Scholar] [CrossRef]
- Scherber, C.; Beduschi, T.; Tscharntke, T. Novel approaches to sampling pollinators in whole landscapes: A lesson for landscape-wide biodiversity monitoring. Landsc. Ecol. 2019, 34, 1057–1067. [Google Scholar] [CrossRef]
- Beyer, N.; Kulow, J.; Dauber, J. The contrasting response of cavity-nesting bees, wasps and their natural enemies to biodiversity conservation measures. Insect Conserv. Divers. 2023, 16, 468–482. [Google Scholar] [CrossRef]
- Staley, J.T.; Redhead, J.W.; O’Connor, R.S.; Jarvis, S.G.; Siriwardena, G.M.; Henderson, I.G.; Botham, M.S.; Carvell, C.; Smart, S.M.; Phillips, S.; et al. Designing a survey to monitor multi-scale impacts of agri-environment schemes on mobile taxa. J. Environ. Manag. 2021, 290, 112589. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Land Cover and Use: Primary Data; European Union: Brussels, Belgium, 2024; Available online: https://ec.europa.eu/eurostat/web/lucas/database/primary-data (accessed on 26 February 2024).
- Oppermann, R.; Schraml, A.; Sutcliffe, L.; Lüdemann, J. Final Report—European Monitoring of Biodiversity in Agricultural Landscapes (EMBAL); Institute for Agroecology and Biodiversity (IFAB): Mannheim, Germany, 2018. [Google Scholar]
- Brus, D.J. Statistical approaches for spatial sample survey: Persistent misconceptions and new developments. Eur. J. Soil Sci. 2021, 72, 686–703. [Google Scholar] [CrossRef]
- Redlich, S.; Zhang, J.; Benjamin, C.; Dhillon, M.S.; Englmeier, J.; Ewald, J.; Fricke, U.; Ganuza, C.; Haensel, M.; Hovestadt, T.; et al. Disentangling effects of climate and land use on biodiversity and ecosystem services—A multi-scale experimental design. Meth. Ecol. Evol. 2022, 13, 514–527. [Google Scholar] [CrossRef]
- Geijzendorffer, I.R.; Targetti, S.; Schneider, M.K.; Brus, D.J.; Jeanneret, P.; Jongman, R.H.G.; Knotters, M.; Viaggi, D.; Angelova, S.; Arndorfer, M.; et al. How much would it cost to monitor farmland biodiversity in Europe? J. Appl. Ecol. 2016, 53, 140–149. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. A conditioned Latin hypercube method for sampling in the presence of ancillary information. Comput. Geosci. 2006, 32, 1378–1388. [Google Scholar] [CrossRef]
- Brus, D.J. Sampling for digital soil mapping: A tutorial supported by R scripts. Geoderma 2019, 338, 464–480. [Google Scholar] [CrossRef]
- Ma, T.; Brus, D.J.; Zhu, A.X.; Zhang, L.; Scholten, T. Comparison of conditioned Latin hypercube and feature space coverage sampling for predicting soil classes using simulation from soil maps. Geoderma 2020, 370, 114366. [Google Scholar] [CrossRef]
- Nuñez-Penichet, C.; Cobos, M.E.; Soberón, J.; Gueta, T.; Barve, N.; Barve, V.; Navarro-Sigüenza, A.G.; Peterson, A.T. Selection of sampling sites for biodiversity inventory: Effects of environmental and geographical considerations. Meth. Ecol. Evol. 2022, 13, 1595–1607. [Google Scholar] [CrossRef]
- Bartual, A.M.; Sutter, L.; Bocci, G.; Moonen, A.-C.; Cresswell, J.; Entling, M.; Giffard, B.; Jacot, K.; Jeanneret, P.; Holland, J.; et al. The potential of different semi-natural habitats to sustain pollinators and natural enemies in European agricultural landscapes. Agric. Ecosyst. Environ. 2019, 279, 43–52. [Google Scholar] [CrossRef]
