Ngāokeoke Aotearoa: The Peripatoides Onychophora of New Zealand
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
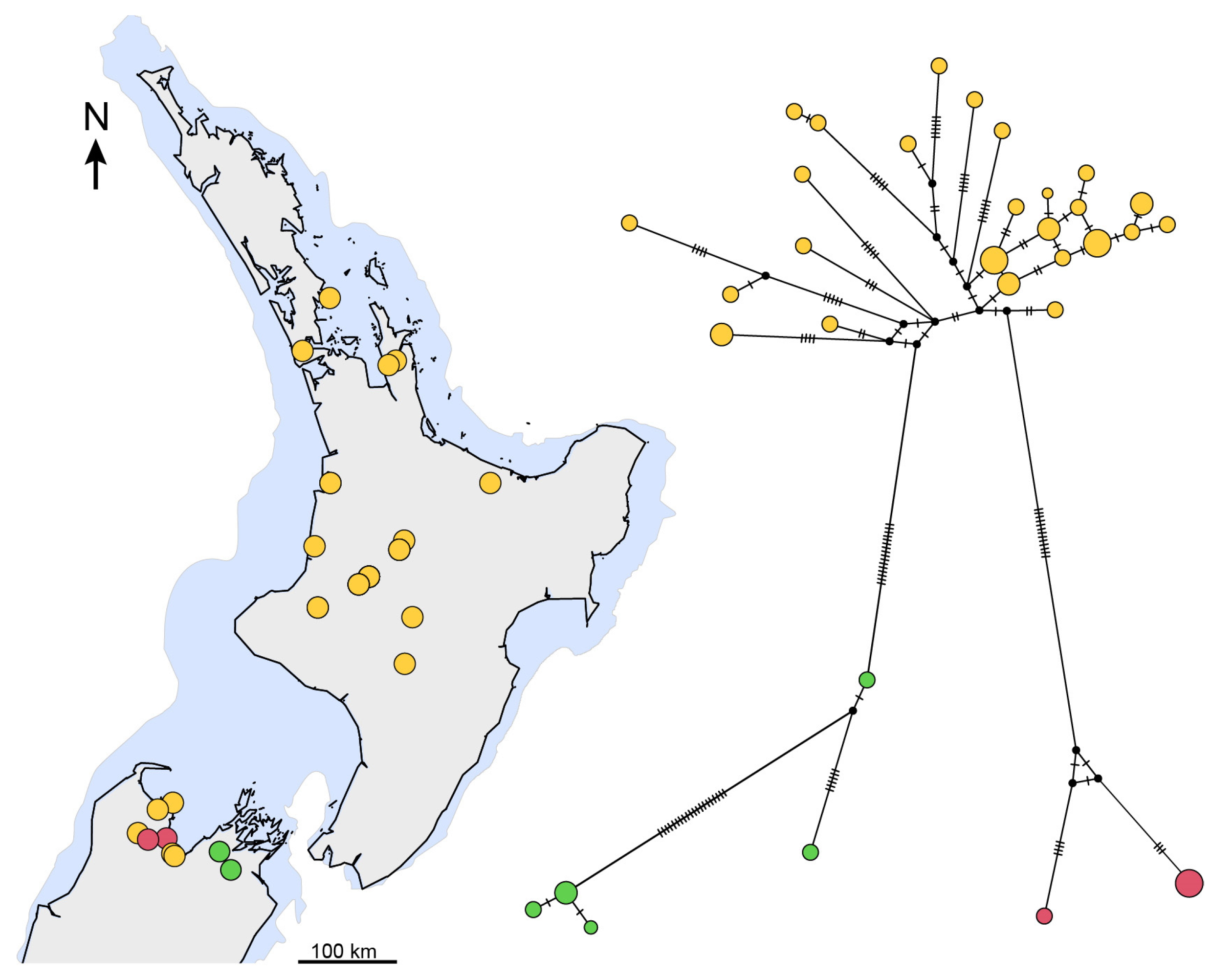
3.1. Allozyme Diversity
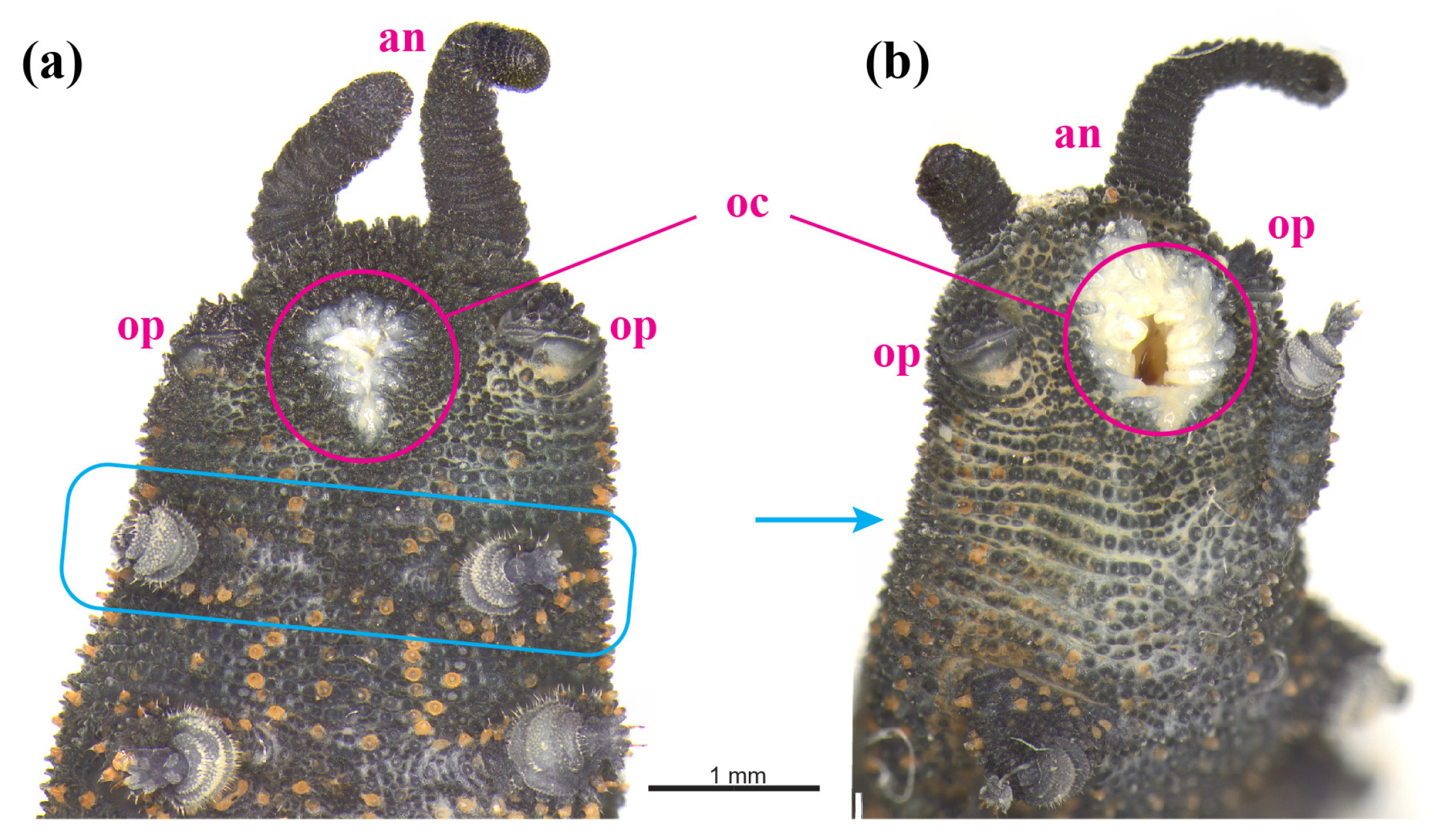
3.2. Phenotypic Variation
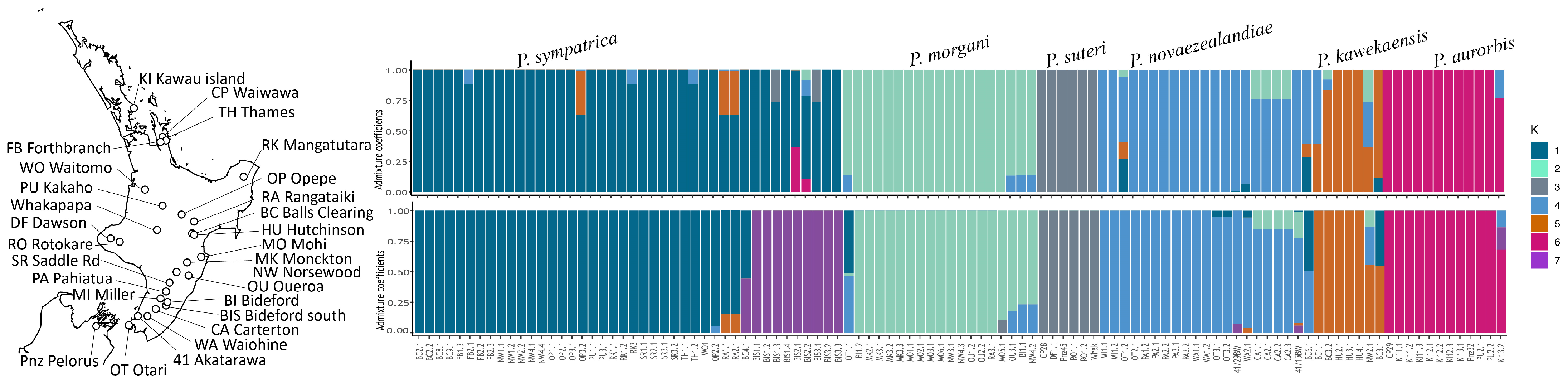
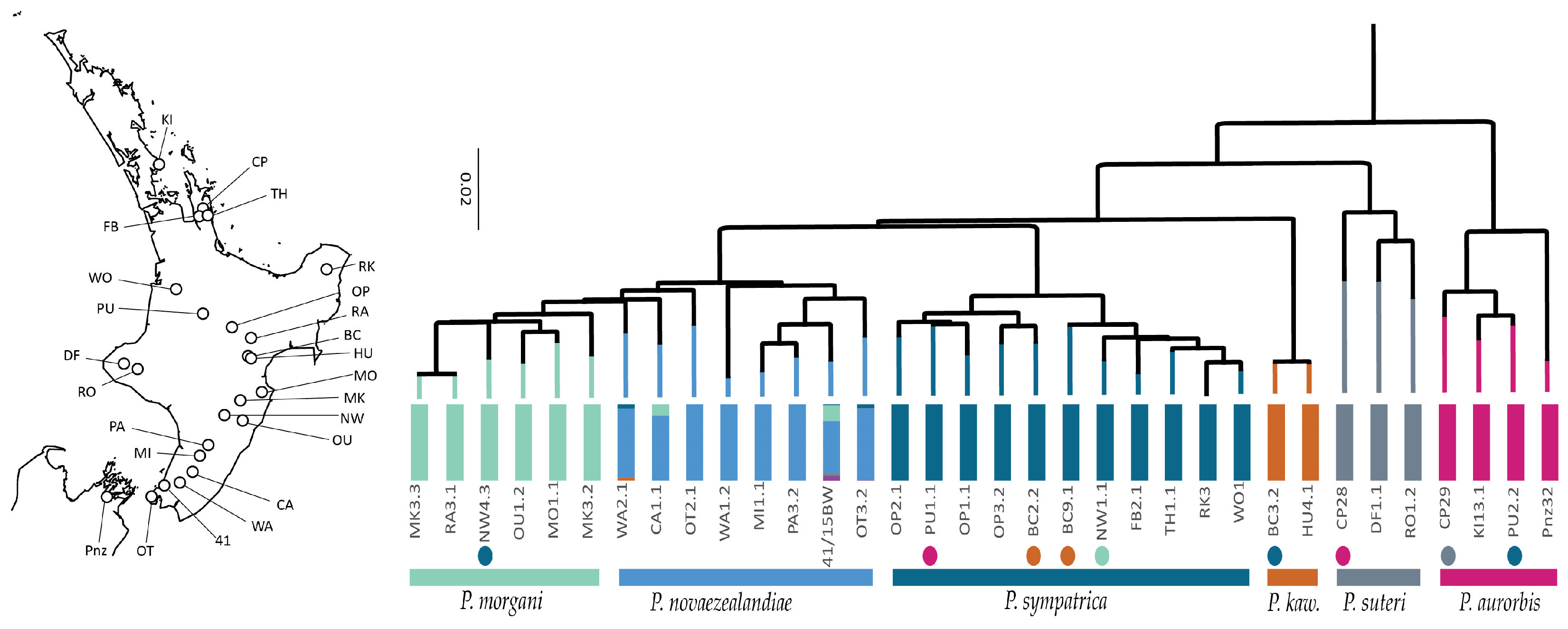
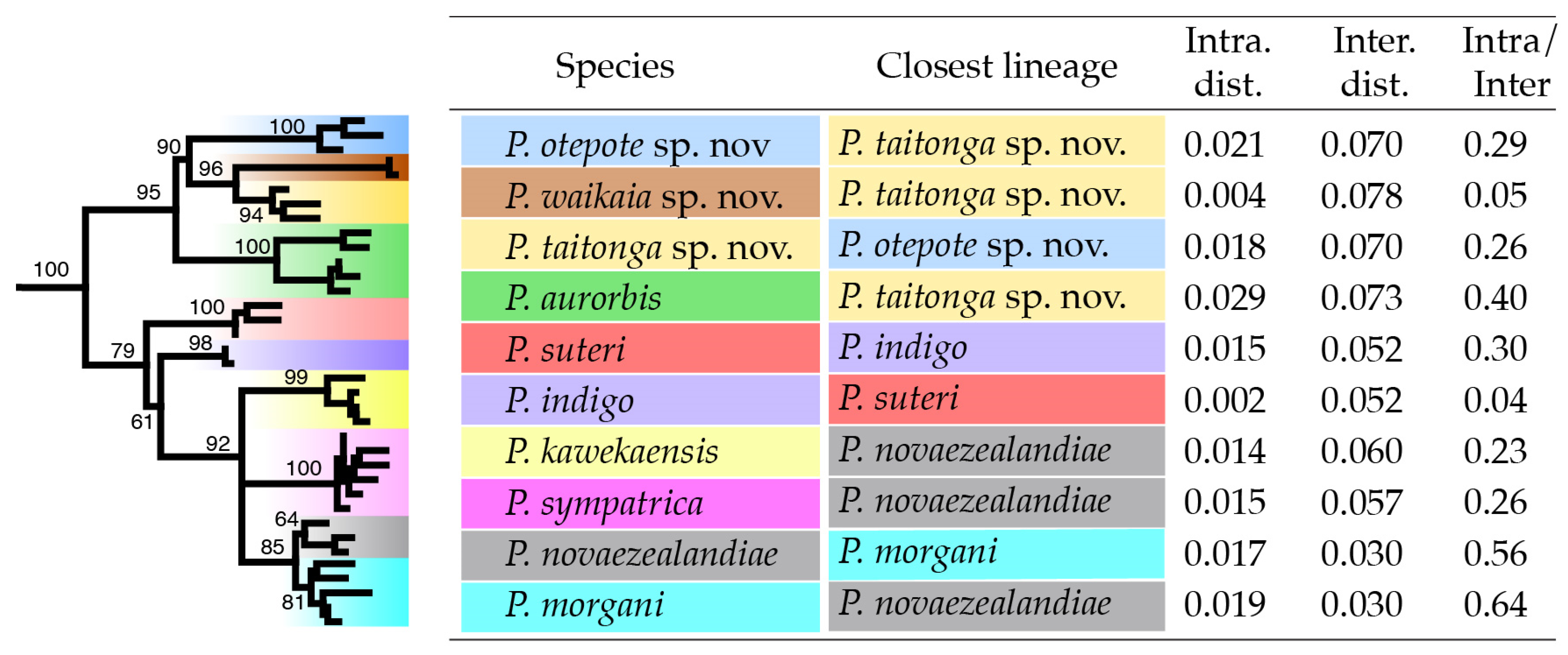
3.3. Phylogenetic Diversity
3.4. Existing Species
3.5. New Species from the South
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guilding, L. Mollusca caribbaeana: An account of a new genus of Mollusca. Zool. J. 1828, 2, 443. [Google Scholar]
- Hutton, F.W. On Peripatus novae-zealandiae. Ann. Mag. Nat. Hist. 1876, 18, 361–369. [Google Scholar] [CrossRef]
- Sedgewick, A. Peripatus. In The Cambridge Natural History; McMillan & Co.: London, UK; New York, NY, USA, 1895. [Google Scholar]
- Dunn, C.W.; Hejnol, A.; Matus, D.Q.; Pang, K.; Browne, W.E.; Smith, S.A.; Seaver, E.; Rouse, G.W.; Obst, M.; Edgecombe, J.D.; et al. Broad phylogenetic sampling improves resolution of animal tree of life. Nature 2008, 452, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Oliveira, I.S. Phylum Onychophora, Grube (1853). Zootaxa 2013, 3703, 15–16. [Google Scholar]
- Manton, S.M.; Heatley, N.G. Studies on the Onychophora. II. The feeding, digestion, excretion and food storage of Peripatopsis with biochemical estimations and analyses. Philos. Trans. R. Soc. Lond. 1937, 227, 411–464. [Google Scholar]
- Oliveira, I.S. An updated world checklist of velvet worms (Onychophora) with notes on nomenclature and status of names. ZooKeys 2023, 1184, 133–260. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.L.; Samways, M.J.; Ruhberg, H. A review of the Onychophora of South Africa, with discussion of their conservation. Ann. Natal Mus. 1997, 38, 283–312. [Google Scholar]
- Watt, J.C. The New Zealand Onycophora. Tane 1960, 8, 95–103. [Google Scholar]
- Dendy, A. Additions to the cryptozoic fauna of New Zealand. Ann. Mag. Nat. Hist. 1894, 14, 393–401. [Google Scholar] [CrossRef]
