Mitogenomic Analysis and Phylogenetic Implications for the Deltocephaline Tribe Chiasmini (Hemiptera: Cicadellidae: Deltocephalinae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxon Sampling
2.2. DNA Extraction and Sequencing
2.3. Mitogenome Sequence Assembly and Annotation
2.4. Mitogenome Sequence Analysis
2.5. Phylogenetic Analysis
3. Results and Discussion
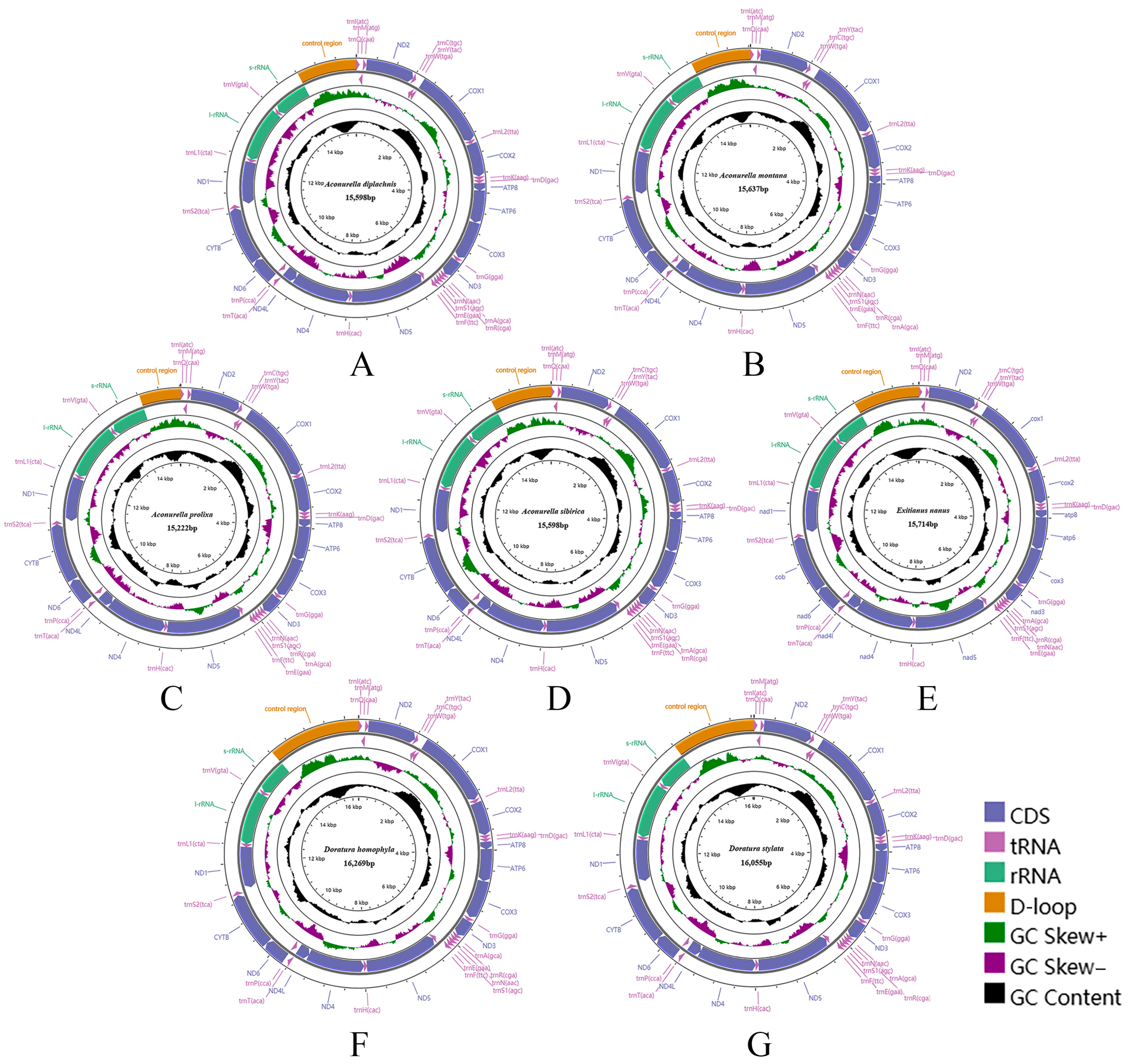
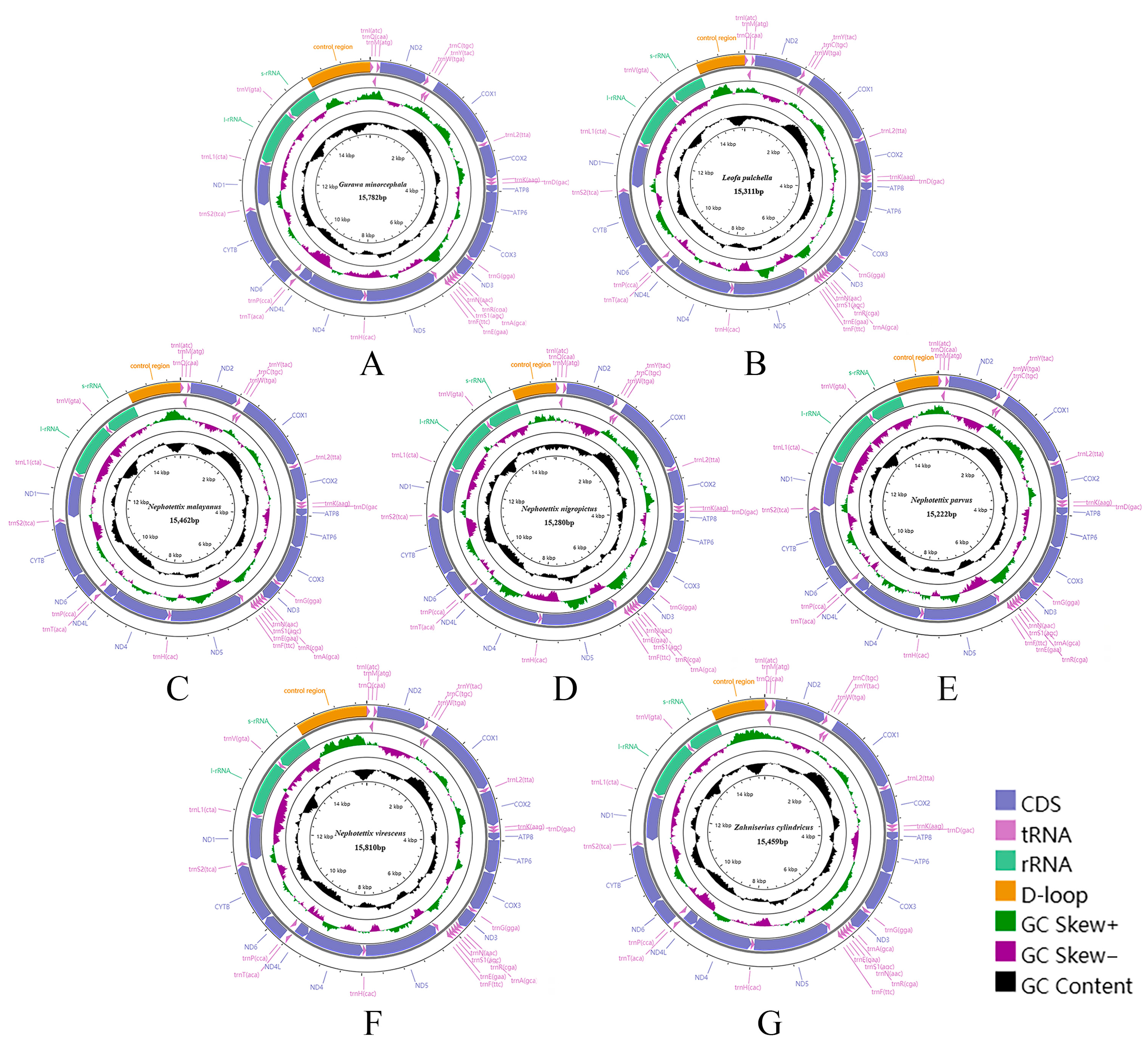
3.1. Mitogenome Analysis
3.2. Mitogenome Organization and Gene Content
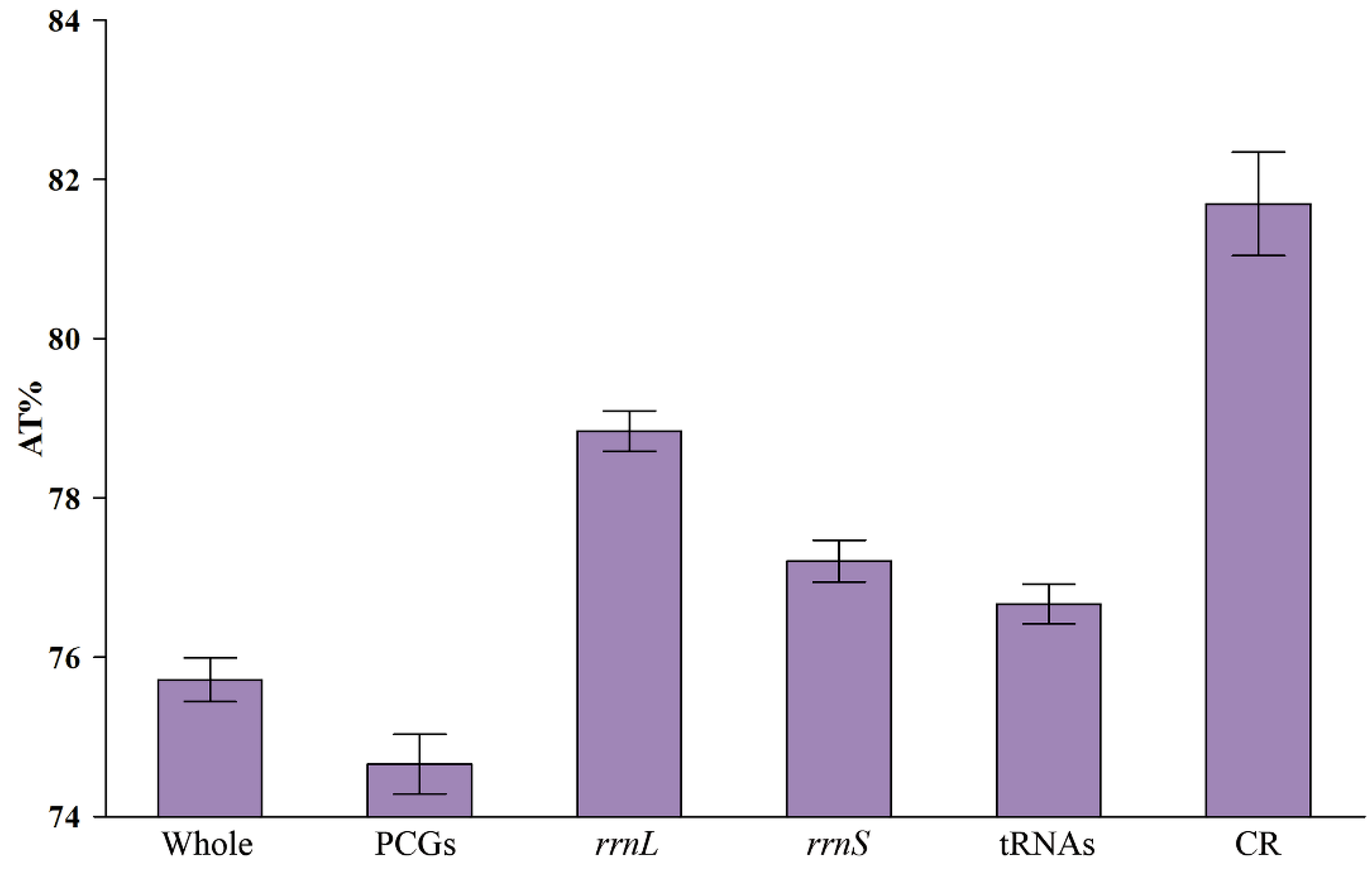
3.3. Nucleotide Composition
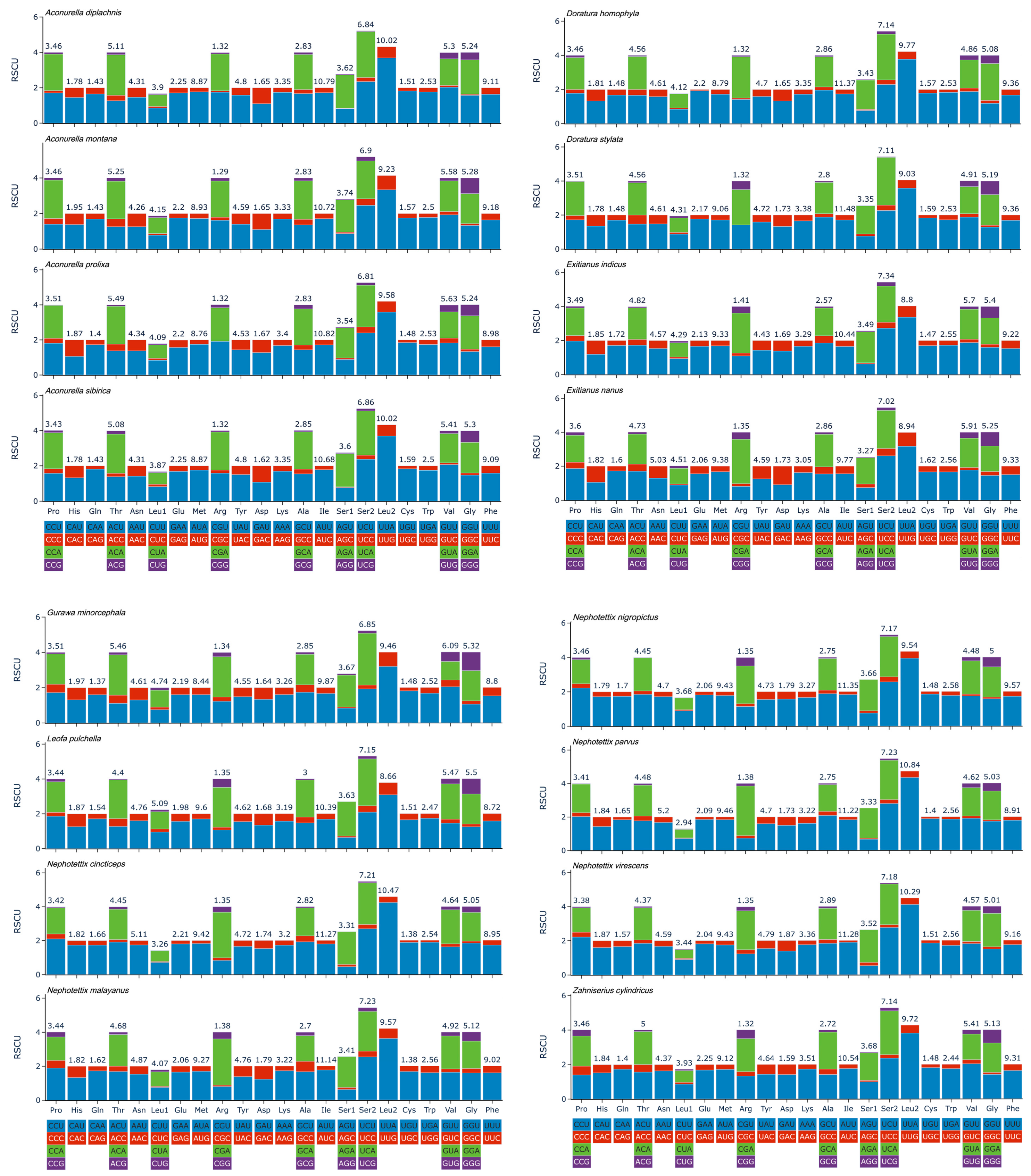
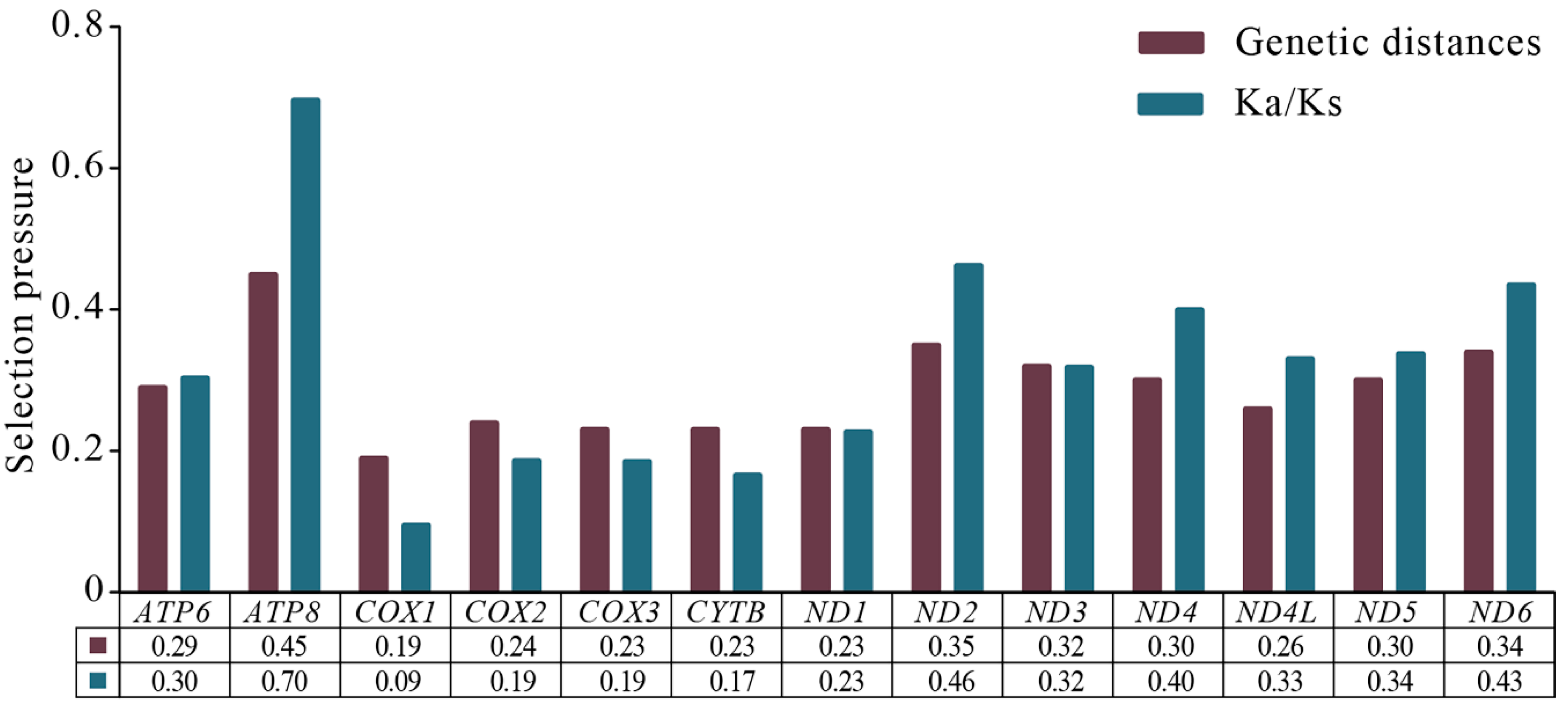
3.4. Protein-Coding Genes and Codon Usage
3.5. RNA Genes
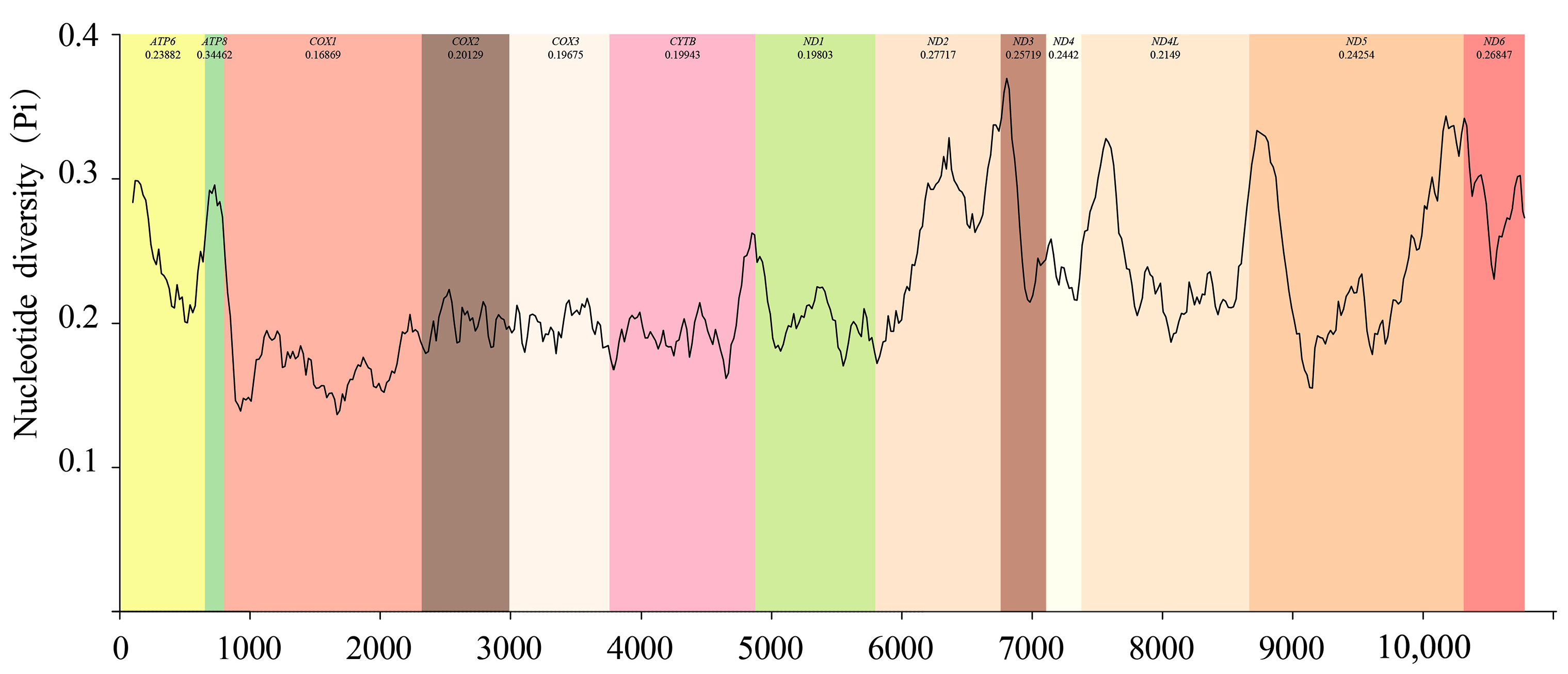
3.6. Gene Overlap and Non-Coding Regions
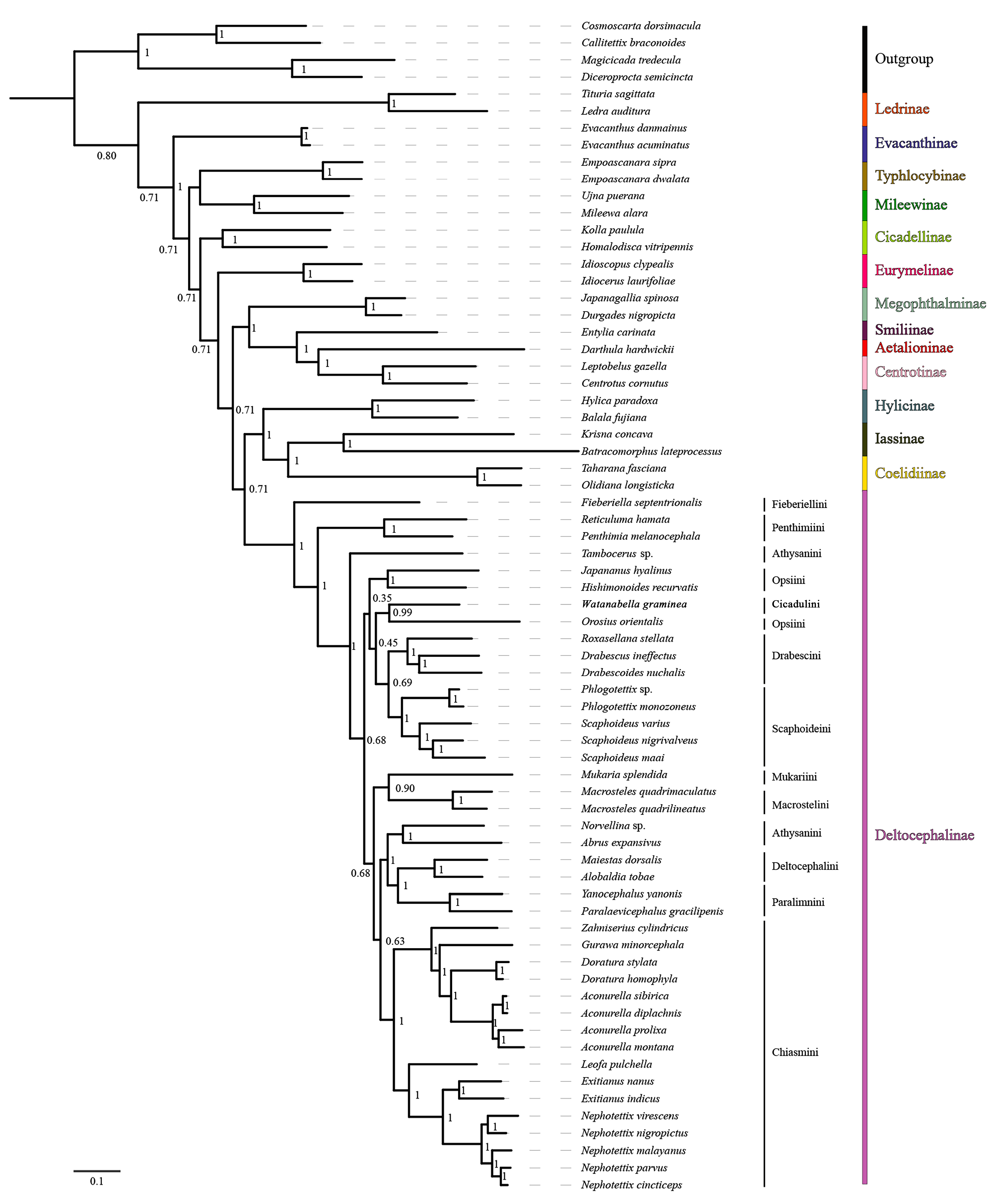
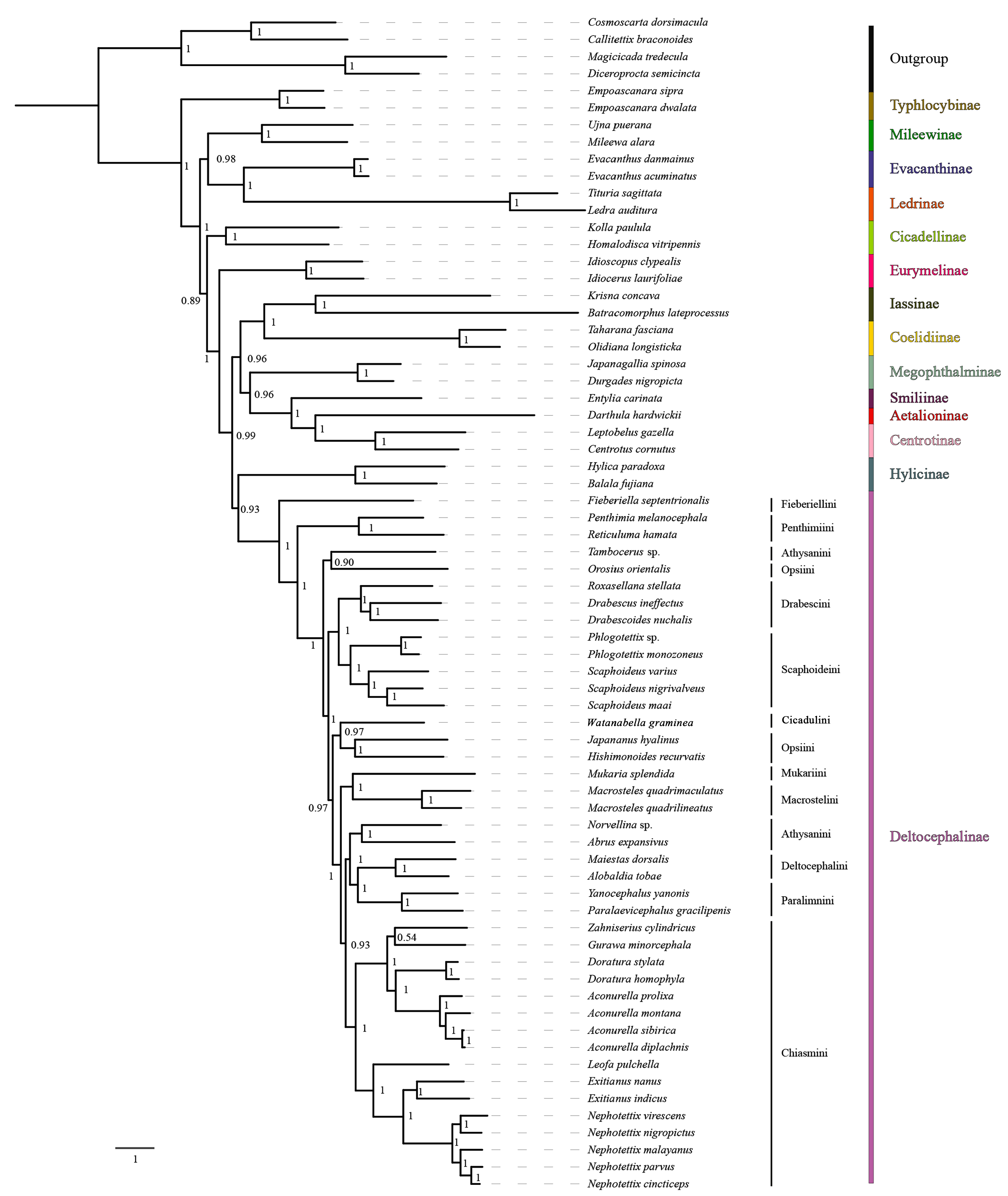
3.7. Phylogenetic Relationships
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]
- Abascal, F.; Posada, D.; Knight, R.D.; Zardoya, R. Parallel evolution of the genetic code in arthropod mitochondrial genomes. PLoS Biol. 2006, 4, e127. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef]
- Wilson, A.C.; Cann, R.L.; Carr, S.M.; George, M.; Gyllensten, U.B.; Helm-Bychowski, K.M.; Higuchi, R.B.; Palumbi, S.R.; Prager, E.M.; Sage, R.D.; et al. Mitochondrial DNA and two perspectives on evolutionary genetics. Biol. J. Linn. Soc. 1985, 26, 375–400. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Gao, Y.; Xiong, J.L.; Dietrich, C.H.; Duan, Y.N. Phylogenetic analyses of the leafhopper tribe Chiasmini and species delimitation of the genus Exitianus Ball (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini) in China based on molecular data. Eur. J. Taxon. 2024, 921, 276–297. [Google Scholar]
- Zahniser, J.N.; Dietrich, C.H. A review of the tribes of Deltocephalinae (Hemiptera: Auchenorrhyncha: Cicadellidae). Eur. J. Taxon. 2013, 45, 1–211. [Google Scholar] [CrossRef]
- Cao, Y.H.; Dietrich, C.H.; Zahniser, J.N.; Dmitriev, D.A. Dense sampling of taxa and characters improves phylogenetic resolution among deltocephaline leafhoppers (Hemiptera: Cicadellidae: Deltocephalinae). Syst. Entomol. 2022, 47, 430–444. [Google Scholar] [CrossRef]
- Duan, Y.N.; Zhang, Y.L.; Webb, M.D. Review of the grassland leafhopper subgenus Leofa (Prasutagus) Distant (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini). Zootaxa 2009, 1972, 35–43. [Google Scholar] [CrossRef]
- Duan, Y.N.; Zhang, Y.L. A taxonomic review of the grassland leafhopper genera Gurawa Distant and Chiasmus Mulsant & Rey (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini) from China with description of a new species. Zootaxa 2012, 3537, 41–52. [Google Scholar]
- Duan, Y.N.; Zhang, Y.L. Redescription of the grassland leafhopper genus Doraturopsis Lindberg (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini) with description of a new genus and species from China. Zootaxa 2012, 3177, 24–32. [Google Scholar] [CrossRef]
- Duan, Y.N.; Zhang, Y.L. New records of the grassland leafhopper genus Doratura Sahlberg (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini) from China. Zootaxa 2012, 3175, 54–62. [Google Scholar] [CrossRef]