- Fijen, T.P.M.; Scheper, J.A.; Boekelo, B.; Raemakers, I.; Kleijn, D. Effects of landscape complexity on pollinators are moderated by pollinators’ association with mass-flowering crops. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190387. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.E.; Sayer, C.D.; Bennion, H.; Axmacher, J.C. Open-canopy ponds benefit diurnal pollinator communities in an agricultural landscape: Implications for farmland pond management. Insect Conserv. Divers. 2021, 14, 307–324. [Google Scholar] [CrossRef]
- Bergholz, K.; Sittel, L.P.; Ristow, M.; Jeltsch, F.; Weiss, L. Pollinator guilds respond contrastingly at different scales to landscape parameters of land-use intensity. Ecol. Evol. 2022, 12, e8708. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, M.A.; Carper, A.L.; Wilson, C.J.; Scott, V.L.; Gibbs, J. Geographic Biases in Bee Research Limits Understanding of Species Distribution and Response to Anthropogenic Disturbance. Front. Ecol. Evol. 2019, 7, 194. [Google Scholar] [CrossRef]
- Mentges, A.; Blowes, S.A.; Hodapp, D.; Hillebrand, H.; Chase, J.M. Effects of site-selection bias on estimates of biodiversity change. Conserv. Biol. 2021, 35, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Burgess, B.J.; Jackson, M.C.; Murrell, D.J. Are experiment sample sizes adequate to detect biologically important interactions between multiple stressors? Ecol. Evol. 2022, 12, e9289. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Haughland, D.L.; Bayne, E.; Schieck, J. Capacity of large-scale, long-term biodiversity monitoring programmes to detect trends in species prevalence. Biodivers. Conserv. 2009, 18, 2961–2978. [Google Scholar] [CrossRef]
- LeBuhn, G.; Droege, S.; Connor, E.F.; Gemmill-Herren, B.; Potts, S.G.; Minckley, R.L.; Griswold, T.; Jean, R.; Kula, E.; Roubik, D.W.; et al. Detecting insect pollinator declines on regional and global scales. Conserv. Biol. 2013, 27, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.D.; Barry, S.J.E.; Ferguson, H.M.; Müller, P. Power analysis for generalized linear mixed models in ecology and evolution. Meth. Ecol. Evol. 2015, 6, 133–142. [Google Scholar] [CrossRef]
- Kain, M.P.; Bolker, B.M.; McCoy, M.W. A practical guide and power analysis for GLMMs: Detecting among treatment variation in random effects. PeerJ 2015, 3, e1226. [Google Scholar] [CrossRef]
- Thomas, J.A. Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 339–357. [Google Scholar] [CrossRef]
- Lindermann, L.; Grabener, S.; Stahl, J.; Hellwig, N.; Dieker, P. Citizen-science based monitoring of wild bees and wasps in nesting aids—Benefits for volunteers, insects and ecological science. Citiz. Sci. Theory Pract. 2023; submitted. [Google Scholar]
- Sickel, W.; Kulow, J.; Krüger, L.; Dieker, P. BEE-quest of the nest: A novel method for eDNA-based, non-lethal detection of cavity-nesting hymenopterans and other arthropods. Environ. DNA 2023, 5, 1163–1176. [Google Scholar] [CrossRef]
- Destatis. Land-und Forstwirtschaft, Fischerei: Bodenfläche nach Art der Tatsächlichen Nutzung, 2020; Fachserie 3 Reihe 5.1; Statistisches Bundesamt (Destatis): Wiesbaden, Germany, 2021.
- AdV. Dokumentation zur Modellierung der Geoinformationen des Amtlichen Vermessungswesens (GeoInfoDok)—Erläuterungen zum ATKIS® Basis-DLM, Version 6.0. ATKIS-Katalogwerke. ATKIS-Objektartenkatalog Basis-DLM; Arbeitsgemeinschaft der Vermessungsverwaltungen der Länder der Bundesrepublik Deutschland: München, Germany, 2008. [Google Scholar]
- BKG. Digitales Basis-Landschaftsmodell 2018; Bundesamt für Kartographie und Geodäsie: Frankfurt am Main, Germany, 2018; Available online: https://gdz.bkg.bund.de/index.php/default/digitales-basis-landschaftsmodell-kompakt-basis-dlm-kompakt.html (accessed on 14 July 2022).
