Antibacterial Activity of Defensive Secretions from the Lace Bug Stephanitis svensoni (Drake) (Hemiptera: Tingidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. General Procedures
2.3. Preparation of the Defensive Extract
2.4. Quantitative Analysis of the Defensive Extract
2.5. Antibacterial Activity Test
2.6. Synthesis
2.6.1. 3-Oxododecanal (4)
2.6.2. 1-(2,6-Dihydroxyphenyl)dodecan-1-one (8)
2.6.3. Synthesis of the Standard 2-alkyl-5-hydroxychromanone
2.6.4. 2-Heptyl-5-hydroxychroman-4-one (6)
2.6.5. 2-Nonyl-5-hydroxychroman-4-one (7)
2.6.6. 2-Undecanyl-5-hydroxychroman-4-one (9)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guidoti, M.; Montemayor, S.I.; Guilbert, É. Lace bugs (Tingidae). In True Bugs (Heteroptera) of the Neotropics. Entomology in Focus; Panizzi, A., Grazia, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 395–419. [Google Scholar] [CrossRef]
- Drake, C.J.; Ruhoff, F.A. Lacebugs of the world: A catalog (Hemiptera: Tingidae). US Natl. Mus. Bull. 1965, 243, 1–634. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Masaki, T.; Motooka, M.; Hino, D.; Ueda, K. Highly toxic seeds of the Japanese star anise Illicium anisatum are dispersed by a seed-caching bird and a rodent. Ecol. Res. 2018, 33, 495–504. [Google Scholar] [CrossRef]
- Lane, J.F.; Koch, W.T.; Leeds, N.S.; Gorin, G. On the toxin of Illicium anisatum. I. The isolation and characterization of a convulsant principle: Anisatin 1. J. Am. Chem. Soc. 1952, 74, 3211–3215. [Google Scholar] [CrossRef]
- Livingstone, D. On the body outgrowths and the phenomenon of ‘sweating’ in the nymphal instars of Tingidae (Hemiptera: Heteroptera). J. Nat. Hist. 1978, 12, 377–394. [Google Scholar] [CrossRef]
- Oliver, J.E.; Neal, J.W., Jr.; Lusby, W.R.; Aldrich, J.R.; Kochansky, J.P. Novel components from secretory hairs of azalea lace bug Stephanitis pyrioides (Hemiptera: Tingidae). J. Chem. Ecol. 1985, 11, 1223–1228. [Google Scholar] [CrossRef]
