Influence of Alternative Prey on the Functional Response of a Predator in Two Contexts: With and without Intraguild Predation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Whitefly Trialeurodes vaporariorum
2.3. Aphid Myzus persicae
2.4. Parasitoid Eretmocerus eremicus
2.5. Predator Geocoris punctipes
2.6. Parasitized Whitefly Nymphs
2.7. Experiment 1: Effect of Alternative Prey on the Functional Response of G. punctipes without IGP
2.7.1. Predator Starving
2.7.2. Experimental Arenas
2.8. Experiment 2: Effect of AP on the Functional Response of G. punctipes When IGP Is Present
2.9. Statistical Analysis
2.9.1. Comparison of Consumed Whiteflies and Aphids
2.9.2. Functional Response (FR) Analysis
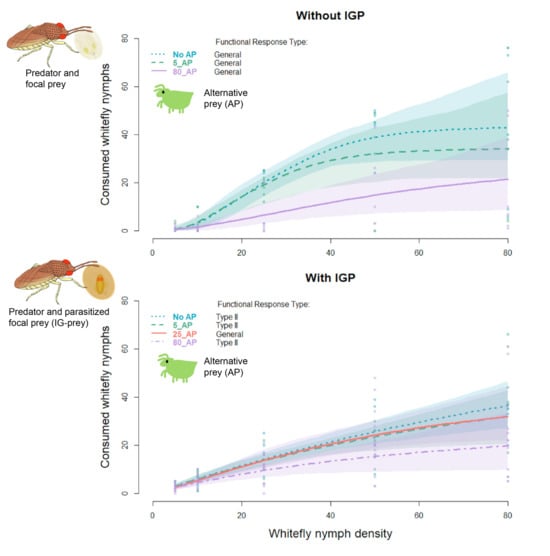
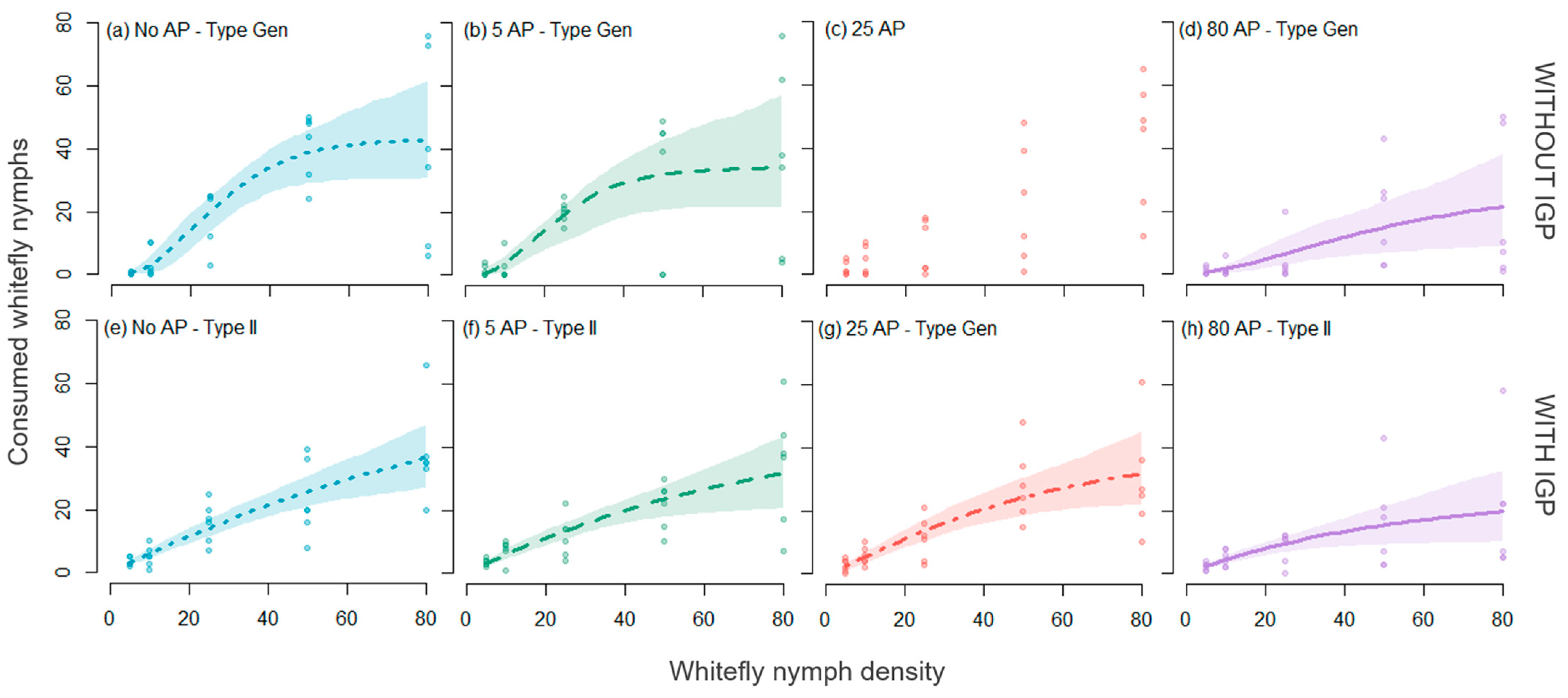
3. Results
3.1. Prey Consumption among AP Treatments
3.2. Functional Response Models
4. Discussion
4.1. Functional Response of G. punctipes without Alternative Prey and No IGP
4.2. Effect of Alternative Prey on the Functional Response of G. punctipes
4.2.1. Without IGP
4.2.2. With IGP
4.3. Functional Response of G. punctipes with and without IGP
4.3.1. Absence of Alternative Prey (AP)
4.3.2. Presence of Alternative Prey (AP)
4.4. Relevance for Biocontrol Programs
4.5. Shortcomings and Prospects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carapia, V.; Castillo-Gutierrez, A. Estudio comparativo sobre la morfología de Trialeurodes vaporariorum (westwood) y Bemisia tabaci (Gennadius) Hemiptera: Aleyrodidae). Acta Zool. Méx. 2013, 29, 178–193. [Google Scholar] [CrossRef]