- Marshall, L.; Carvalheiro, L.G.; Aguirre-Gutierrez, J.; Bos, M.; de Groot, G.A.; Kleijn, D.; Potts, S.G.; Reemer, M.; Roberts, S.; Scheper, J.; et al. Testing projected wild bee distributions in agricultural habitats: Predictive power depends on species traits and habitat type. Ecol. Evol. 2015, 5, 4426–4436. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, M.; Goslee, S.C.; Douglas, M.R.; Tooker, J.F.; Grozinger, C.M. Wild bees as winners and losers: Relative impacts of landscape composition, quality, and climate. Glob. Chang. Biol. 2021, 27, 1250–1265. [Google Scholar] [CrossRef]
- Meier, S.; Walz, U.; Syrbe, R.-U.; Grunewald, K. Das bundesweite Habitatpotenzial für Wildbienen. Ein Indikator für Bestäubungsleistung. Naturschutz Landschaftsplan. 2021, 53, 12–19. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Neumann, K.; Seidelmann, K. Microsatellites for the inference of population structures in the Red Mason bee Osmia rufa (Hymenoptera: Megachilidae). Apidologie 2006, 37, 75–83. [Google Scholar] [CrossRef][Green Version]
- Zurbuchen, A.; Landert, L.; Klaiber, J.; Müller, A.; Hein, S.; Dorn, S. Maximum foraging ranges in solitary bees: Only few individuals have the capability to cover long foraging distances. Biol. Conserv. 2010, 143, 669–676. [Google Scholar] [CrossRef]
- Hofmann, M.M.; Fleischmann, A.; Renner, S.S. Foraging distances in six species of solitary bees with body lengths of 6 to 15 mm, inferred from individual tagging, suggest 150 m-rule-of-thumb for flower strip distances. J. Hymenopt. Res. 2020, 77, 105–117. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Likens, G.E. Adaptive monitoring: A new paradigm for long-term research and monitoring. Trends Ecol. Evol. 2009, 24, 482–486. [Google Scholar] [CrossRef]
- Henriques, S.; Böhm, M.; Collen, B.; Luedtke, J.; Hoffmann, M.; Hilton-Taylor, C.; Cardoso, P.; Butchart, S.H.M.; Freeman, R. Accelerating the monitoring of global biodiversity: Revisiting the sampled approach to generating Red List Indices. Conserv. Lett. 2020, 13, e12703. [Google Scholar] [CrossRef]
- van Strien, A.J.; van Zweden, J.S.; Sparrius, L.B.; Odé, B. Improving citizen science data for long-term monitoring of plant species in the Netherlands. Biodivers. Conserv. 2022, 31, 2781–2796. [Google Scholar] [CrossRef]
- Stokstad, G.; Fjellstad, W. Experiences from a National Landscape Monitoring Programme—Maintaining Continuity Whilst Meeting Changing Demands and Opportunities. Land 2019, 8, 77. [Google Scholar] [CrossRef]
- Weiser, E.L.; Diffendorfer, J.E.; López-Hoffman, L.; Semmens, D.; Thogmartin, W.E. Consequences of ignoring spatial variation in population trend when conducting a power analysis. Ecography 2019, 42, 836–844. [Google Scholar] [CrossRef]
- Kevan, P.G. Pollinators as bioindicators of the state of the environment: Species, activity and diversity. Agric. Ecosyst. Environ. 1999, 74, 373–393. [Google Scholar] [CrossRef]
- Westrich, P. Habitat requirements of central European bees and the problems of partial habitats. In The Conservation of Bees; Matheson, A., Buchmann, S.L., O’Toole, C., Westrich, P., Williams, I.H., Eds.; Academic Press: London, UK, 1996; pp. 1–16. [Google Scholar]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Exploring the interactions between resource availability and the utilisation of semi-natural habitats by insect pollinators in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2017, 246, 157–167. [Google Scholar] [CrossRef]
- Naeem, M.; Huang, J.; Zhang, S.; Luo, S.; Liu, Y.; Zhang, H.; Luo, Q.; Zhou, Z.; Ding, G.; An, J. Diagnostic indicators of wild pollinators for biodiversity monitoring in long-term conservation. Sci. Total Environ. 2020, 708, 135231. [Google Scholar] [CrossRef] [PubMed]
- van Swaay, C.A.M.; Nowicki, P.; Settele, J.; van Strien, A.J. Butterfly monitoring in Europe: Methods, applications and perspectives. Biodivers. Conserv. 2008, 17, 3455–3469. [Google Scholar] [CrossRef]
- Segre, H.; Kleijn, D.; Bartomeus, I.; WallisDeVries, M.F.; de Jong, M.; van der Schee, M.F.; Román, J.; Fijen, T.P.M. Butterflies are not a robust bioindicator for assessing pollinator communities, but floral resources offer a promising way forward. Ecol. Indic. 2023, 154, 110842. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Münzenberg, U.; Bürger, C.; Thies, C.; Tscharntke, T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 2002, 83, 1421–1432. [Google Scholar] [CrossRef]