- Duan, Y.N.; Zhang, Y.L. A taxonomic review of the grassland leafhopper genus Aconurella Ribaut (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini) from China with the description of two new species. Zootaxa 2012, 3397, 28–44. [Google Scholar] [CrossRef]
- Duan, Y.N.; Zhang, Y.L. Review of the grassland leafhopper genus Exitianus Ball (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini) from China. ZooKeys 2013, 333, 31–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duan, Y.N.; Zhang, Y.L. Review of the grassland leafhopper genus Nephotettix Matsumura (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini) from the Chinese mainland. Zootaxa 2014, 3755, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.L.; Dietrich, C.H.; Duan, Y.N. Phylogenetic analyses and species delimitation of Nephotettix Matsumura (Hemiptera: Cicadellidae: Deltocephalinae: Chiasmini) in China based on molecular data. Zool. Anz. 2021, 293, 202–214. [Google Scholar] [CrossRef]
- Carloni, E.; Virla, E.; Paradell, S.; Carpane, P.; Nome, C.; Laguna, I.; Giménez Pecci, M.P. Exitianus obscurinervis (Hemiptera: Cicadellidae), a new experimental vector of Spiroplasma kunkelii. J. Econom. Entomol. 2011, 104, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Izadpanah, K.; Siampour, M.; Taghizadeh, M. Molecular characterization, and transmission of Bermuda grass white leaf phytoplasma in Iran. J. Plant Pathol. 2009, 91, 655–661. [Google Scholar]
- Zahniser, J.N. Seven new species and new distributions of Old World Chiasmini (Hemiptera: Cicadellidae: Deltocephalinae), with a redescription, key to genera, and species checklist for the tribe. Zootaxa 2008, 1808, 1–32. [Google Scholar] [CrossRef]
- Zahniser, J.N. An enigmatic new leafhopper genus, Protochiasmus (Hemiptera: Cicadellidae: Deltocephalinae), from Brazil. Dtsch. Entomol. Z. 2010, 57, 271–274. [Google Scholar] [CrossRef]
- Zahniser, J.N. New generic synonymies and combinations in Chiasmini (Cicadellidae: Deltocephalinae). Int. J. Trop. Insect Sci. 2012, 32, 173–176. [Google Scholar] [CrossRef]
- Zahniser, J.N.; Dietrich, C.H. Phylogeny of the leafhopper subfamily Deltocephalinae (Insecta: Auchenorrhyncha: Cicadellidae) and related subfamilies based on morphology. Syst. Biodivers. 2008, 6, 1–24. [Google Scholar] [CrossRef]
- Zahniser, J.N.; Dietrich, C.H. Phylogeny of the leafhopper subfamily Deltocephalinae (Hemiptera: Cicadellidae) based on molecular and morphological data with a revised family-group classification. Syst. Entomol. 2010, 35, 489–511. [Google Scholar] [CrossRef]
- Zahniser, J.N.; Dietrich, C.H. Phylogeny, evolution, and historical biogeography of the grassland leafhopper tribe Chiasmini (Hemiptera: Cicadellidae: Deltocephalinae). Zool. J. Linn. Soc. 2015, 175, 473–495. [Google Scholar] [CrossRef]
- Du, Y.M.; Dietrich, C.H.; Dai, W. Complete mitochondrial genome of Macrosteles quadrimaculatus (Matsumura) (Hemiptera: Cicadellidae: Deltocephalinae) with a shared tRNA rearrangement and its phylogenetic implications. Int. J. Biol. Macromol. 2019, 122, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Cai, W.Z.; Li, H. Insufficient power of mitogenomic data in resolving the auchenorrhynchan monophyly. Zool. J. Linn. Soc. 2018, 183, 776–790. [Google Scholar] [CrossRef]