- Byrne, D.N.; Bellows, T.S. Whitefly biology. Annu. Rev. Entomol. 1991, 36, 431–457. [Google Scholar] [CrossRef]
- Velasco-Hernández, M.; Ramirez-Romero, R.; Cicero, L.; Michel-Rios, C.; Desneux, N. Intraguild predation on the whitefly parasitoid Eretmocerus eremicus by the generalist predator Geocoris punctipes: A behavioral approach. PLoS ONE 2013, 8, e80679. [Google Scholar] [CrossRef]
- Gerling, D.; Alomar, O.; Arn, J. Biological control of Bemisia tabaci using predators and parasitoids. Crop Prot. 2001, 20, 779–799. [Google Scholar] [CrossRef]
- Bao-Fundora, L.; Ramirez-Romero, R.; Sánchez-Hernández, C.V.; Sánchez-Martínez, J.; Desneux, N. Intraguild predation of Geocoris punctipes on Eretmocerus eremicus and its influence on the control of the whitefly Trialeurodes vaporariorum. Pest. Manag. Sci. 2016, 72, 1110–1116. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, C.V.; Desneux, N.; Bao-Fundora, L.; Ramirez-Romero, R. Alternative extraguild prey modifies focal extraguild prey consumption and parasitism but not intraguild predation intensity. Biol. Control 2021, 153, 104475. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Hoddle, M.S.; Center, T.D. Control de Plagas y Malezas por Enemigos Naturales; USDA 2007; FHTET-2007-02; US Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2007; 751p.
