Natural Diversity of Cuticular Pheromones in a Local Population of Drosophila after Laboratory Acclimation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Flies
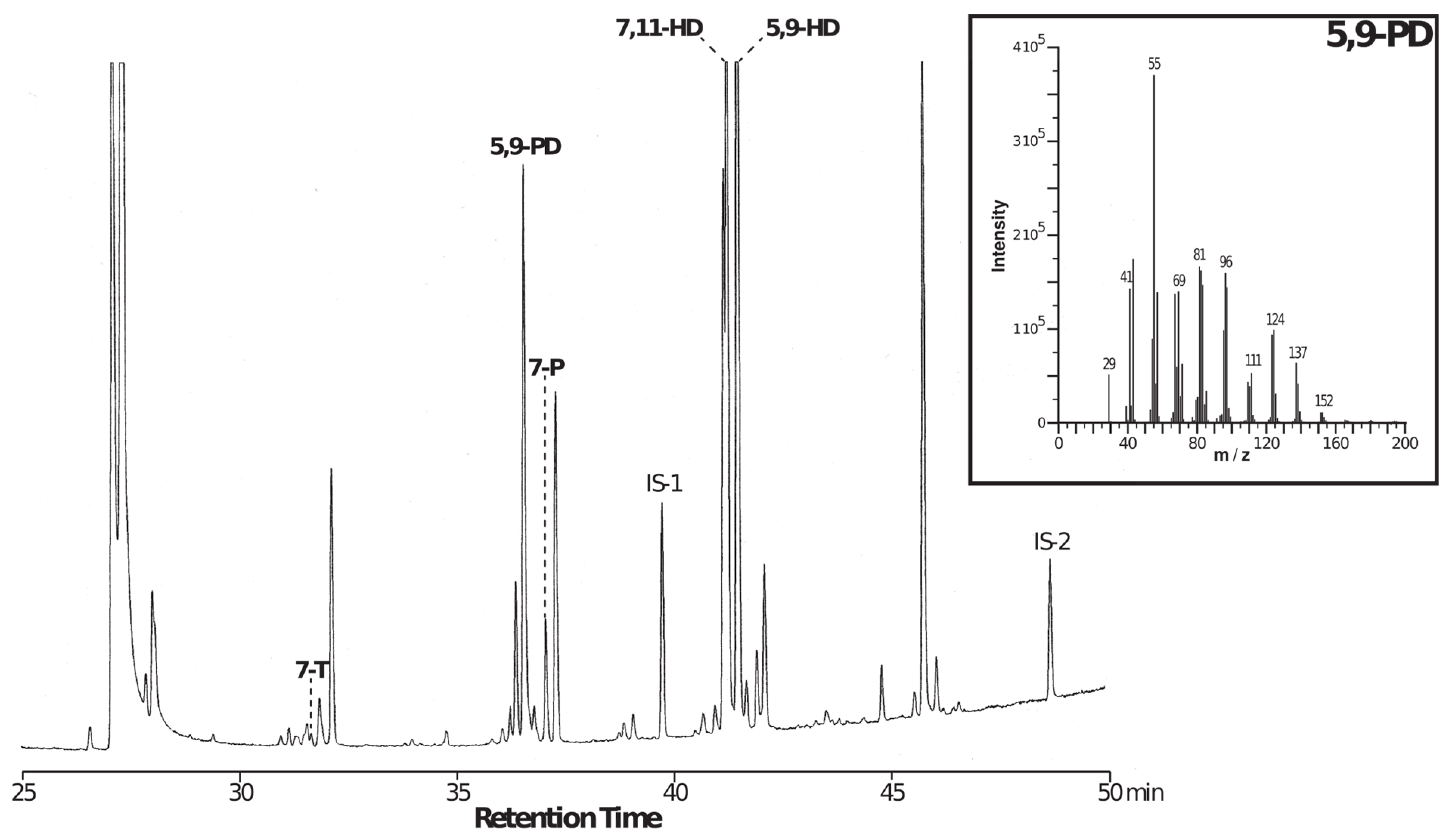
2.2. Chemical Analysis
2.3. Statistics
3. Results
3.1. Comparison between Males in Individual Lines
3.2. Comparison between Males in the Four “Fruit Lines” Groups
3.3. Females of Individual Lines
3.4. Analysis of other CHCs in Males
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chown, S.L.; Sorensen, J.G.; Terblanche, J.S. Water loss in insects: An environmental change perspective. J. Insect Physiol. 2011, 57, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Menzel, F.; Blaimer, B.B.; Schmitt, T. How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161727. [Google Scholar] [CrossRef] [PubMed]
- Otte, T.; Hilker, M.; Geiselhardt, S. Phenotypic plasticity of cuticular hydrocarbon profiles in insects. J. Chem. Ecol. 2018, 44, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Rouault, J.D.; Marican, C.; Wicker-Thomas, C.; Jallon, J.M. Relations between cuticular hydrocarbon (HC) polymorphism, resistance against desiccation and breeding temperature; a model for hc evolution in D. melanogaster and D simulans. Genetica 2004, 120, 195–212. [Google Scholar] [CrossRef]
- Savarit, F.; Ferveur, J.F. Genetic study of the production of sexually dimorphic cuticular hydrocarbons in relation with the sex-determination gene transformer in Drosophila melanogaster. Genet. Res. 2002, 79, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Toolson, E.C.; Kuper-Simbron, R. Laboratory evolution of epicuticular hydrocarbon composition and cuticular permeability in Drosophila pseudoobscura: Effects on sexual dimorphism and thermal acclimation ability. Evolution 1989, 43, 468–473. [Google Scholar]
- Ferveur, J.-F.; Cortot, J.; Rihani, K.; Cobb, M.; Everaerts, C. Desiccation resistance: Effect of cuticular hydrocarbons and water content in Drosophila melanogaster adults. PeerJ 2018, 6, e4318. [Google Scholar] [CrossRef]
- Gibbs, A.G.; Fukuzato, F.; Matzkin, L.M. Evolution of water conservation mechanisms in drosophila. J. Exp. Biol. 2003, 206, 1183–1192. [Google Scholar] [CrossRef]
- Krupp, J.J.; Nayal, K.; Wong, A.; Millar, J.G.; Levine, J.D. Desiccation resistance is an adaptive life-history trait dependent upon cuticular hydrocarbons, and influenced by mating status and temperature in D. melanogaster. J. Insect Physiol. 2020, 121, 103990. [Google Scholar] [CrossRef]
- Stinziano, J.; Sové, R.J.; Rundle, H.D.; Sinclair, B.J. Rapid desiccation hardening changes the cuticular hydrocarbon profile of Drosophila melanogaster. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 180, 38–42. [Google Scholar] [CrossRef]