- Kohler, F.; Verhulst, J.; Van Klink, R.; Kleijn, D. At what spatial scale do high-quality habitats enhance the diversity of forbs and pollinators in intensively farmed landscapes? J. Appl. Ecol. 2008, 45, 753–762. [Google Scholar] [CrossRef]
- Andrade, C.; Villers, A.; Balent, G.; Bar-Hen, A.; Chadoeuf, J.; Cylly, D.; Cluzeau, D.; Fried, G.; Guillocheau, S.; Pillon, O.; et al. A real-world implementation of a nationwide, long-term monitoring program to assess the impact of agrochemicals and agricultural practices on biodiversity. Ecol. Evol. 2021, 11, 3771–3793. [Google Scholar] [CrossRef] [PubMed]
- Bowler, E.; Lefebvre, V.; Pfeifer, M.; Ewers, R.M. Optimising sampling designs for habitat fragmentation studies. Meth. Ecol. Evol. 2021, 13, 217–229. [Google Scholar] [CrossRef]
- Henry, P.-Y.; Lengyel, S.; Nowicki, P.; Julliard, R.; Clobert, J.; Čelik, T.; Gruber, B.; Schmeller, D.S.; Babij, V.; Henle, K. Integrating ongoing biodiversity monitoring: Potential benefits and methods. Biodivers. Conserv. 2008, 17, 3357–3382. [Google Scholar] [CrossRef]
- Couvet, D.; Devictor, V.; Jiguet, F.; Julliard, R. Scientific contributions of extensive biodiversity monitoring. Comptes Rendus Biol. 2011, 334, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kühl, H.S.; Bowler, D.E.; Bösch, L.; Bruelheide, H.; Dauber, J.; Eichenberg, D.; Eisenhauer, N.; Fernández, N.; Guerra, C.A.; Henle, K.; et al. Effective Biodiversity Monitoring Needs a Culture of Integration. One Earth 2020, 3, 462–474. [Google Scholar] [CrossRef]
- Wintle, B.A.; Runge, M.C.; Bekessy, S.A. Allocating monitoring effort in the face of unknown unknowns. Ecol. Lett. 2010, 13, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.V.; Viana, B.; Bommarco, R.; Brosi, B.; del Coro Arizmendi, M.; Cunningham, S.A.; Galetto, L.; Hill, R.; Lopes, A.V.; Pires, C.; et al. Ten policies for pollinators. Science 2016, 354, 975–976. [Google Scholar] [CrossRef]
- Lengyel, S.; Kobler, A.; Kutnar, L.; Framstad, E.; Henry, P.-Y.; Babij, V.; Gruber, B.; Schmeller, D.; Henle, K. A review and a framework for the integration of biodiversity monitoring at the habitat level. Biodivers. Conserv. 2008, 17, 3341–3356. [Google Scholar] [CrossRef]
- Petrou, Z.I.; Manakos, I.; Stathaki, T. Remote sensing for biodiversity monitoring: A review of methods for biodiversity indicator extraction and assessment of progress towards international targets. Biodivers. Conserv. 2015, 24, 2333–2363. [Google Scholar] [CrossRef]
- Skidmore, A.K.; Coops, N.C.; Neinavaz, E.; Ali, A.; Schaepman, M.E.; Paganini, M.; Kissling, W.D.; Vihervaara, P.; Darvishzadeh, R.; Feilhauer, H.; et al. Priority list of biodiversity metrics to observe from space. Nat. Ecol. Evol. 2021, 5, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Zarnetske, P.L.; Read, Q.D.; Record, S.; Gaddis, K.D.; Pau, S.; Hobi, M.L.; Malone, S.L.; Costanza, J.; Dahlin, K.M.; Latimer, A.M.; et al. Towards connecting biodiversity and geodiversity across scales with satellite remote sensing. Glob. Ecol. Biogeogr. 2019, 28, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Geijzendorffer, I.R.; Roche, P.K. Can biodiversity monitoring schemes provide indicators for ecosystem services? Ecol. Indic. 2013, 33, 148–157. [Google Scholar] [CrossRef]
- Jetz, W.; McGeoch, M.A.; Guralnick, R.; Ferrier, S.; Beck, J.; Costello, M.J.; Fernandez, M.; Geller, G.N.; Keil, P.; Merow, C.; et al. Essential biodiversity variables for mapping and monitoring species populations. Nat. Ecol. Evol. 2019, 3, 539–551. [Google Scholar] [CrossRef]
- Westphal, C.; Bommarco, R.; Carré, G.; Lamborn, E.; Morison, N.; Petanidou, T.; Potts, S.G.; Roberts, S.P.M.; Szentgyörgyi, H.; Tscheulin, T.; et al. Measuring bee diversity in different European habitats and biogeographical regions. Ecol. Monogr. 2008, 78, 653–671. [Google Scholar] [CrossRef]
- Fabian, Y.; Sandau, N.; Bruggisser, O.T.; Aebi, A.; Kehrli, P.; Rohr, R.P.; Naisbit, R.E.; Bersier, L.F. Plant diversity in a nutshell: Testing for small-scale effects on trap nesting wild bees and wasps. Ecosphere 2014, 5, 1–18. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.M.; Weisser, W.W.; Tscharntke, T. Multitrophic effects of experimental changes in plant diversity on cavity-nesting bees, wasps, and their parasitoids. Oecologia 2012, 169, 453–465. [Google Scholar] [CrossRef]