- Tan, X.; Hu, N.; Zhang, F.; Ramirez-Romero, R.; Desneux, N.; Ge, F. Mixed release of two parasitoids and a polyphagous ladybird as a potential strategy to control the tobacco whitefly Bemisia tabaci. Sci. Rep. 2016, 6, 28245. [Google Scholar] [CrossRef]
- Polis, G.A.; Holt, R.D. Intraguild predation: The dynamics of complex trophic interactions. Trends Ecol. Evolut. 1992, 7, 151–154. [Google Scholar] [CrossRef]
- Holt, R.D.; Polis, G.A. A theoretical framework for intraguild predation. Am. Nat. 1997, 149, 745–764. [Google Scholar] [CrossRef]
- Polis, G.A.; Myers, C.A.; Holt, R.D. The ecology and evolution of intraguild predation: Potential competitors that eat each other. Annu. Rev. Ecol. Syst. 1989, 20, 297–330. [Google Scholar] [CrossRef]
- Lester, P.J.; Harmsen, R. Functional and numerical responses do not always indicate the most effective predator for biological control: An analysis of two predators in a two-prey system. J. Appl. Ecol. 2002, 39, 455–468. [Google Scholar] [CrossRef]
- Koss, A.; Chang, G.; Snyder, W. Predation of the green peach aphids by generalist predators in the presence of alternative, Colorado potato beetle egg prey. Biol. Control 2004, 31, 237–244. [Google Scholar] [CrossRef]
- Solomon, M. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Kalinkat, G.; Schneider, F.D.; Digel, C.; Guill, C.; Rall, B.C.; Brose, U. Body masses, functional responses and predator–prey stability. Ecol. Lett. 2013, 16, 1126–1134. [Google Scholar] [CrossRef]
- van Leeuwen, E.; Jansen, V.A.A.; Bright, P.W. How population dynamics shape the functional response in a one-predator–two-prey system. Ecology 2007, 88, 1571–1581. [Google Scholar] [CrossRef]
- Fernández-arhex, V.; Corley, J. The functional response of parasitoids and its implications for biological control. Biocontrol Sci. Technol. 2003, 13, 403–413. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Hassell, M.P. The Dynamics of Arthropod Predator-Prey Systems; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Ail-Catzim, C.E.; Cerna-Chávez, E.; Landeros-Flores, J.; Ochoa-Fuentes, Y.; Rodríguez-González, R.E.; Rueda Puente, E.O. Respuesta funcional de Chrysoperla carnea en ninfas de estadio inicial de Bactericera cockerelli. Southwest Entomol. 2018, 43, 723–731. [Google Scholar] [CrossRef]
- Real, L. The kinetics of functional response. Am. Nat. 1977, 111, 289–300. [Google Scholar] [CrossRef]
- Rosenbaum, B.; Rall, B.C. Fitting functional responses: Direct parameter estimation by simulating differential equations. Methods Ecol. Evol. 2018, 9, 2076–2090. [Google Scholar] [CrossRef]
- Oaten, A.; Murdoch, W.W. Functional response and stability in predator-prey systems. Am. Nat. 1975, 109, 289–298. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Kopp, M.; Tollrian, R. Predator functional responses: Discriminating between handling and digesting prey. Ecol. Monogr. 2002, 71, 95–112. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity-stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef]
- van Baalen, M.; Krivan, V.; van Rijn, P.C.J.; Sabelis, M.W. Food, switching predators, and the persistence of predator-prey systems. Am. Nat. 2001, 157, 512–524. [Google Scholar] [CrossRef]