- Oliver, J.E.; Neal, J.W., Jr.; Lusby, W.R. Phenolic acetogenins secreted by rhododendron lace bug, Stephanitis rhododendri Horvath (Hemiptera: Tingidae). J. Chem. Ecol. 1987, 13, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Lusby, W.R.; Oliver, J.E.; Neal, J.W., Jr.; Heath, R.R. Isolation and identification of the major component of setal exudate from Corythucha ciliata. J. Nat. Prod. 1987, 50, 1126–1130. [Google Scholar] [CrossRef]
- Lusby, W.R.; Oliver, J.E.; Neal, J.W., Jr.; Heath, R.R. Acyclohexanediones from setal exudate of Hawthorn lace bug nymph Corythucha cydoniae (Hemiptera: Tingidae). J. Chem. Ecol. 1989, 15, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Lusby, W.R.; Neal, J.W., Jr. Exocrine secretions of the andromeda lace bug Stephanitis takeyai (Hemiptera: Tingidae). J. Chem. Ecol. 1990, 16, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Lusby, W.R. Synthesis of 2-acyl-3,6-dihydroxy-2-cyclohexen-1-ones. Tetrahedron 1988, 44, 1591–1596. [Google Scholar] [CrossRef]
- Lusby, W.R.; Oliver, J.E.; Neal, J.W., Jr. Isolation, identification, and synthesis of 2-acyl-3,6-dihydroxycyclohex-2-ene-1-ones. In Synthesis and Chemistry of Agrochemicals II; ACS Symposium Series; Baker, D.R., Fenyes, W.G., Moberg, W.K., Eds.; ACS Publications: Washington, DC, USA, 1991; Volume 443, pp. 413–421. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Neal, J.W., Jr.; Oliver, J.E.; Lusby, W.R. Chemistry via-à-vis maternalism in lace bugs (Heteroptera: Tingidae): Alarm pheromones and exudate defense in Corythucha and Gargaphia species. J. Chem. Ecol. 1991, 17, 2307–2322. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.G. Pheromones of true bugs. In The Chemistry of Pheromones and Other Semiochemicals, Vol. II; Schulz, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 37–84. [Google Scholar]
- Neal, J.W., Jr.; Oliver, J.E.; Fetterer, R.H. In vitro antimicrobial and nematocidal activity of acetogenins identified from exocrine secretions of Stephanitis and Corythucha lace bug nymphs (Heteroptera: Tingidae). Ann. Entomol. Soc. Am. 1995, 88, 496–501. [Google Scholar] [CrossRef]