- Wu, K.Q.; Yan, M.H.; Zhang, Y.L.; Dietrich, C.H.; Duan, Y.N. The complete mitochondrial genome of Aconurella prolixa (Lethierry 1885) (Deltocephalinae: Chiasmini). Mitochondrial DNA Part B 2022, 7, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Tan, Z.X.; Shen, R.R.; Xing, J.C. Comparative mitochondrial genome analysis of three leafhopper species of the genus Abrus Dai & Zhang (Hemiptera: Cicadellidae: Deltocephalinae) from China with phylogenetic implication. BMC Genomics 2023, 24, 714. [Google Scholar]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313319. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.L.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Kück, P.; Meid, S.A.; Groß, C.; Wägele, J.W.; Misof, B. AliGROOVE-visualization of heterogeneous sequence divergence within multiple sequence alignments and detection of inflated branch support. BMC Bioinformatics 2014, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; IQ-TREE, R.L. 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Liu, J.; Bu, C.P.; Wipfler, B.; Liang, A.P. Comparative analysis of the mitochondrial genomes of Callitettixini spittlebugs (Hemiptera: Cercopidae) confirms the overall high evolutionary speed of the AT-Rich region but reveals the presence of short conservative elements at the tribal level. PLoS ONE 2014, 9, e109140. [Google Scholar] [CrossRef] [PubMed]
- Su, T.J.; He, B.; Li, K.; Liang, A.P. Comparative analysis of the mitochondrial genomes of oriental spittlebug trible Cosmoscartini: Insights into the relationships among closely related taxa. BMC Genom. 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Hasegawa, H.; Cooley, J.R.; Simon, C.; Yoshimura, J.; Cai, W.Z.; Sota, T.; Li, H. Mitochondrial genomics reveals shared phylogeographic patterns and demographic history among three periodical cicada species groups. Mol. Biol. Evol. 2019, 36, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.P.; Gao, J.; Zhao, X. Characterization of the complete mitochondrial genome of the treehopper Darthula hardwickii (Hemiptera: Aetalionidae). Mitochondrial DNA Part A 2016, 27, 3291–3292. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, J.J.; Dai, R.H. Mitogenomics of five Olidiana leafhoppers (Hemiptera: Cicadellidae: Coelidiinae) and their phylogenetic implications. Peerj 2021, 9, e11086. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, H.; Dai, R.H. Complete mitochondrial genome of Taharana fasciana (Insecta, Hemiptera: Cicadellidae) and comparison with other Cicadellidae insects. Genetica 2017, 145, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Xing, J.C. Complete mitochondrial genome of Abrus expansivus (Hemiptera: Cicadellidae: Deltocephalinae) from China. Mitochondrial DNA Part B 2019, 4, 197–198. [Google Scholar] [CrossRef]
- Song, N.; Cai, W.Z.; Li, H. Deep-level phylogeny of Cicadomorpha inferred from mitochondrial genomes sequenced by NGS. Sci. Rep. 2017, 7, 10429. [Google Scholar] [CrossRef]
- Yu, P.F.; Wang, M.X.; Cui, L.; Chen, X.X.; Han, B.Y. The complete mitochondrial genome of Tambocerus sp. (Hemiptera: Cicadellidae). Mitochondrial DNA Part A 2015, 28, 133–134. [Google Scholar] [CrossRef]