- Tschanz, B.; Bersier, L.F.; Bacher, S. Functional responses: A question of alternative prey and predator density. Ecology 2007, 88, 1300–1308. [Google Scholar] [CrossRef]
- Desneux, N.; O’Neil, R.J. Potential of an alternative prey to disrupt predation of the generalist predator, Orius insidiosus, on the pest aphid, Aphis glycines, via short-term indirect interactions. Bull. Entomol. Res. 2008, 98, 631–639. [Google Scholar] [CrossRef]
- Bompard, A.; Jaworski, C.C.; Bearez, P.; Desneux, N. Sharing a predator: Can an invasive alien pest affect predation on a local pest? Popul. Ecol. 2013, 55, 433–440. [Google Scholar] [CrossRef]
- Norrdahl, K.; Korpimaki, E. Do predators limit the abundance of alternative prey? Experiments With Vole-Eating Avian and Mammalian Predators. Oikos 2000, 91, 528–540. [Google Scholar] [CrossRef]
- Faria, L.D.B.; Tuller, J.; Maia, L.F.; Reigada, C.; Godoy, W.A.C. Alternative prey and abundance covariance switches an intraguild predator’s functional response. J. Insect. Behav. 2014, 27, 503–513. [Google Scholar] [CrossRef]
- Cohen, A.C.; Byrne, D.N. Geocoris punctipes as a predator of Bemisia tabaci: A laboratory evaluation. Entomol. Exp. Appl. 1992, 64, 195–202. [Google Scholar] [CrossRef]
- Parajulee, M.N.; Shrestha, R.B.; Leser, J.F.; Wester, D.B.; Blanco, C.A. Evaluation of the functional response of selected Arthropod predators on bollworm eggs in the laboratory and effect of temperature on their predation efficiency. Environ. Entomol. 2006, 35, 379–386. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Goggin, F.L.; Williamson, V.M.; Ullman, D.E. Variability in the response of Macrosiphum euphorbiae and Myzus persicae (Hemiptera: Aphididae) to the tomato resistance gene Mi. Environ. Entomol. 2001, 30, 101–106. [Google Scholar] [CrossRef]
- Leite, G.L.D.; Picanço, M.; Jham, G.N.; Ecole, C.C. Effect of leaf characteristics, natural enemies and climatic conditions on the intensities of Myzus persicae and Frankliniella schulzei attacks on Lycopersicon esculentum. Arq. Inst. Biol. 2002, 69, 71–82. [Google Scholar]
- Calixto, A.; Bueno, V.; Montes, F.; van Lenteren, J.C. Development and termal requirements of the Nearctic predator Geocoris punctipes (Hemiptera: Geocoridae) reared at constant and alternating temperatures and fed on Anagasta kuehniella (Lepidoptera: Pyralidae) eggs. Eur. J. Entomol. 2014, 111, 521–528. [Google Scholar] [CrossRef]
- Torres, J.B.; Ruberson, J.R. Interactions of Bt-cotton and the omnivorous big-eyed bug Geocoris punctipes (Say), a key predator in cotton fields. Biol. Control 2006, 39, 47–57. [Google Scholar] [CrossRef]
- Champlain, R.A.; Sholdt, L.L. Life history of Geocoris punctipes (Hemiptera: Lygaeidae) in the laboratory. Ann. Entomol. Soc. Am. 1967, 60, 881–883. [Google Scholar] [CrossRef]
- Rose, M.; Zolnerowich, G. Eretmocerus haldeman (Hymenoptera: Aphelinidae) in the United States with Descriptions of New Species Attacking Bemisia (tabaci complex) (Homoptera: Aleyrodidae). Proc. Entomol. Soc. 1997, 99, 1–27. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19971107613 (accessed on 25 April 2024).