- Araújo, M.B.; Ferri-Yáñez, F.; Bozinovic, F.; Marquet, P.A.; Valladares, F.; Chown, S.L. Heat freezes niche evolution. Ecol. Lett. 2013, 16, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Boukal, D.S.; Bideault, A.; Carreira, B.M.; Sentis, A. Species interactions under climate change: Connecting kinetic effects of temperature on individuals to community dynamics. Curr. Opin. Insect Sci. 2019, 35, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.L.; Brown, G.P.; Webb, J.K.; Shine, R. Invasion and the evolution of speed in toads. Nature 2006, 439, 803. [Google Scholar] [CrossRef] [PubMed]
- Umina, P.A.; Weeks, A.R.; Kearney, M.R.; McKechnie, S.W.; Hoffmann, A.A. A rapid shift in a classic clinal pattern in drosophila reflecting climate change. Science 2005, 308, 691–693. [Google Scholar] [CrossRef]
- White, S.A.; Dillon, M.E. Climate warming and bumble bee declines: The need to consider sub-lethal heat, carry-over effects, and colony compensation. Front. Physiol. 2023, 14, 1251235. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.G. Lipid melting and cuticular permeability: New insights into an old problem. J. Insect Physiol. 2002, 48, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Young, H.P.; Larabee, J.K.; Gibbs, A.G.; Schal, C. Relationship between tissue-specific hydrocarbon profiles and lipid melting temperatures in the cockroach Blattella germanica. J. Chem. Ecol. 2000, 26, 1245–1263. [Google Scholar] [CrossRef]
- Andersen, S.O.; Peter, M.G.; Roepstorff, P. Cuticular sclerotization in insects. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 1996, 113, 689–705. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Morozumi, Y.; Murakami, T.; Watanabe, M.; Ohta, S.; Omura, H. Absence of cuticular alkenes allows lycaenid larvae to avoid predation by Formica japonica ants. Entomol. Sci. 2019, 22, 126–136. [Google Scholar] [CrossRef]
- Wang, Y.; Norum, M.; Oehl, K.; Yang, Y.; Zuber, R.; Yang, J.; Farine, J.-P.; Gehring, N.; Flötenmeyer, M.; Ferveur, J.-F.; et al. Dysfunction of oskyddad causes harlequin-type ichthyosis-like defects in Drosophila melanogaster. PLoS Genet. 2020, 16, e1008363. [Google Scholar] [CrossRef] [PubMed]
- Billeter, J.C.; Wolfner, M.F. Chemical cues that guide female reproduction in Drosophila melanogaster. J. Chem. Ecol. 2018, 44, 750–769. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.V.; López, H.; Jaffe, K. Nestmate recognition signals of the leaf-cutting ant Atta laevigata. J. Insect Physiol. 2002, 48, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Sillam-Dussès, D.; Sémon, E.; Moreau, C.; Valterová, I.; Šobotník, J.; Robert, A.; Bordereau, C. Neocembrene a, a major component of the trail-following pheromone in the genus Prorhinotermes (Insecta, Isoptera, Rhinotermitidae). Chemoecology 2005, 15, 1–6. [Google Scholar] [CrossRef]