- Yang, W.J.; Gao, Y.R.; Li, C.; Song, Y.H. The complete mitochondrial genome of Chlorotettix nigromaculatus (Hemiptera: Cicadellidae: Deltocephalinae) with phylogenetic consideration. Mitochondrial DNA Part B 2019, 4, 624–625. [Google Scholar] [CrossRef]
- Du, Y.M.; Zhang, C.N.; Dietrich, C.H.; Zhang, Y.L.; Dai, W. Characterization of the complete mitochondrial genomes of Maiestas dorsalis and Japananus hyalinus (Hemiptera: Cicadellidae) and comparison with other Membracoidea. Sci. Rep. 2017, 7, 14197. [Google Scholar] [CrossRef]
- Wu, Y.F.; Dai, R.H.; Zhan, H.P.; Qu, L. Complete mitochondrial genome of Drabescoides nuchalis (Hemiptera: Cicadellidae). Mitochondrial DNA Part A 2016, 27, 3626–3627. [Google Scholar] [CrossRef]
- Xu, D.L.; Yu, T.H.; Zhang, Y.L. Characterization of the complete mitochondrial genome of Drabescus ineffectus and Roxasellana stellata (Hemiptera: Cicadellidae: Deltocephalinae: Drabescini) and their phylogenetic implications. Insects 2020, 11, 534. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Y.; Di, X.C.; Shan, L.C.Y.; Wang, S.S. The complete mitochondrial genome of Fieberiella septentrionalis (Hemiptera: Cicadellidae: Deltocephalinae). Mitochondrial DNA Part B 2021, 6, 1426–1428. [Google Scholar] [CrossRef]
- Mao, M.; Yang, X.S.; Bennett, G. The complete mitochondrial genome of Macrosteles quadrilineatus (Hemiptera: Cicadellidae). Mitochondrial DNA Part B 2017, 2, 173–175. [Google Scholar] [CrossRef]
- Yang, H.Y.; Dai, R.H. Complete mitochondrial genome of Mukaria splendida Distant (Hemiptera: Cicadellidae: Deltocephalinae: Mukariini) and phylogenetic analysis. Mitochondrial DNA Part B 2021, 6, 622–623. [Google Scholar] [CrossRef]
- Xing, J.C.; Wang, J.J. Complete mitochondrial genome of Paralaevicephalus gracilipenis (Hemiptera: Cicadellidae: Deltocephalinae) from China. Mitochondrial DNA Part B 2019, 4, 1372–1373. [Google Scholar] [CrossRef]
- Xu, T.L.; Dai, R.H. Complete mitochondrial genome of Penthimia melanocephala Motschulsky, 1863 (Hemiptera: Cicadellidae: Deltocephalinae). Mitochondrial DNA Part B 2021, 6, 568–569. [Google Scholar] [CrossRef]
- Xu, T.L.; Dai, R.H. Complete mitogenome of Reticuluma hamata (Hemiptera: Cicadellidae: Deltocephalinae) from China. Mitochondrial DNA Part B 2020, 5, 1437–1438. [Google Scholar] [CrossRef]
- Du, Y.M.; Dai, W.; Dietrich, C.H. Mitochondrial genomic variation and phylogenetic relationships of three groups in the genus Scaphoideus (Hemiptera: Cicadellidae: Deltocephalinae). Sci. Rep. 2017, 7, 16908. [Google Scholar] [CrossRef][Green Version]
- Wang, J.J.; Yang, M.F.; Dai, R.H.; Li, H.; Wang, X.Y. Characterization and phylogenetic implications of the complete mitochondrial genome of Idiocerinae (Hemiptera: Cicadellidae). Int. J. Biol. Macromol. 2018, 120, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.H.; Wang, J.J.; Yang, M.F. The complete mitochondrial genome of the leafhopper Idioscopus clypealis (Hemiptera: Cicadellidae: Idiocerinae). Mitochondrial DNA Part B 2018, 3, 32–33. [Google Scholar]