- Jurenka, R.A.; Neal, J.W., Jr.; Howard, R.W.; Oliver, J.E.; Blomquist, G.J. In vitro inhibition of prostaglandin H synthase by compounds from the exocrine secretions of lace bugs. Comp. Biochem. Physiol. 1989, 93C, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.R.; Neal, J.; Oliver, J.E.; Lusby, W.R. Bird-repellent properties of secretions from nymphs of the azalea lace bug. Ecol. Appl. 1991, 1, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Kawai, A.; Shimizu, N.; Tokumaru, S.; Ueyama, H. Geraniol, E-3,7-dimethyl-2,6-octadien-1-ol, as the alarm pheromone of the sycamore lace bug Corythucha ciliata (Say). J. Chem. Ecol. 2011, 37, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shimizu, N. Alarm pheromone activity of nymph-specific geraniol in chrysanthemum lace bug Corythucha marmorata against adults and nymphs. Nat. Prod. Commun. 2015, 10, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shimizu, N. Identification of a sex pheromone of the chrysanthemum lace bug Corythucha marmorata (Hemiptera: Tingidae). Sci. Rep. 2017, 7, 7302. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, T. Structure of an antimicrobial substance isolated from Houttuynia cordata Thunb. Yakugaku Zasshi 1952, 72, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Wilzer, K.R.; Waters, R.M. Synthesis of 1-(2,6-dihydroxyphenyl)-1-alkanones and benzophenone by aromatization of 2-acyl-3-hydroxy-2-cyclohexene-1-ones with mercuric acetate. Synthesis 1990, 1990, 1117–1119. [Google Scholar] [CrossRef]
- Okombi, S.; Schmidt, J.; Mariotte, A.M.; Perrier, E.; Boumendjel, A. A one-step synthesis of 2-alkyl-5-hydroxychromones and 3-alkoyl-2-alkyl-5-hydroxychromones. Chem. Pharm. Bull. 2005, 53, 1460–1462. [Google Scholar] [CrossRef]