- Steinmetz, I.; Schmolz, E.; Ruther, J. Cuticular lipids as trail pheromone in a social wasp. Proc. Biol. Sci. 2003, 270, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, X.; Mehren, J.; Hiroi, M.; Billeter, J.C.; Miyamoto, T.; Amrein, H.; Levine, J.D.; Anderson, D.J. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat. Neurosci. 2011, 14, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Ginzel, M.D. Chemical ecology, biochemistry, and molecular biology of insect hydrocarbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Jallon, J.M. A few chemical words exchanged bydrosophila during courtship and mating. Behav. Genet. 1984, 14, 441–478. [Google Scholar] [CrossRef]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific p450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef] [PubMed]
- Chertemps, T.; Duportets, L.; Labeur, C.; Ueda, R.; Takahashi, K.; Saigo, K.; Wicker-Thomas, C. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2007, 104, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Dallerac, R.; Labeur, C.; Jallon, J.M.; Knippie, D.C.; Roelofs, W.L.; Wicker-Thomas, C. A delta 9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 9449–9454. [Google Scholar] [CrossRef]
- Marcillac, F.; Bousquet, F.; Alabouvette, J.; Savarit, F.; Ferveur, J.F. A mutation with major effects on Drosophila melanogaster sex pheromones. Genetics 2005, 171, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Antony, C.; Jallon, J.M. The chemical basis for sex recognition in Drosophila melanogaster. J. Insect Physiol. 1982, 28, 873–880. [Google Scholar] [CrossRef]
- Ferveur, J.F.; Cobb, M.; Boukella, H.; Jallon, J.M. World-wide variation in Drosophila melanogaster sex pheromone: Behavioural effects, genetic bases and potential evolutionary consequences. Genetica 1996, 97, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jallon, J.M.; Pechine, J.M. A novel chemical race of Drosophila melanogaster in Africa. C. R. Acad. Sci. Paris Sér.ii 1989, 309, 1551–1556. [Google Scholar]
- Yukilevich, R.; True, J.R. Incipient sexual isolation among cosmopolitan Drosophila melanogaster populations. Evolution 2008, 62, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortot, J.; Farine, J.-P.; Houot, B.; Everaerts, C.; Ferveur, J.-F. Experimental introgression to evaluate the impact of sex specific traits on Drosophila melanogaster incipient speciation. G3 2019, 9, 2561. [Google Scholar] [CrossRef]
- Grillet, M.; Everaerts, C.; Houot, B.; Ritchie, M.G.; Cobb, M.; Ferveur, J.F. Incipient speciation in Drosophila melanogaster involves chemical signals. Sci. Rep. 2012, 2, 224. [Google Scholar] [CrossRef]
- Sureau, G.; Ferveur, J.F. Go-adaptation of pheromone production and behavioural responses in Drosophila melanogaster males. Genet. Res. 1999, 74, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Houot, B.; Svetec, N.; Godoy-Herrera, R.; Ferveur, J.F. Effect of laboratory acclimation on the variation of reproduction-related characters in Drosophila melanogaster. J. Exp. Biol. 2010, 213, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Shields, A.; Straker, M.; Dalrymple, H.; Dhillon, P.K.; Harbinder, S. Variation in the male pheromones and mating success of wild caught Drosophila melanogaster. PLoS ONE 2011, 6, e23645. [Google Scholar] [CrossRef] [PubMed]