- Velasco-Hernández, M.; Desneux, N.; Ramírez-Martínez, M.M.; Cicero, L.; Ramirez-Romero, R. Host species suitability and instar preference of Aphidius ervi and Aphelinus abdominalis. Entomol. Gen. 2017, 36, 347–367. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Jones, W.A.; Liu, T.X. Interactions among two species of Eretmocerus (Hymenoptera: Aphelinidae), two species of whitefly (Homoptera: Aleyrodidae), and tomato. Environ. Entomol. 2002, 31, 397–402. [Google Scholar] [CrossRef]
- Asplen, M.K.; Bellamy, D.E.; Byrne, D.N. Eggs of Eretmocerus Eremicus, a Whitefly Parasitoid. Vegetable Report. 2001, pp. 1–3. Available online: https://repository.arizona.edu/bitstream/handle/10150/214910/az1252-2b-2001.pdf?sequence=1&isAllowed=y (accessed on 17 March 2024).
- Cohen, A.C. Simple Method for Rearing the Insects Predators Geocoris punctipes (Heteroptera: Lygaeidae) on a Meat Diet. J. Econ. Entomol. 1985, 78, 1173–1175. [Google Scholar] [CrossRef]
- Tillman, P.G.; Mullinix, B.G. Effect of prey species on plant feeding behavior by the big-eyed bug, Geocoris punctipes (Say) (Heteroptera: Geocoridae), on cotton. Environ. Entomol. 2003, 32, 1399–1403. [Google Scholar] [CrossRef]
- Contreras-Garduño, J.; Torres-Enciso, P.; Ramirez-Romero, R. The immune response of the whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) when parasitized by Eretmocerus eremicus (Hymenoptera: Aphelinidae). PLoS ONE 2023, 18, e0296157. [Google Scholar] [CrossRef]
- Meisner, M.; Harmon, J.P.; Harvey, C.T.; Ives, A.R. Intraguild predation on the parasitoid Aphidius ervi by the generalist predator Harmonia axyridis: The threat and its avoidance. Entomol. Exp. Appl. 2010, 138, 193–201. [Google Scholar] [CrossRef]
- Holling, C.S. The functional response of invertebrate predators to prey density. Mem. Entomol. Soc. Can. 1966, 98, 5–86. [Google Scholar] [CrossRef]
- Rogers, D. Random search and insect population models. J. Anim. Ecol. 1972, 41, 369–383. [Google Scholar] [CrossRef]
- Juliano, S.A. Non-linear curve fitting: Predation and functional response curves. In Design and Analysis of Ecological Experiments; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020; pp. 159–182. [Google Scholar]
- Hassell, M.P.; Lawton, J.H.; Beddington, J.R. Sigmoid functional responses by invertebrate predators and parasitoids. J. Anim. Ecol. 1977, 46, 249–262. [Google Scholar] [CrossRef]
- Real, L.A. Ecological determinants of functional response. Ecology 1979, 60, 481–485. [Google Scholar] [CrossRef]
- Pritchard, D. frair: Tools for Functional Response Analysis. R Package Version 0.5.100. 2017. Available online: https://CRAN.R-project.org/package=frair (accessed on 25 April 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 25 April 2024).