- Dendy, A. A new peripatus from New Zealand. Nature 1900, 61, 444. [Google Scholar] [CrossRef]
- Ruhberg, H. Die Peripatopsidae (Onychophora). Systematik, okologie, chorologie und phylogenetische aspekte. Zoologica 1985, 137, 1–183. [Google Scholar]
- Tutt, K.; Daugherty, C.H.; Gibbs, G.W. Life history characteristics of male and female Peripatoides novaezealandiae (Onychophora: Peripatopsidae). J. Zool. 2002, 258, 257–267. [Google Scholar] [CrossRef]
- Manton, S.M. Studies on the Onychophora, IV—The passage of spermatozoa into the ovary on Peripatopsis and the early developments of the ova. Philos. Trans. R. Soc. 1938, 228, 421–441. [Google Scholar]
- Ruhberg, H. Onychophora. In Reproductive Biology of Invertebrates; Adiyodi, K.G., Adiyodi, R.G., Eds.; Oxford & IBH Publishing Co.: New Dehli, India, 1990; pp. 61–76. [Google Scholar]
- Mayer, G. Metaperipatus inae sp. nov. (Onychophora: Peripatopsidae) from Chile with a novel ovarian type and dermal insemination. Zootaxa 2007, 11440, 21–37. [Google Scholar] [CrossRef]
- Curach, N.; Sunnucks, P. Molecular anatomy of an onychophoran: Compartmentalized sperm storage and heterogeneous paternity. Mol. Ecol. 1999, 8, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Southwood, T.R.E.; May, R.M.; Hassell, M.P.; Conway, G.R. Ecological strategies and population parameters. Am. Nat. 1974, 108, 791–804. [Google Scholar] [CrossRef]
- McLennan, J.A. Breeding of North Island brown kiwi, Apteryx australis mantelli, in Hawke’s Bay, New Zealand. N. Z. J. Ecol. 1988, 11, 89–97. [Google Scholar]
- Pianka, E.R. On r- and K-selection. Am. Nat. 1970, 104, 592–597. [Google Scholar] [CrossRef]
- Bull, J.K.; Sands, C.J.; Garrick, R.C.; Gardner, M.G.; Tait, N.N.; Briscoe, D.A.; Rowell, D.M.; Sunnucks, P. Environmental complexity and biodiversity: The multi-layered evolutionary history of a log-dwelling velvet worm in montane temperate Australia. PLoS ONE 2013, 8, e84559. [Google Scholar] [CrossRef] [PubMed]