- Cortot, J.; Farine, J.-P.; Cobb, M.; Everaerts, C.; Ferveur, J.-F. Factors affecting the biosynthesis and emission of a Drosophila pheromone. J. Exp. Biol. 2022, 225, jeb244422. [Google Scholar] [CrossRef] [PubMed]
- Everaerts, C.; Farine, J.P.; Cobb, M.; Ferveur, J.F. Drosophila cuticular hydrocarbons revisited: Mating status alters cuticular profiles. PLoS ONE 2010, 5, e9607. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Bernier, U.R.; Sutton, B.D. Elution patterns from capillary gc for methyl-branched alkanes. J. Chem. Ecol. 1998, 24, 1845–1865. [Google Scholar] [CrossRef]
- Guiraudie-Capraz, G.; Pho, D.B.; Jallon, J.M. Role of the ejaculatory bulb in biosynthesis of the male pheromone cis-vaccenyl acetate in Drosophila melanogaster. Integr. Zool. 2007, 2, 89–99. [Google Scholar] [CrossRef]
- Mullen, S.P.; Mendelson, T.C.; Schal, C.; Shaw, K.L. Rapid evolution of cuticular hydrocarbons in a species radiation of acoustically diverse hawaiian crickets (gryllidae: Tigonidiinae: Laupala). Evolution 2007, 61, 223–231. [Google Scholar] [CrossRef]
- Rouault, J.; Capy, P.; Jallon, J.M. Variations of male cuticular hydrocarbons with geoclimatic variables: An adaptative mechanism in Drosophila melanogaster? Genetica 2001, 110, 117–130. [Google Scholar] [CrossRef]
- Martin, S.J.; Vitikainen, E.; Shemilt, S.; Drijfhout, F.P.; Sundström, L. Sources of variation in cuticular hydrocarbons in the ant Formica exsecta. J. Chem. Ecol. 2013, 39, 1415–1423. [Google Scholar] [CrossRef]
- Pavković-Lučić, S. Is there ethological isolation among Drosophila melanogaster strains reared for more than 35 generations on different food? Arch. Biol. Sci. 2009, 61, 105–112. [Google Scholar] [CrossRef]
- Pavković-Lučić, S.; Trajković, J.; Miličić, D.; Anđelković, B.; Lučić, L.; Savić, T.; Vujisić, L. “Scent of a fruit fly”: Cuticular chemoprofiles after mating in differently fed Drosophila melanogaster (Diptera: Drosophilidae) strains. Arch. Insect Biochem. Physiol. 2022, 109, e21866. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.W.; Jackson, L.L.; Banse, H.; Blows, M.W. Cuticular hydrocarbons of Drosophila birchii and D. serrata: Identification and role in mate choice in D. serrata. J. Chem. Ecol. 2003, 29, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Farine, J.P.; Ferveur, J.F.; Everaerts, C. Volatile Drosophila cuticular pheromones are affected by social but not sexual experience. PLoS ONE 2012, 7, e40396. [Google Scholar] [CrossRef] [PubMed]
- Jallon, J.M.; David, J.R. Variations in cuticular hydrocarbons among the 8 species of the Drosophila melanogaster subgroup. Evolution 1987, 41, 294–302. [Google Scholar] [PubMed]