- Pritchard, D.W.; Paterson, R.A.; Bovy, H.C.; Barrios-O’Neill, D. Frair: An R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 2017, 8, 1528–1534. [Google Scholar] [CrossRef]
- Okuyama, T. On selection of functional response models: Holling’s models and more. BioControl 2013, 58, 293–298. [Google Scholar] [CrossRef]
- Hammill, E.; Petchey, O.L.; Anholt, B.R. Predator functional response changed by induced defenses in prey. Am. Nat. 2010, 176, 723–731. [Google Scholar] [CrossRef]
- Jeavons, E.; Le Lann, C.; van Baaren, J. Interactions between natural enemies and pollinators: Combining ecological theory with agroecological management. Entomol. Gen. 2023, 43, 243–259. [Google Scholar] [CrossRef]
- Kumar, B.; Mishra, G. Functional response and predatory interactions in conspecific and heterospecific combinations of two congeneric species (Coleoptera: Coccinellidae). Eur. J. Entomol. 2014, 111, 257–265. [Google Scholar] [CrossRef]
- Bressendorff, B.B.; Toft, S. Dome-shaped functional response induced by nutrient imbalance of the prey. Biol. Lett. 2011, 7, 517–520. [Google Scholar] [CrossRef]
- Merlin, B.L.; Ferreira, L.P.; Godoy, W.A.; Moraes, G.J.; Cônsoli, F.L. Functional response of Neoseiulus californicus preying on Tetranychus urticae is affected by prey quality and host-plant acclimation. Biol. Control 2022, 165, 104811. [Google Scholar] [CrossRef]
- Chesson, J. The effect of alternative prey on the functional response of Notonecta hoffmani. Ecology 1989, 70, 1227–1235. [Google Scholar] [CrossRef]
- Eubanks, M.D.; Denno, R.F. Health food versus fast food: The effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecol. Entomol. 2000, 25, 140–146. [Google Scholar] [CrossRef]
- Koss, A.M.; Snyder, W.E. Alternative prey disrupt biocontrol by a guild of generalist predators. Biol. Control 2005, 32, 243–251. [Google Scholar] [CrossRef]