- Trewick, S.A.; Morgan-Richards, M. Artificial weta roosts: A technique for ecological study and population monitoring of tree weta (Hemideina) and other invertebrates. N. Z. J. Ecol. 2000, 24, 201–208. Available online: https://newzealandecology.org/nzje/2111 (accessed on 3 March 2024).
- Mayer, G.; Oliveira, I.S.; Baer, A.; Hammel, J.U.; Gallant, J.; Hochberg, R. Capture of prey, feeding, and functional Anatomy of the jaws in velvet worms (Onychophora). Integr. Comp. Biol. 2015, 55, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Tait, N.N.; Briscoe, D.A. Genetic differentiation within New Zealand Onychophora and their relationships to the Australian fauna. Zool. J. Linn. Soc. 1995, 114, 103–113. [Google Scholar] [CrossRef]
- Trewick, S.A. Sympatric cryptic species in New Zealand Onychophora. Biol. J. Linn. Soc. 1998, 63, 307–329. [Google Scholar] [CrossRef]
- Trewick, S.A. Molecular diversity of Dunedin peripatus (Onychophora: Peripatopsidae). N. Z. J. Ecol. 1999, 26, 381–393. [Google Scholar] [CrossRef]
- Trewick, S.A. Mitochondrial DNA sequences support allozyme evidence for cryptic radiation of New Zealand Peripatoides (Onychophora). Mol. Ecol. 2000, 9, 269–281. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 8 November 2023).
- Becker, R.A.; Wilks, A.R.; Brownrigg, R.; Minka, T.P.; Deckmyn, A. Maps: Draw Geographical Maps, R Package Version 3.4.1; 2022. Available online: https://CRAN.R-project.org/package=maps (accessed on 8 November 2023).
- Becker, R.A.; Wilks, A.R.; Brownrigg, R. Mapdata: Extra Map Databases, R Package Version 2.3.1; 2022. Available online: https://CRAN.R-project.org/package=mapdata (accessed on 8 November 2023).
- May, B. Starch gel electrophoresis of allozymes. In Molecular Genetic Analysis of Populations: A Practical Approach; Hoelzel, A.R., Ed.; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Frichot, E.; François, O. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Sunnucks, P.; Hales, D.F. Numerous transposed sequences of mitochondrial cytochrome oxidase I–II in aphids of the genus Sitobion (Hemiptera: Aphididae). Mol. Biol. Evol. 1996, 13, 510–524. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Am. Entomol. Soc. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Pripnow, B.; Ruhberg, H. Peripatopsidae (Onychophora) from New Zealand—Observations on selected morphs of the ‘Peripatoides novaezealandiae—complex’ in culture: Morphological and reproductive aspects. Afr. Invertebr. 2003, 44, 103–114. [Google Scholar]
- Ruhberg, H.; Daniels, S.R. Morphological assessment supports the recognition of four novel species in the widely distributed velvet worm Peripatopsis moseleyi sensu lato (Onychophora: Peripatopsidae). Invertebr. Syst. 2012, 27, 131–145. [Google Scholar] [CrossRef]
- Oliveira, I.; Ruhberg, H.; Rowell, D.M.; Mayer, G. Revision of Tasmanian viviparous velvet worms (Onychophora: Peripatopsidae) with descriptions of two new species. Invertebr. Syst. 2018, 32, 909–932. [Google Scholar] [CrossRef]
- Sherbon, B.J.; Walker, M.H. A new species of Peripatopsis from South Africa, P. stelliporata, with observations on embryonic development and sperm degradation (Onychophora, Peripatopsidae). J. Zool. 2004, 264, 295–305. [Google Scholar] [CrossRef]
- Silva, J.R.M.C.; Coelho, M.P.D.; Nogueira, M.I. Induced inflammatory process in Peripatus acacioi Marcus et Marcus (Onychophora). J. Invertebr. Pathol. 2000, 75, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Trewick, S.A.; Bland, K.J. Fire and Slice: Palaeogeography for biogeography at New Zealand’s North Island/ South Island juncture. J. R. Soc. N. Z. 2012, 42, 153–183. [Google Scholar] [CrossRef]
- Masters, B.C.; Fan, V.; Ross, H.A. Species delimitation—A Geneious plugin for the exploration of species boundaries. Mol. Ecol. Resour. 2011, 11, 154–157. [Google Scholar] [CrossRef]
- Trewick, S.; Hitchmough, R.; Rolfe, J.; Stringer, I. Conservation status of New Zealand Onychophora (‘Peripatus’ or Velvet Worm); New Zealand Threat Classification Series 26; Department of Conservation: Wellington, New Zealand, 2018; p. 3.