- Coyne, J.A.; Wicker-Thomas, C.; Jallon, J.M. A gene responsible for a cuticular hydrocarbon polymorphism in Drosophila melanogaster. Genet. Res. 1999, 73, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Michalak, P.; Malone, J.H.; Lee, I.T.; Hoshino, D.; Ma, D.N. Gene expression polymorphism in Drosophila populations. Mol. Ecol. 2007, 16, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Tsaur, S.C.; Coyne, J.A.; Wu, C.I. The nucleotide changes governing cuticular hydrocarbon variation and their evolution in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2001, 98, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Balanyá, J.; Oller, J.M.; Huey, R.B.; Gilchrist, G.W.; Serra, L. Global genetic change tracks global climate warming in Drosophila subobscura. Science 2006, 313, 1773–1775. [Google Scholar] [CrossRef]
- Rodríguez-Trelles, F.; Rodríguez, M.A. Rapid micro-evolution and loss of chromosomal diversity in Drosophila in response to climate warming. Evol. Ecol. 1998, 12, 829–838. [Google Scholar] [CrossRef]
- Capy, P.; David, J.R.; Allemand, R.; Carton, Y.; Febvay, G.; Kermarec, A. Genetic analysis of Drosophila melanogaster in the french west indies and comparison with populations from other parts of the world. Genetica 1986, 69, 167–176. [Google Scholar] [CrossRef]
- David, J.R.; Capy, P. Genetic variation of Drosophila melanogaster natural populations. Trends Genet. 1988, 4, 106–111. [Google Scholar] [CrossRef]
- Cortot, J.; Farine, J.-P.; Ferveur, J.-F.; Everaerts, C. Aging-related variation of cuticular hydrocarbons in wild type and variant Drosophila melanogaster. J. Chem. Ecol. 2022, 48, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.G. Water-proofing properties of cuticular lipids. Am. Zool. 1998, 38, 471–482. [Google Scholar] [CrossRef]
- Dillwith, J.W.; Blomquist, G.J.; Nelson, D.R. Biosynthesis of the hydrocarbon components of the sex pheromone of the housefly, Musca domestica L. Insect Biochem. 1981, 11, 247–253. [Google Scholar] [CrossRef]
- Jeffcoat, R. The biosynthesis of unsaturated fatty acids and its control in mammalian liver. Essays Biochem. 1979, 15, 1–36. [Google Scholar] [PubMed]
- Tillman-Wall, J.A.; Vanderwel, D.; Kuenzli, M.E.; Reitz, R.C.; Blomquist, G.J. Regulation of sex pheromone biosynthesis in the housefly, Musca domestica: Relative contribution of the elongation and reductive steps. Arch. Biochem. Biophys. 1992, 299, 92–99. [Google Scholar] [CrossRef]



| Line | Number of F0 Flies | F3 Progeny | F3 Male | Adult Lethality (F12 & F25) | |||||
|---|---|---|---|---|---|---|---|---|---|
| “Fruit Lines” | # | Female | Male | Females/Males | Progeny | Female | Male | ||
| Fig 1 | #1 | 10 | 8 | 60/40 | 102 | 0 | 29 | 0 | 50 |
| Fig 2 | #2 | 9 | 4 | 60/40 | 107 | 7 | 40 | 5 | 80 |
| Fig 3 | #3 | 7 | 6 | 50/50 | 97 | 13 | 53 | 0 | 60 |
| Fig 4 | #4 | 12 | 8 | 50/50 | 101 | 1 | 42 | 1 | 45 |
| Fig 5 | #5 | 10 | 10 | 50/50 | 40 | 5 | 34 | 0 | 61 |
| Fig 6 | #6 | 10 | 6 | 50/50 | 73 | 2 | 38 | 2 | 75 |
| Fig 7 | #7 | 9 | 9 | 50/50 | 84 | 0 | 22 | 5 | 62 |
| Fig 8 | #8 | 7 | 11 | 50/50 | 79 | 6 | 40 | 4 | 68 |
| Peach 1 | #9 | 5 | 10 | 50/50 | 32 | 7 | 39 | 1 | 70 |
| Peach 2 | #10 | 11 | 10 | 50/50 | 95 | 10 | 31 | 1 | 53 |
| Peach 3 | #11 | 6 | 11 | 55/45 | 89 | 4 | 34 | 0 | 85 |
| Peach 4 | #12 | 2 | 3 | 55/45 | 60 | 5 | 27 | 3 | 60 |
| Peach 5 | #13 | 5 | 4 | 50/50 | 31 | 5 | 37 | 1 | 55 |