- Yuan, Z.W.; Yang, X.; Li, C.; Song, Y.H. The complete mitochondrial genome of the leafhopper Evacanthus acuminatus (Hemiptera: Cicadellidae: Evacanthinae). Mitochondrial DNA Part B 2019, 4, 3866–3867. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.M.; Liang, Z.L.; Dietrich, C.H.; Dai, W. Comparative analysis of mitochondrial genomes of Nirvanini and Evacanthini (Hemiptera: Cicadellidae) reveals an explicit evolutionary relationship. Genomics 2021, 113, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Huang, W.J.; Zhang, Y.L. The complete mitochondrial genome of four Hylicinae (Hemiptera: Cicadellidae): Structural features and phylogenetic implications. Insects 2020, 11, 869. [Google Scholar] [CrossRef]
- Wang, J.J.; Wu, Y.F.; Dai, R.H.; Yang, M.F. Comparative mitogenomes of six species in the subfamily Iassinae (Hemiptera: Cicadellidae) and phylogenetic analysis. Int. J. Biol. Macromol. 2020, 149, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Li, D.F.; Li, H.; Yang, M.F.; Dai, R.H. Structural and phylogenetic implications of the complete mitochondrial genome of Ledra auditura. Sci. Rep. 2019, 9, 15746. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, Y.L. Characterization of two complete mitochondrial genomes of Ledrinae (Hemiptera: Cicadellidae) and phylogenetic analysis. Insects 2020, 11, 609. [Google Scholar] [CrossRef]
- Wang, J.J.; Dai, R.H.; Li, H.; Zhan, H.P. Characterization of the complete mitochondrial genome of Japanagallia spinosa and Durgades nigropicta (Hemiptera: Cicadellidae: Megophthalminae). Biochem. Syst. Ecol. 2017, 74, 33–41. [Google Scholar] [CrossRef]
- He, H.L.; Yang, M.F. Characterization of the leafhopper mitogenome of Mileewa alara (Hemiptera: Cicadellidae: Mileewinae) and its phylogenetic analysis. Mitochondrial DNA Part B 2021, 6, 1265–1266. [Google Scholar] [CrossRef]
- Yu, T.H.; Zhang, Y.L. Two complete mitochondrial genomes of Mileewinae (Hemiptera: Cicadellidae) and a phylogenetic analysis. Insects 2021, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Yuan, Z.W.; Li, C.; Song, Y.H. Complete mitochondrial genome sequence of Empoascanara dwalata (Hemiptera: Cicadellidae: Typhlocybinae). Mitochondrial DNA Part B 2020, 5, 2260–2261. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Chen, X.X.; Li, C.; Song, Y.H. The complete mitochondrial genome of Empoascanara sipra (Hemiptera: Cicadellidae: Typhlocybinae) with phylogenetic consideration. Mitochondrial DNA Part B 2020, 5, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, A.P. Complete DNA sequence of the mitochondrial genome of the treehopper Leptobelus gazella (Membracoidea: Hemiptera). Mitochondrial DNA Part A 2016, 27, 3318–3319. [Google Scholar] [CrossRef]
- Mao, M.; Yang, X.H.; Bennett, G. The complete mitochondrial genome of Entylia carinata (Hemiptera: Membracidae). Mitochondrial DNA Part B 2016, 1, 662–663. [Google Scholar] [CrossRef]

| Organism | Locality | Code | GenBank Accession | Reference |
|---|---|---|---|---|
| Aconurella diplachnis | Xinjiang | Hm086374 | OK105069 | This study |
| Aconurella montana | Yunnan | Hm087561 | OK105070 | This study |
| Aconurella prolixa | Guangxi | HN027 | MZ433366 | [26] |
| Aconurella sibirica | Anhui | Hm083232 | OK105071 | This study |
| Doratura homophyla | Xinjiang | HN010 | OK105076 | This study |
| Doratura stylata | Xinjiang | HN029 | OK105077 | This study |
| Exitianus indicus | Henan | KY039128 | [25] | |
| Exitianus nanus | Guangxi | YN002 | OK105078 | This study |
| Gurawa minorcephala | Guangxi | Hm086937 | OK105072 | This study |