- DOC. New Zealand Peripatus/Ngaokeoke Current Knowledge, Conservation and Future Research Needs; Department of Conservation, Ōtepoti/Dunedin Office: Dunedin, New Zealand, 2014; ISBN 978-0-478-15009-4.
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.L.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 3, 148–155. [Google Scholar] [CrossRef]
- Wallace, A.R. On the phenomena of variation and geographical distribution as illustrated by the Papilioniae of the Malayan region. Trans. Linn. Soc. Lond. 1965, 25, 1–71. [Google Scholar] [CrossRef]
- Luo, A.; Ling, C.; Ho, S.Y.W.; Zhu, C.-D. Comparison of methods for molecular species delimitation across a range of speciation scenarios. Syst. Biol. 2018, 67, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M. Species delimitation in herpetology. J. Herpetol. 2019, 53, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M.; Chambers, E.A.; Devitt, T.J. Contemporary methods and evidence for species delimitation. Ichthyol. Herpetol. 2021, 109, 895–903. [Google Scholar] [CrossRef]
- Vaux, F.; Trewick, S.A.; Morgan-Richards, M. Speciation through the looking-glass. Biol. J. Linn. Soc. 2017, 120, 480–488. [Google Scholar] [CrossRef]
- Harrison, R.G.; Larson, E.L. Hybridisation, introgression, and the nature of species boundaries. J. Hered. 2014, 105, 795–809. [Google Scholar] [PubMed]
- Morgan-Richards, M.; Wallis, G.P. A comparison of five hybrid zones of the weta Hemideina thoracica (orthoptera: Anostostomatidae): Degree of cytogenetic differentiation fails to predict zone width. Evolution 2007, 57, 849–861. [Google Scholar] [CrossRef]
- Cunha, W.T.R.; Santos, R.C.O.; Araripe, J.; Sampaio, I.; Schneider, H.; Rêgo, P.S. Molecular analyses reveal the occurrence of three new sympatric lineages of velvet worms (Onychophora: Peripatidae) in the eastern Amazon basin. Genet. Mol. Biol. 2017, 40, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Barquero-González, J.P.; Sánchez-Vargas, S.; Morera-Brenes, B. A new giant velvet worm from Costa Rica suggests absence of the genus Peripatus (Onychophora: Peripatidae) in Central America. Rev. Biol. Trop. 2020, 68, 300–320. [Google Scholar] [CrossRef]
- Costa, C.S.; Giribet, G. Panamanian velvet worms in the genus Epiperipatus, with notes on their taxonomy and distribution and the description of a new species (Onychophora, Peripatidae). Invertebr. Biol. 2021, 140, e12336. [Google Scholar] [CrossRef]
- Sato, S.; Buckman-Young, R.S.; Harvey, M.S.; Giribet, G. Cryptic speciation in a biodiversity hotspot: Multilocus molecular data reveal new velvet worm species from Western Australia (Onychophora: Peripatopsidae: Kumbadjena). Invertebr. Syst. 2018, 32, 1249–1264. [Google Scholar] [CrossRef]
- Reid, A.L. Review of the Peripatopsidae (Onychophora) in Australia, with comments on Peripatopsid relationships. Invertebr. Syst. 1996, 10, 663–936. [Google Scholar] [CrossRef]