| Mirabel 1 | #14 | 5 | 6 | 50/50 | 72 | 1 | 40 | 3 | 70 |
| Mirabel 2 | #15 | 4 | 5 | 55/45 | 110 | 7 | 29 | 1 | 54 |
| Mirabel 3 | #16 | 5 | 1 | 60/40 | 83 | 5 | 30 | 5 | 60 |
| Mirabel 4 | #17 | 4 | 6 | 45/55 | 108 | 7 | 31 | 3 | 69 |
| Banana 1 | #18 | 5 | 7 | 50/50 | 105 | 1 | 42 | 1 | 65 |
| Banana 2 | #19 | 1 | 1 | 45/55 | 80 | 4 | 36 | 0 | 65 |
| Banana 3 | #20 | 2 | 5 | 45/55 | 51 | 5 | 34 | 3 | 64 |
| Melon 1 | #21 | 3 | 3 | 55/45 | 46 | 3 | 34 | 2 | 60 |
| Melon 2 | #22 | 3 | 2 | ||||||
| F5 | F12 | F25 | F40 | |||||
|---|---|---|---|---|---|---|---|---|
| Phenotypes | Phenotypes | Phenotypes | Phenotypes | |||||
| Line # | Temperate-like | Tropical- like | Temperate- like | Tropical- like | Temperate- like | Tropical- like | Temperate- like | Tropical- like |
| #1 | 7 | 4 | 6 | 2 | 7 | 7 (+2) | 6 (+3) | |
| #2 | 5 | 10 | 11 | 5 | 10 (+5) | |||
| #3 | 3 | 10 | 15 | |||||
| #4 | 3 | 10 | 15 | |||||
| #5 | 2 | 3 | 7 | 3 | 9 | 2 | ||
| #6 | 3 | 10 | 13 | |||||
| #7 | 2 | 2 | 8 | 2 | 8 | |||
| #8 | 9 | 10 | 10 | 2 | ||||
| #9 | 3 | 10 | 12 | |||||
| #10 | 3 | 10 | 8 | 7 (+5) | ||||
| #11 | 3 | 2 | 8 | 5 | 5 | 3 | 7 (+5) | |
| #12 | 4 | 1 | 10 | 8 | ||||
| #13 | 4 | 10 | 12 | |||||
| #14 | 4 | 2 | 13 | 2 | 12 | 3 | ||
| #15 | 3 | 10 | 11 | |||||
| #16 | 5 | 1 | 10 | 11 | ||||
| #17 | 7 | 10 | 13 | |||||
| #18 | 5 | 9 | 15 | |||||
| #19 | 5 | 10 | 17 | |||||
| #20 | 3 | 10 | 15 | |||||
| #21 | 4 | 5 | 14 | |||||
| Temperate-like | Tropical-like | |||||||
|---|---|---|---|---|---|---|---|---|
| 7-P/5,9PD | 7,11-HD/5,9-HD | 7-P/5,9PD | 7,11-HD/5,9-HD | |||||
| Slope | p | Slope | p | Slope | p | Slope | p | |
| Fig | 0.014 | 0.0001 | −0.540 | 0.015 | 0.003 | 0.128 | −0.017 | 0.066 |
| Peach | 0.003 | 0.469 | −1.006 | 0.006 | −0.001 | 0.783 | −0.109 | 0.069 |
| Mirabel | −0.007 | 0.346 | −0.153 | 0.298 | 0.011 | 0.033 | −0.484 | 0.051 |
| Banana | 0.007 | 0.252 | −1.138 | 0.048 | ||||
| Melon | 0.007 | 0.273 | −0.219 | 0.009 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferveur, J.-F.; Cortot, J.; Cobb, M.; Everaerts, C. Natural Diversity of Cuticular Pheromones in a Local Population of Drosophila after Laboratory Acclimation. Insects 2024, 15, 273. https://doi.org/10.3390/insects15040273
Ferveur J-F, Cortot J, Cobb M, Everaerts C. Natural Diversity of Cuticular Pheromones in a Local Population of Drosophila after Laboratory Acclimation. Insects. 2024; 15(4):273. https://doi.org/10.3390/insects15040273
Chicago/Turabian StyleFerveur, Jean-François, Jérôme Cortot, Matthew Cobb, and Claude Everaerts. 2024. "Natural Diversity of Cuticular Pheromones in a Local Population of Drosophila after Laboratory Acclimation" Insects 15, no. 4: 273. https://doi.org/10.3390/insects15040273
APA StyleFerveur, J.-F., Cortot, J., Cobb, M., & Everaerts, C. (2024). Natural Diversity of Cuticular Pheromones in a Local Population of Drosophila after Laboratory Acclimation. Insects, 15(4), 273. https://doi.org/10.3390/insects15040273