- Garg, N.K.; Caspi, D.D.; Stoltz, B.M. The Total synthesis of (+)-dragmacidin F. J. Am. Chem. Soc. 2004, 126, 9552–9553. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.B.; Howard, A.S. The essential oil of Illicium anisatum Linn. Can. J. Chem. 1966, 44, 2461–2464. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.S.; Oh, T.H.; Baik, J.S.; Song, G.; Lee, N.H.; Hyun, C.G. Chemical composition, antioxidant, anti-elastase, and anti-inflammatory activities of Illicium anisatum essential oil. Acta Pharm. 2009, 59, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Bae, Y.S. Phenolic compounds from Japanese anise (Illicium anisatum L.) twigs. J. Korean Wood Sci. Technol. 2017, 45, 456–462. [Google Scholar] [CrossRef]
- Min, H.J.; Kim, C.S.; Hyun, H.J.; Bae, Y.S. Essential oil analysis of Illicium anistum L. extracts. J. Korean Wood Sci. Technol. 2017, 45, 682–688. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Wu, Q.; Ma, C.; Wang, Z.; Peng, W.; Song, H. Synthesis and antibacterial evaluation of novel 4-alkyl substituted phenyl β-aldehyde ketone derivatives. Eur. J. Med. Chem. 2009, 44, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
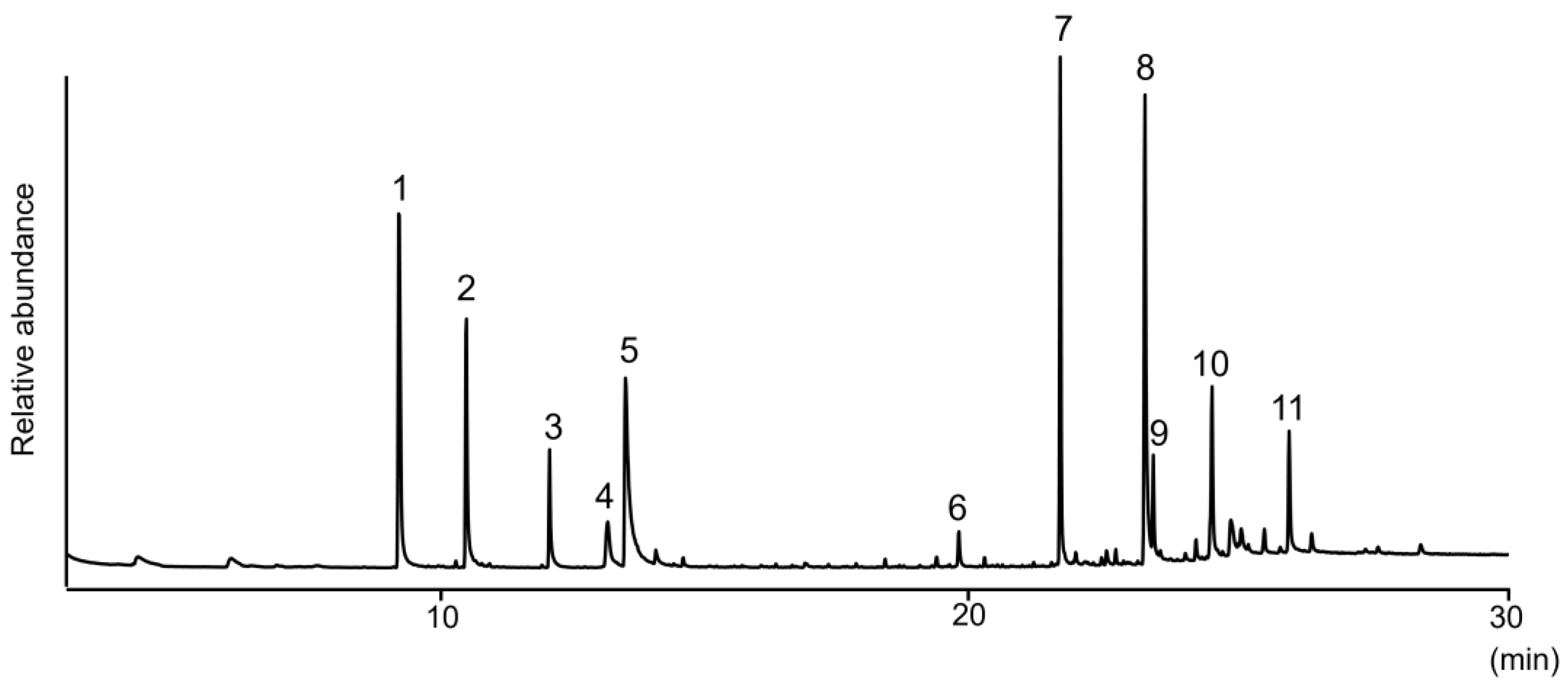
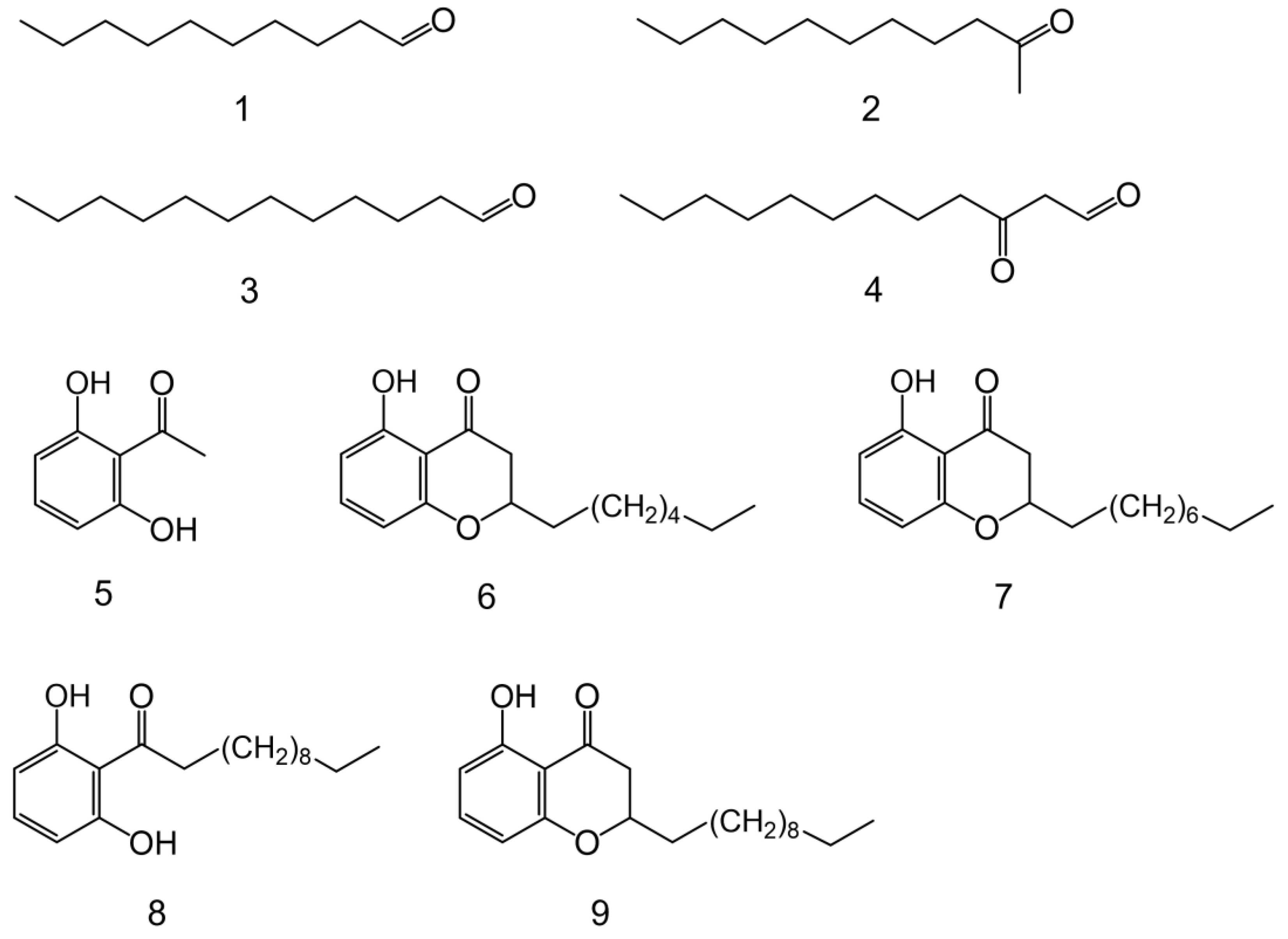
| Peak | Compound | GC tR (min) | Molecular and Diagnostic Ions m/z (Intensity %) |
|---|---|---|---|
| 1 | decanal | 9.44 | 41 (100), 57(82), 70 (44), 82 (44), 95 (25), 112 (25), 128 (6), 138 (M+—18, 2) |
| 2 | 2-undecanone | 10.70 | 43 (97), 58 (100), 71 (37), 85 (8), 112 (6), 127 (4), 155 (3), 170 (M+, 7) |
| 3 | dodecanal | 12.25 | 41 (100), 57 (93), 67 (44), 82 (63), 96 (37), 110 (16), 123 (7), 140 (14), 156 (2), 166 (M+—18, 1) |
| 4 | 3-oxododecanal | 13.33 | 43 (30), 55 (15), 71 (100), 86 (76), 99 (14), 124 (6), 155 (3), 180 (M+—18, 2) |
| 5 | 2,6-dihydroxyacetophenone | 13.64 | 43 (8), 81 (14), 108 (3), 137 (100), 152 (M+, 42) |
| 6 | 5-hydroxy-2-heptylchromanone | 19.92 | 81 (6), 108 (16), 137 (100), 152 (4), 163 (79), 262 (M+, 48) |
| 7 | 5-hydroxy-2-nonylchromanone | 21.83 | 41 (7), 108 (16), 137 (100), 163 (97), 290 (M+, 51) |