| Leofa pulchella | Guangxi | GX031 | OK105073 | This study |
| Nephotettix cincticeps | Henan | KP749836 | [25] | |
| Nephotettix malayanus | Guangxi | GX073 | OK105079 | This study |
| Nephotettix nigropictus | Yunnan | YN041 | OK105080 | This study |
| Nephotettix parvus | Yunnan | Hm086799 | OK105074 | This study |
| Nephotettix virescens | Yunnan | YN–009 | OK105081 | This study |
| Zahniserius cylindricus | Yunnan | Hm080184 | OK105075 | This study |
| Organism | Length (bp) | AT% | AT Skew | GC Skew |
|---|---|---|---|---|
| Aconurella diplachnis | 15,598 | 76.4 | 0.123 | −0.186 |
| Aconurella montana | 15,637 | 75.2 | 0.128 | −0.169 |
| Aconurella prolixa | 15,222 | 75.2 | 0.125 | −0.169 |
| Aconurella sibirica | 15,598 | 76.0 | 0.124 | −0.183 |
| Doratura homophyla | 16,269 | 76.9 | 0.090 | −0.160 |
| Doratura stylata | 16,055 | 76.3 | 0.093 | −0.165 |
| Exitianus indicus | 16,089 | 75.1 | 0.119 | −0.157 |
| Exitianus nanus | 15,714 | 74.3 | 0.133 | −0.144 |
| Gurawa minorcephala | 15,782 | 73.6 | 0.128 | −0.220 |
| Leofa pulchella | 15,311 | 74.6 | 0.129 | −0.150 |
| Nephotettix cincticeps | 14,805 | 75.1 | 0.071 | −0.094 |
| Nephotettix malayanus | 15,462 | 75.4 | 0.074 | −0.089 |
| Nephotettix nigropictus | 15,280 | 77.0 | 0.065 | −0.087 |
| Nephotettix parvus | 15,222 | 77.4 | 0.070 | −0.106 |
| Nephotettix virescens | 15,810 | 77.3 | 0.069 | −0.084 |
| Zahniserius cylindricus | 15,459 | 75.7 | 0.096 | −0.136 |
| Species | Start Codon/Stop Codon (ATN/TAN) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATP6 | ATP8 | COX1 | COX2 | COX3 | CYTB | ND1 | ND2 | ND3 | ND4 | ND4L | ND5 | ND6 | |
| Aconurella diplachnis | G/A | A/A | G/G | A/T- | G/A | G/A | T/A | A/A | A/A | G/T- | T/A | TTG/A | T/A |
| Aconurella montana | G/A | C/A | G/A | A/T- | G/A | G/G | T/A | A/A | A/A | G/T- | G/A | TTG/A | A/A |
| Aconurella prolixa | G/A | C/A | G/A | A/T- | G/A | G/G | C/A | A/A | A/A | G/T- | T/A | TTG/G | T/A |
| Aconurella sibirica | G/A | A/A | G/G | A/T- | G/A | G/A | T/A | A/A | A/A | G/T- | T/A | TTG/A | T/A |
| Doratura homophyla | G/A | A/A | G/A | A/A | G/A | G/A | T/A | A/A | T/A | G/T- | C/A | GTG/G | A/A |
| Doratura stylata | G/A | T/A | G/A | A/A | G/A | G/A | T/A | A/A | T/A | G/T- | T/A | GTG/G | A/A |
| Exitianus indicus | G/A | C/A | G/A | A/T- | G/A | G/A | T/A | T/A | A/A | A/T- | T/G | A/A | T/A |
| Exitianus nanus | G/A | C/A | G/G | A/A | G/A | G/A | T/A | T/A | A/G | G/T- | T/G | G/G | TTG/T- |
| Gurawa minorcephala | G/A | A/A | G/G | G/A | G/A | G/A | G/A | T/A | T/A | G/A | T/A | TTG/A | T/T- |
| Leofa pulchella | G/A | T/A | G/A | A/A | G/G | G/G | A/A | T/A | T/G | G/A | T/A | TTG/A | A/A |
| Nephotettix cincticeps | G/A | G/A | G/A | A/A | G/A | G/A | T/A | T/A | A/TA | G/A | G/G | A/A | A/A |
| Nephotettix malayanus | G/A | T/A | G/A | A/A | G/A | G/A | T/A | T/A | A/A | G/A | C/A | TTG/A | TTG/A |
| Nephotettix nigropictus | GTG/A | A/A | G/A | A/A | G/A | G/A | T/A | T/A | A/A | G/A | G/A | TTG/A | TTG/A |
| Nephotettix parvus | G/A | C/A | G/A | A/A | G/G | G/A | T/A | T/A | A/G | G/G | G/A | TTG/A | TTG/A |
| Nephotettix virescens | G/A | A/A | G/A | A/A | G/A | G/A | T/A | T/A | A/A | G/A | G/A | TTG/A | TTG/A |