- Diekötter, T.; Peter, F.; Jauker, B.; Wolters, V.; Jauker, F. Mass-flowering crops increase richness of cavity-nesting bees and wasps in modern agro-ecosystems. GCB Bioenergy 2014, 6, 219–226. [Google Scholar] [CrossRef]
- Gathmann, A.; Greiler, H.J.; Tscharntke, T. Trap-nesting bees and wasps colonizing set-aside fields: Succession and body size, management by cutting and sowing. Oecologia 1994, 98, 8–14. [Google Scholar] [CrossRef]
- Krewenka, K.M.; Holzschuh, A.; Tscharntke, T.; Dormann, C.F. Landscape elements as potential barriers and corridors for bees, wasps and parasitoids. Biol. Conserv. 2011, 144, 1816–1825. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Grazing Intensity and the Diversity of Grasshoppers, Butterflies, and Trap-Nesting Bees and Wasps. Conserv. Biol. 2002, 16, 1570–1580. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I. Importance of Habitat Area and Landscape Context for Species Richness of Bees and Wasps in Fragmented Orchard Meadows. Conserv. Biol. 2003, 17, 1036–1044. [Google Scholar] [CrossRef]
- Uzman, D.; Reineke, A.; Entling, M.H.; Leyer, I. Habitat area and connectivity support cavity-nesting bees in vineyards more than organic management. Biol. Conserv. 2020, 242, 108419. [Google Scholar] [CrossRef]
- Steckel, J.; Westphal, C.; Peters, M.K.; Bellach, M.; Rothenwoehrer, C.; Erasmi, S.; Scherber, C.; Tscharntke, T.; Steffan-Dewenter, I. Landscape composition and configuration differently affect trap-nesting bees, wasps and their antagonists. Biol. Conserv. 2014, 172, 56–64. [Google Scholar] [CrossRef]
| Term | Explanation | Example (Referring to Case Study Presented in Section 3) |
|---|---|---|
| Monitoring area | Spatial frame that defines criteria for sampling units to be considered suitable for inclusion in the sample set | Agricultural landscape of Germany, defined as at least 30% agricultural area per landscape quadrat |
| Sample set | All sampling units where the monitoring will be realised | 950 sites with survey locations (Section 3.5.) and survey times |
| Sampling unit | Landscape segment with any number of pollinator surveys observed at one or more survey plots inside the sampling unit over a specific time | Landscape quadrat oriented towards the LUCAS grid with nesting aids observed over one season (Section 3.1.) |
| Survey | Collection of species data following a predefined method at a specific survey plot and time | Collection of data on cavity-nesting wild bees by taking photos of a pair of nesting aids at a specific location and time |
| Survey plot | Location of a survey with a predefined spatial extent | Location of a pair of nesting aids |
| Sampling strategy | Random or model-based method to select sampling units for the sample set | Systematic random sampling |
| Sample size | Number of sampling units included in the sample set | 950 |
| Sampling interval | Time between the beginning of two consecutive sampling periods, i.e., the temporal resolution of the monitoring | 2 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellwig, N.; Sommerlandt, F.M.J.; Grabener, S.; Lindermann, L.; Sickel, W.; Krüger, L.; Dieker, P. Six Steps towards a Spatial Design for Large-Scale Pollinator Surveillance Monitoring. Insects 2024, 15, 229. https://doi.org/10.3390/insects15040229
Hellwig N, Sommerlandt FMJ, Grabener S, Lindermann L, Sickel W, Krüger L, Dieker P. Six Steps towards a Spatial Design for Large-Scale Pollinator Surveillance Monitoring. Insects. 2024; 15(4):229. https://doi.org/10.3390/insects15040229
Chicago/Turabian StyleHellwig, Niels, Frank M. J. Sommerlandt, Swantje Grabener, Lara Lindermann, Wiebke Sickel, Lasse Krüger, and Petra Dieker. 2024. "Six Steps towards a Spatial Design for Large-Scale Pollinator Surveillance Monitoring" Insects 15, no. 4: 229. https://doi.org/10.3390/insects15040229
APA StyleHellwig, N., Sommerlandt, F. M. J., Grabener, S., Lindermann, L., Sickel, W., Krüger, L., & Dieker, P. (2024). Six Steps towards a Spatial Design for Large-Scale Pollinator Surveillance Monitoring. Insects, 15(4), 229. https://doi.org/10.3390/insects15040229