| 8 | 1-(2,6-dihydroxyphenyl)dodecan-1-one | 23.39 | 41 (6), 81 (6), 137 (100), 152 (23), 165 (25), 189 (11), 274 (7), 292 (M+, 7) |
| 9 | 5-hydroxy-2-undecanylchromanone | 23.57 | 41 (13), 108 (17), 137 (92), 163 (100), 282 (3), 318 (M+, 61) |
| 10 | unknown | 24.67 | 43 (31), 108 (10), 137 (31), 162 (82), 163 (100), 177 (10), 205 (5), 332 (35) |
| 11 | nonacosane | 26.16 | 43 (62), 57 (100), 71 (76), 85 (54), 99 (20), 113 (11), 127 (8), 267 (4), 408 (M+, 3) |
| Larval Secretion | S. aureus | E. coli | ||
|---|---|---|---|---|
| 30 μg | 300 μg | 30 μg | 300 μg | |
| decanal (1) | – (2) | – (2) | – (2) | – (4) |
| 2-undecanone (2) | – (2) | – (2) | – (2) | – (4) |
| dodecanal (3) | – (2) | – (2) | – (2) | – (4) |
| 3-oxododecanal (4) | – (2) | 11.0 (4) | – (2) | – (4) |
| 2,6-dihydroxyacetophenone (5) | – (6) | 18.6 (2) | – (2) | 14.5 (12) |
| 5-hydroxy-2-heptylchromanone (6) | – (2) | 10.0 (4) | – (2) | – (4) |
| 5-hydroxy-2-nonylchromanone (7) | – (2) | – (2) | – (2) | – (4) |
| 1-(2,6-dihydroxyphenyl)dodecan-1-one (8) | 10.6 (8) | 11.7 (10) | 11.8 (8) | 12.2 (12) |
| 5-hydroxy-2-undecanylchromanone (9) | – (2) | – (2) | – (2) | – (4) |
| nonacosane (11) | – (2) | – (2) | – (2) | – (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, N.; Takahara, C.; Ogami, H. Antibacterial Activity of Defensive Secretions from the Lace Bug Stephanitis svensoni (Drake) (Hemiptera: Tingidae). Insects 2024, 15, 257. https://doi.org/10.3390/insects15040257
Shimizu N, Takahara C, Ogami H. Antibacterial Activity of Defensive Secretions from the Lace Bug Stephanitis svensoni (Drake) (Hemiptera: Tingidae). Insects. 2024; 15(4):257. https://doi.org/10.3390/insects15040257
Chicago/Turabian StyleShimizu, Nobuhiro, Chihiro Takahara, and Hiroki Ogami. 2024. "Antibacterial Activity of Defensive Secretions from the Lace Bug Stephanitis svensoni (Drake) (Hemiptera: Tingidae)" Insects 15, no. 4: 257. https://doi.org/10.3390/insects15040257