| Zahniserius cylindricus | G/A | T/A | G/G | A/G | G/G | G/A | G/A | T/A | C/A | G/T- | T/A | TTG/A | T/T- |
| Species | tRNA Base Mismatch | rrnL (bp) | rrnS (bp) | |||||
|---|---|---|---|---|---|---|---|---|
| A–A | A–C | A–G | G–U | U–C | U–U | |||
| Aconurella diplachnis | 25 | 2 | 1201 | 801 | ||||
| Aconurella montana | 2 | 2 | 25 | 3 | 1203 | 783 | ||
| Aconurella prolixa | 1 | 27 | 1 | 1210 | 793 | |||
| Aconurella sibirica | 1 | 25 | 3 | 1206 | 780 | |||
| Doratura homophyla | 3 | 26 | 1 | 3 | 1205 | 743 | ||
| Doratura stylata | 2 | 21 | 5 | 1206 | 746 | |||
| Exitianus indicus | 29 | 5 | 1208 | 751 | ||||
| Exitianus nanus | 1 | 1 | 1 | 21 | 1 | 5 | 1209 | 744 |
| Gurawa minorcephala | 1 | 1 | 1 | 31 | 3 | 1211 | 750 | |
| Leofa pulchella | 2 | 25 | 3 | 1198 | 737 | |||
| Nephotettix cincticeps | 2 | 1 | 20 | 6 | 1201 | 741 | ||
| Nephotettix malayanus | 2 | 23 | 4 | 1193 | 745 | |||
| Nephotettix nigropictus | 2 | 18 | 4 | 1207 | 748 | |||
| Nephotettix parvus | 3 | 23 | 5 | 1206 | 745 | |||
| Nephotettix virescens | 2 | 23 | 3 | 1206 | 745 | |||
| Zahniserius cylindricus | 2 | 33 | 3 | 1216 | 741 | |||
| Superfamily/Family/ Subfamily | Tribe | Species | Accession Number | Reference |
|---|---|---|---|---|
| Cercopoidea | ||||
| Cercopidae | ||||
| Callitettixinae | Callitettixini | Callitettix braconoides | JX844628 | [41] |
| Cercopinae | Cosmoscartini | Cosmoscarta dorsimacula | MG599490 | [42] |
| Cicadoidea | ||||
| Cicadidae | ||||
| Cicadinae | Fidicinini | Diceroprocta semicincta | KM000131 | Unpublished |
| Cicadettinae | Lamotialnini | Magicicada tredecula | MH937695 | [43] |
| Membracoidea | ||||
| Aetalionidae | ||||
| Aetalioninae | Darthulini | Darthula hardwickii | KP316404 | [44] |
| Cicadellidae | ||||
| Cicadellinae | Cicadellini | Kolla paulula | MW542170 | Unpublished |
| Proconiini | Homalodisca vitripennis | AY875213 | Unpublished | |
| Coelidiinae | Coelidiini | Olidiana longisticka | MN780582 | [45] |
| Taharana fasciana | KY886913 | [46] | ||
| Deltocephalinae | Athysanini | Abrus expansivus | MK033020 | [47] |
| Norvellina sp. | KY039131 | [48] | ||
| Tambocerus sp. | KT827824 | [49] | ||
| Chiasmini | Aconurella diplachnis | OK105069 | This study | |
| Aconurella montana | OK105070 | This study | ||
| Aconurella prolixa | MZ433366 | [26] | ||
| Aconurella sibirica | OK105071 | This study | ||
| Doratura homophyla | OK105076 | This study | ||
| Doratura stylata | OK105077 | This study | ||
| Exitianus indicus | KY039128 | [25] | ||
| Exitianus nanus | OK105078 | This study | ||
| Gurawa minorcephala | OK105072 | This study | ||
| Leofa pulchella | OK105073 | This study | ||
| Nephotettix cincticeps | KP749836 | [25] | ||
| Nephotettix malayanus | OK105079 | This study | ||
| Nephotettix nigropictus | OK105080 | This study | ||
| Nephotettix parvus | OK105074 | This study | ||
| Nephotettix virescens | OK105081 | This study | ||
| Zahniserius cylindricus | OK105075 | This study | ||
| Cicadulini | Watanabella graminea | MK234840 | [50] | |
| Deltocephalini | Alobaldia tobae | KY039116 | [48] | |
| Maiestas dorsalis | KX786285 | [51] | ||
| Drabescini | Drabescoides nuchalis | KR349344 | [52] | |
| Drabescus ineffectus | MT527188 | [53] | ||
| Roxasellana stellata | MT527187 | [53] | ||