- Badii, M.H.; Landeros, J.; Rodríguez, H.; Cerna, E.; Valenzuela, J.; Ochoa, Y. Some aspects of predation. Int. J. Good Consc. 2013, 8, 148–158. [Google Scholar]
- Hassell, M.P. The Spatial and Temporal Dynamics of Host Parasitoid Interactions; Oxford Series in Ecology and Evolution; Oxford University Press: London, UK, 2000. [Google Scholar]
- Nordlund, D.A.; Morrison, R.K. Handling time, prey preference and functional response of Chrysoperla rufilabris in the laboratory. Entomol. Exp. Appl. 1990, 57, 237–242. [Google Scholar] [CrossRef]
- Kalinkat, G.; Rall, B.C.; Uiterwaal, S.F.; Uszko, W. Empirical evidence of type III functional responses and why it remains rare. Front. Ecol. Evol. 2023, 11, 1033818. [Google Scholar] [CrossRef]
- Schenk, D.; Bacher, S. Functional response of a generalist insect predator to one of its prey species in the field. J. Anim. Ecol. 2002, 71, 524–531. [Google Scholar] [CrossRef]
- Abraços-Duarte, G.; Ramos, S.; Valente, F.; Borges da Silva, E.; Figuereido, E. Functional response and predation rate of Dycyphus cerastii Wagner (Hemiptera: Miridae). Insects 2021, 12, 530. [Google Scholar] [CrossRef] [PubMed]
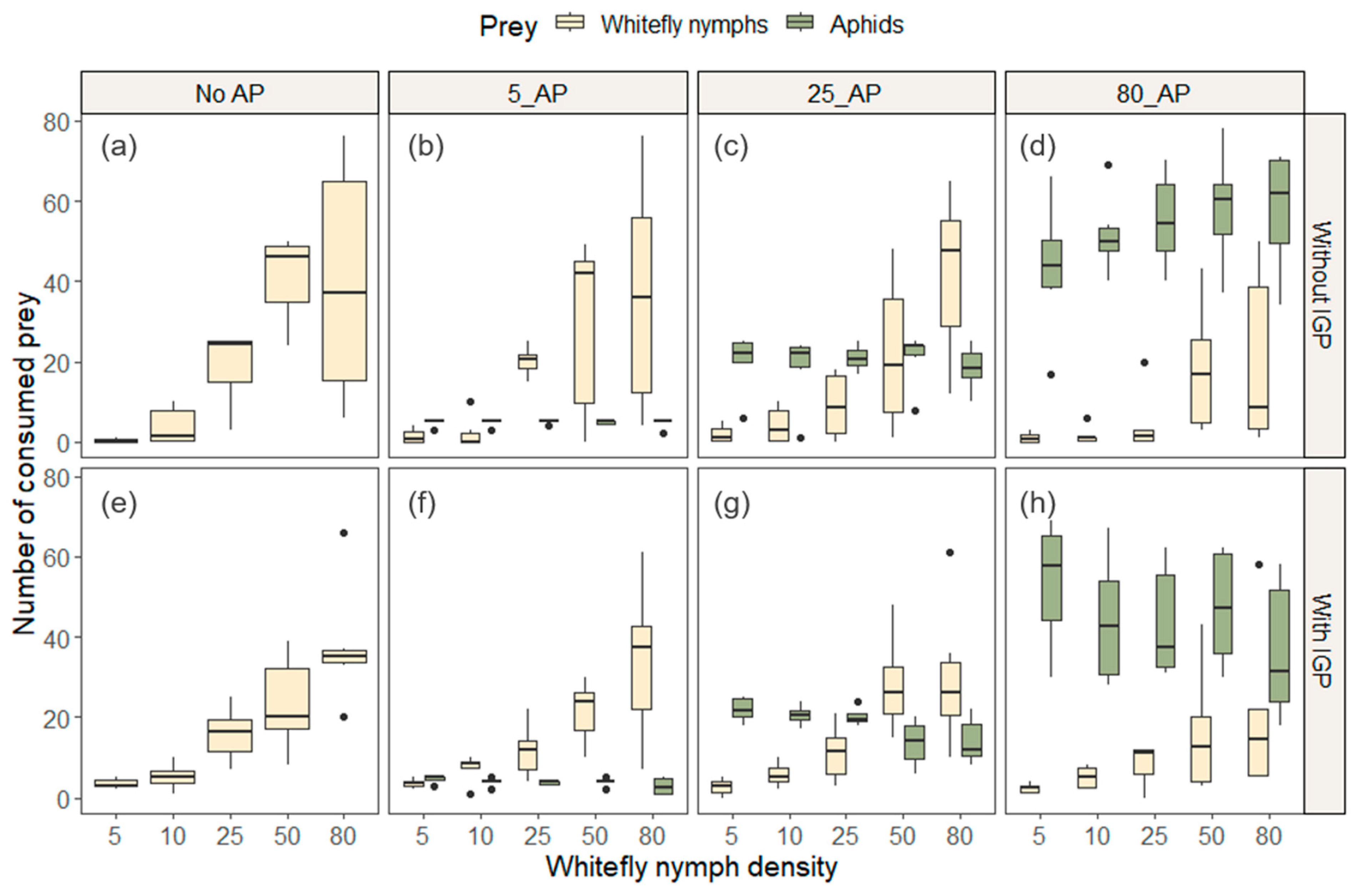

| Experiment | Treatment | Prey Density | Consumed Whitefly Nymphs * | Consumed Aphids * | Total Consumed Prey |
|---|---|---|---|---|---|
| (1) Without IGP | No AP | 5 | 0.33 ± 0.211 a | 0.33 | |
| 10 | 3.83 ± 1.973 b | 3.83 | |||
| 25 | 19 ± 3.821 c | 19 | |||
| 50 | 41.17 ± 4.369 c | 41.17 | |||
| 80 | 39.67 ± 12.298 c | 39.67 | |||
| 5_AP | 5 | 1.33 ± 0.715 a | 4.67 ± 0.333 a | 6 | |
| 10 | 2.17 ± 1.641 a | 4.67 ± 0.333 a | 6.84 | ||
| 25 | 20.17 ± 1.4 b | 4.83 ± 0.167 a | 25 | ||
| 50 | 29.67 ± 9.472 b | 4.67 ± 0.211 a | 34.34 | ||
| 80 | 36.5 ± 11.927 b | 4.5 ± 0.5 a | 41 | ||
| 25_AP | 5 | 1.83 ± 0.872 a | 20 ± 2.96 a | 21.83 | |
| 10 | 4.17 ± 1.851 a | 18.5 ± 3.62 a | 22.67 | ||
| 25 | 9 ± 3.464 ab | 20.8 ± 1.22 a | 29.80 | ||
| 50 | 22 ± 7.698 bc | 21 ± 2.66 a | 43 | ||