- Reid, A.L. Eight new Planipapillus (Onychophora: Peripatopsidae) from southeastern Australia. Proc. Linn. Soc. New South Wales 2000, 122, 1–32. [Google Scholar]
- Reid, A.L.; Tait, N.N.; Briscoe, D.A.; Rowell, D.M. Morphological, cytogenetic and allozymic variation within Cephalofovea (Onychophora: Peripatopsidae) with descriptions of three new species. Zool. J. Linn. Soc. 1995, 114, 115–138. [Google Scholar] [CrossRef]
- Daniels, S.R.; Picker, M.D.; Cowlin, R.M.; Hamer, M.L. Unravelling evolutionary lineages among South African velvet worms (Onychophora: Peripatopsis) provides evidence for widespread cryptic speciation. Biol. J. Linn. Soc. 2009, 97, 200–216. [Google Scholar] [CrossRef]
- Barnes, A.; Reiss, T.; Daniels, S.R. Systematics of the Peripatopsis clavigera species complex (Onychophora: Peripatopsidae) reveals cryptic cladogenic patterning, with the description of five new species. Invertebr. Syst. 2020, 34, 569–590. [Google Scholar] [CrossRef]
- Mcdonald, D.E.; Daniels, S.R. Phylogeography of the Cape velvet worm (Onychophora: Peripatopsis capensis) reveals the impact of Pliocene/Pleistocene climatic oscillations on Afromontane forest in the Western Cape, South Africa. J. Evol. Biol. 2012, 25, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Grobler, P.C.J.; Myburgh, A.M.; Barnes, A.; Savel, R. Integrative taxonomy provides evidence for a cryptic lineage in the velvet worm Peripatopsis birgeri species complex (Onychophora: Peripatopsidae) in KwaZulu-Natal, South Africa. Syst. Biodivers. 2023, 21, 2207574. [Google Scholar] [CrossRef]
- Barnes, A.; Daniels, S.R. On the importance of fine-scale sampling in detecting alpha taxonomic diversity among saproxylic invertebrates: A velvet worm (Onychophora: Opisthopatus amaxhosa) template. Zool. Scr. 2019, 48, 243–262. [Google Scholar] [CrossRef]
- Taylor-Smith, B.L.; Morgan-Richards, M.; Trewick, S.A. Patterns of regional endemism among New Zealand invertebrates. N. Z. J. Ecol. 2019, 47, 1–19. [Google Scholar] [CrossRef]
- Morgan-Richards, M.; Bulgarella, M.; Sivyer, L.; Dowle, E.; Hale, M.; McKean, N.; Trewick, S.A. Explaining large mitochondrial sequence differences within a population sample. R. Soc. Open Access 2017, 4, 179739. [Google Scholar] [CrossRef] [PubMed]
- Sivyer, L.; Morgan-Richards, M.; Koot, E.; Trewick, S.A. Anthropogenic cause of range shifts and gene flow between two grasshopper species revealed by environmental modelling, geometric morphometrics and population genetics. Insect Conserv. Divers. 2018, 11, 415–434. [Google Scholar] [CrossRef]
- Trewick, S.A.; Wallis, G.P. Bridging the ‘beech-gap’: Invertebrate phylogeography implicates recent rather than ancient processes in New Zealand biogeographic patterns. Evolution 2001, 55, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Mallet, J. Hybridization, ecological races and the nature of species: Empirical evidence for the ease of speciation. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2971–2986. [Google Scholar] [CrossRef] [PubMed]