| Fieberiellini | Fieberiella septentrionalis | MW078430 | [54] | |
| Macrostelini | Macrosteles quadrilineatus | KY645960 | [55] | |
| Macrosteles quadrimaculatus | MG727894 | [24] | ||
| Mukariini | Mukaria splendida | MG813485 | [56] | |
| Opsiini | Hishimonoides recurvatis | KY364883 | Unpublished | |
| Japananus hyalinus | KY129954 | [51] | ||
| Orosius orientalis | KY039146 | [48] | ||
| Deltocephalinae | Paralimnini | Paralaevicephalus gracilipenis | MK450366 | [57] |
| Yanocephalus yanonis | KY039113 | [48] | ||
| Penthimiini | Penthimia melanocephala | MT768010 | [58] | |
| Reticuluma hamata | MN922303 | [59] | ||
| Scaphoideini | Phlogotettix monozoneus | MH427717 | Unpublished | |
| Phlogotettix sp. | KY039135 | [48] | ||
| Scaphoideus maai | KY817243 | [60] | ||
| Scaphoideus nigrivalveus | KY817244 | [60] | ||
| Scaphoideus varius | KY817245 | [60] | ||
| Eurymelinae | Idiocerini | Idiocerus laurifoliae | MH433622 | [61] |
| Idioscopus clypealis | MF784430 | [62] | ||
| Evacanthinae | Evacanthini | Evacanthus acuminatus | MK948205 | [63] |
| Evacanthus danmainus | MN227166 | [64] | ||
| Hylicinae | Hylicini | Hylica paradoxa | MW218660 | [65] |
| Sudrini | Balala fujiana | MW218661 | [65] | |
| Iassinae | Batracomorphini | Batracomorphus lateprocessus | MG813489 | [66] |
| Krisnini | Krisna concava | MN577635 | [66] | |
| Ledrinae | Ledrini | Ledra auditura | MK387845 | [67] |
| Tituria sagittata | MT610900 | [68] | ||
| Megophthalminae | Agalliini | Durgades nigropicta | KY123686 | [69] |
| Japanagallia spinosa | KY123687 | [69] | ||
| Mileewinae | Mileewini | Mileewa alara | MW533151 | [70] |
| Ujna puerana | MZ326688 | [71] | ||
| Typhlocybinae | Erythroneurini | Empoascanara dwalata | MT350235 | [72] |
| Empoascanara sipra | MN604278 | [73] | ||
| Membracidae | ||||
| Centrotinae | Centrotini | Centrotus cornutus | KX437728 | [25] |
| Leptobelini | Leptobelus gazella | JF801955 | [74] | |
| Smiliinae | Polyglyptini | Entylia carinata | KX495488 | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, B.; Hassan, M.A.; Xie, B.; Wu, K.; Naveed, H.; Yan, M.; Dietrich, C.H.; Duan, Y. Mitogenomic Analysis and Phylogenetic Implications for the Deltocephaline Tribe Chiasmini (Hemiptera: Cicadellidae: Deltocephalinae). Insects 2024, 15, 253. https://doi.org/10.3390/insects15040253
Shah B, Hassan MA, Xie B, Wu K, Naveed H, Yan M, Dietrich CH, Duan Y. Mitogenomic Analysis and Phylogenetic Implications for the Deltocephaline Tribe Chiasmini (Hemiptera: Cicadellidae: Deltocephalinae). Insects. 2024; 15(4):253. https://doi.org/10.3390/insects15040253
Chicago/Turabian StyleShah, Bismillah, Muhammad Asghar Hassan, Bingqing Xie, Kaiqi Wu, Hassan Naveed, Minhui Yan, Christopher H. Dietrich, and Yani Duan. 2024. "Mitogenomic Analysis and Phylogenetic Implications for the Deltocephaline Tribe Chiasmini (Hemiptera: Cicadellidae: Deltocephalinae)" Insects 15, no. 4: 253. https://doi.org/10.3390/insects15040253
APA StyleShah, B., Hassan, M. A., Xie, B., Wu, K., Naveed, H., Yan, M., Dietrich, C. H., & Duan, Y. (2024). Mitogenomic Analysis and Phylogenetic Implications for the Deltocephaline Tribe Chiasmini (Hemiptera: Cicadellidae: Deltocephalinae). Insects, 15(4), 253. https://doi.org/10.3390/insects15040253