- Eliott, S.; Tait, N.N.; Briscoe, D.A. A pheromonal function for the crural glands of the onychophoran Cephalofovea tomahmontis (Onychophora: Peripatopsidae). J. Zool. 1993, 231, 1–9. [Google Scholar] [CrossRef]
- Rockman, M.V.; Rowell, D.M. Episodic chromosomal evolution in Planipapillus (Onychophora: Peripatopsidae): A phylogenetic approach to evolutionary dynamics and speciation. Evolution 2002, 56, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Rowell, D.M.; Rockman, M.V.; Tait, N.N. Extensive Robertsonian rearrangement: Implications for the radiation and biogeography of Planipapillus Reid (Onychophora: Peripatopsidae). J. Zool. 2002, 257, 171–179. [Google Scholar] [CrossRef]
- Rowell, D.M.; Higgins, A.V.; Briscoe, D.A.; Tait, N.N. The use of chromosomal data in the systematics of viviparous onychophorans from Australia (Onychophora: Peripatopsidae). Zool. J. Linn. Soc. 1995, 114, 139–153. [Google Scholar] [CrossRef]
- Briscoe, D.A.; Tait, N.N. Allozyme evidence for extensive and ancient radiations in Australian Onychophora. Zool. J. Linn. Soc. 1995, 114, 91–102. [Google Scholar] [CrossRef]
- Hebert, P.D.; Billington, N.; Finston, T.L.; Boileau, M.G.; Beaton, M.J.; Barrette, R.J. Genetic variation in the onychophoran Plicatoperipatus jamaicensis. Heredity 1991, 67, 221–229. [Google Scholar] [CrossRef]
- Garrick, R.C.; Rowell, D.M.; Sunnucks, P. Phylogeography of saproxylic and forest floor invertebrates from Tallaganda, South-eastern Australia. Insects 2012, 3, 270–294. [Google Scholar] [CrossRef] [PubMed]
- Barquero González, J.P.; Cabrera Alvarado, A.A.; Valle-Cubero, S.; Monge Nájera, J.; Morera, B. The geographic distribution of Costa Rican velvet worms (Onychophora: Peripatidae). Rev. Biol. Trop. 2016, 64, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Wille, A.; Fuentes, G. Efecto de la ceniza del Volcán Irazú (Costa Rica) en algunos insectos. Rev. Biol. Trop. 1975, 23, 165–175. [Google Scholar]
- Harris, A.C. A large aggregation of Peripatoides novaezealandiae (Hutton, 1876) (Onycophora: Peripatopsidae). J. R. Soc. New Zealand 1991, 21, 405–406. [Google Scholar] [CrossRef]
- Marshall, J.C.; Martin, H. Velvet worm (Phylum Onychophora) on a sand island, in a wetland: Flushed from a Pleistocene refuge by recent rainfall? Austral Ecol. 2020, 45, 264–267. [Google Scholar] [CrossRef]
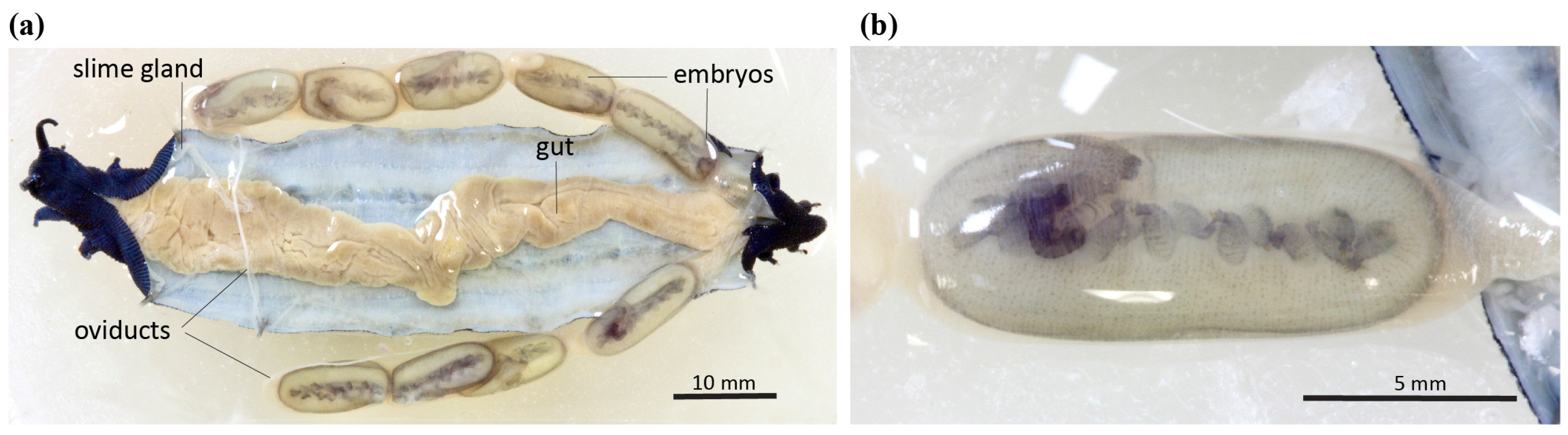

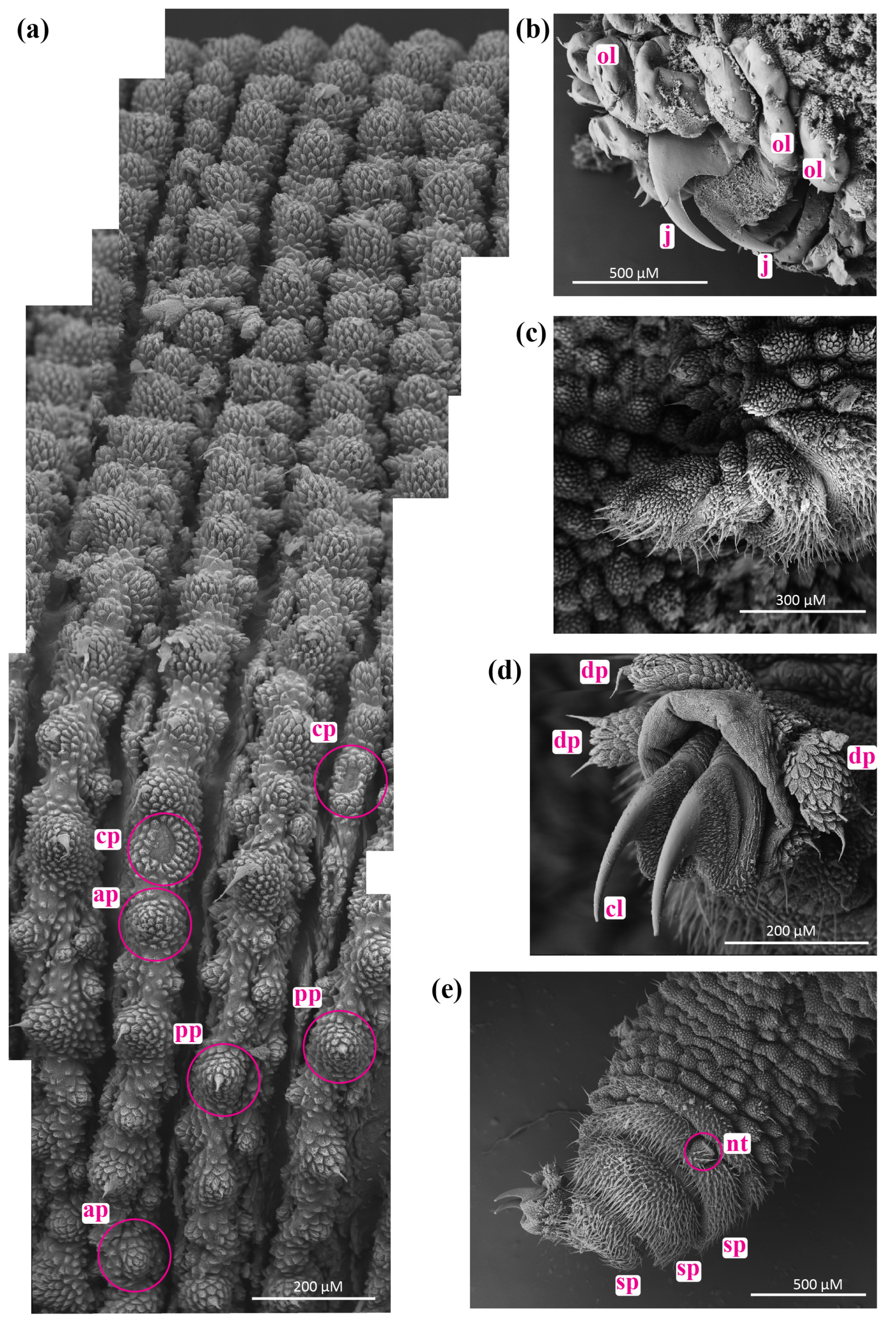
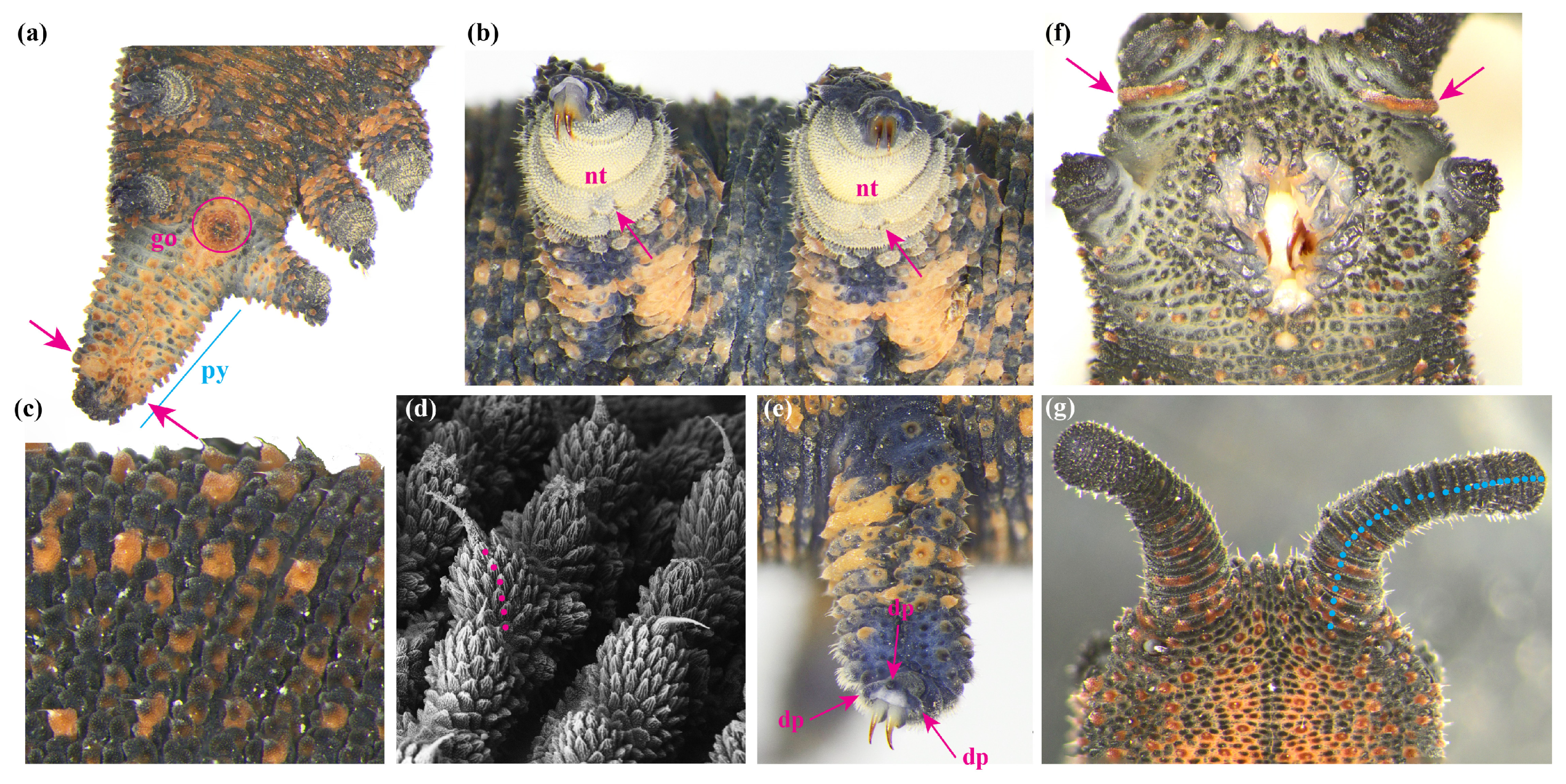










| Species | n | Leg Pairs | Orange Papillae , Range | Orange Antennal Base | Genital Opening | Spinous Pads | Distal Papillae | Nephridial Tubercle Division |
Antenna Rings Range |
Papillary Scales Mode, Range |
Average Length mm | Colour Pattern | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4th | 5th | Lateral | Ventral | Dorsal | |||||||||||
| P. sympatrica | 16 | 15 | 23 12–47 | Absent, partial, or complete | Blue–grey or orange mix | 3 | 3 | Complete or rarely partial | Complete | 24–33 | 8, 6–10 | 20.7 | Absent or partial | Absent, partial or strong | Absent or partial |
| P. indigo | 10 | 15 | 0 | Absent | Blue–grey, rarely orange | 4 | 5 | Complete or rarely partial | Complete or rarely partial | 30–40 | 9, 6–12 | 26.6 | None | None | None |
| P. aurorbis | 13 | 15 | 14.3 4–25 | Absent | Orange or very pale | 3 | 3 | Complete or rarely partial | Complete | 24–33 | 6, 5–8 | 17.8 | None | Partial or strong | Strong or partial |
| P. kawekaensis | 4 | 15 | 30.5 24–44 | Partial | Blue–grey or orange mix | 3 | 3 | Complete or rarely partial | Complete or rarely partial | 20–31 | 6, 6–10 | 19.3 | Absent or partial | Partial or strong | Absent or partial |
| P. suteri | 3 | 16 | 15.7 15–17 | Absent | Blue–grey | 3 | 4 | Partial, rarely complete | Complete | 27–32 | 6, 6–10 | 33 | Absent | Absent | Absent |
| P. novaezealandiae | 8 | 15 | 20.4 15–34 | Absent or partial | Blue–grey | 3 | 3 | Partial, rarely complete | Partial, rarely complete | 21–35 | 8, 7–10 | 24.4 | Absent, partial or strong | Absent, partial or strong | Absent, partial or strong |
| P. morgani | 8 | 15 | 22.1 12–38 | Partial or absent | Blue–grey or orange mix | 3 | 3 | Partial or complete | Partial or complete | 24–32 | 8, 6–10 | 24.4 | Absent, partial or strong | Absent, partial or strong | Absent or partial |
| Species | n | h | Hd | Pi | HKY |
|---|---|---|---|---|---|
| P. sympatrica | 129 | 69 | 0.984 | 0.01613 | 0.0194 |
| P. indigo | 4 | 3 | 0.833 | 0.00191 | 0.0019 |
| P. aurorbis | 43 | 33 | 0.986 | 0.0322 | 0.0333 |
| P. kawekaensis | 26 | 16 | 0.938 | 0.01287 | 0.0130 |
| P. suteri | 18 | 12 | 0.948 | 0.01752 | 0.0189 |
| P. novaezealandiae | 24 | 18 | 0.975 | 0.01989 | 0.0204 |
| P. morgani | 29 | 16 | 0.938 | 0.02075 | 0.0212 |
| P. taitonga sp. nov. | 10 | 10 | 1.0 | 0.0197 | 0.0210 |
| P. otepote sp. nov. | 20 | 13 | 0.926 | 0.02087 | 0.0214 |
| P. waikaia sp. nov. | 6 | 2 | 0.333 | 0.00127 | 0.0013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trewick, S.A.; Koot, E.M.; Morgan-Richards, M. Ngāokeoke Aotearoa: The Peripatoides Onychophora of New Zealand. Insects 2024, 15, 248. https://doi.org/10.3390/insects15040248
Trewick SA, Koot EM, Morgan-Richards M. Ngāokeoke Aotearoa: The Peripatoides Onychophora of New Zealand. Insects. 2024; 15(4):248. https://doi.org/10.3390/insects15040248
Chicago/Turabian StyleTrewick, Steven A., Emily M. Koot, and Mary Morgan-Richards. 2024. "Ngāokeoke Aotearoa: The Peripatoides Onychophora of New Zealand" Insects 15, no. 4: 248. https://doi.org/10.3390/insects15040248
APA StyleTrewick, S. A., Koot, E. M., & Morgan-Richards, M. (2024). Ngāokeoke Aotearoa: The Peripatoides Onychophora of New Zealand. Insects, 15(4), 248. https://doi.org/10.3390/insects15040248