| 80 | 42 ± 8.327 c | 18.5 ± 2.2 a | 60.50 | ||
| 80_AP | 5 | 1 ± 0.516 a | 43.3 ± 6.65 a | 44.30 | |
| 10 | 1.5 ± 0.922 a | 51.7 ± 3.96 a | 53.20 | ||
| 25 | 4.33 ± 3.169 ab | 55.3 ± 4.76 a | 59.63 | ||
| 50 | 18.17 ± 6.426 b | 58.3 ± 5.73 a | 76.47 | ||
| 80 | 19.67 ± 9.376 b | 57.8 ± 6.19 a | 77.47 | ||
| (2) With IGP | No AP2 | 5 | 3.5 ± 0.5 a | 3.50 | |
| 10 | 5.17 ± 2.676 a | 5.17 | |||
| 25 | 15.83 ± 2.676 b | 15.83 | |||
| 50 | 23.17 ± 4.888 bc | 23.17 | |||
| 80 | 37.67 ± 6.195 c | 37.67 | |||
| 5_AP2 | 5 | 3.5 ± 0.428 a | 4.5 ± 0.342 a | 8 | |
| 10 | 7.33 ± 1.333 ab | 3.83 ± 0.401 a | 11.16 | ||
| 25 | 11.67 ± 2.654 bc | 3.67 ± 0.211 a | 15.34 | ||
| 50 | 21.5 ± 3.096 cd | 3.83 ± 0.401 a | 25.33 | ||
| 80 | 34 ± 7.894 d | 2.83 ± 0.833 a | 36.83 | ||
| 25_AP2 | 5 | 2.67 ± 0.803 a | 21.8 ± 1.19 a | 24.47 | |
| 10 | 5.67 ± 1.202 ab | 20.5 ± 0.992 a | 26.17 | ||
| 25 | 11.17 ± 2.821 b | 20.2 ± 0.872 a | 31.37 | ||
| 50 | 28.17 ± 4.778 c | 13.5 ± 2.31 b | 41.67 | ||
| 80 | 29.67 ± 7.191 c | 14 ± 2.32 b | 43.67 | ||
| 80_AP2 | 5 | 2.33 ± 0.494 a | 53.5 ± 6.33 a | 55.83 | |
| 10 | 5 ± 1.125 ab | 44 ± 6.6 a | 49 | ||
| 25 | 8.33 ± 2.076 abc | 43.3 ± 5.77 a | 51.63 | ||
| 50 | 15.83 ± 6.263 bc | 47.2 ± 5.9 a | 63.03 | ||
| 80 | 19.83 ± 8.308 c | 36.5 ± 7.14 a | 56.33 |
| Experiment | Treatment | Coefficient | Estimate | SE | z Value | p Value |
|---|---|---|---|---|---|---|
| (1) Without IGP | No AP | Intercept | 0.038 | 0.1194 | 0.316 | 0.7518 |
| Linear | 3.2579 | 0.6141 | 5.31 | 0.0000 | ||
| Quadratic | −5.7776 | 0.5659 | −10.209 | <0.0001 | ||
| Cubic | 1.29764 | 0.54117 | 2.398 | 0.0165 | ||
| 5_AP | Intercept | −0.9046 | 0.109 | −8.3 | <0.0001 | |
| Linear | 1.504 | 0.5768 | 2.608 | 0.0091 | ||
| Quadratic | −2.1778 | 0.4715 | −4.619 | <0.0001 | ||
| Cubic | 1.3379 | 0.3849 | 3.476 | 0.0005 | ||
| 25_AP | Intercept | −0.8954 | 0.07989 | −11.21 | <0.0001 | |
| Linear | 0.8066 | 0.34921 | 2.31 | 0.0209 | ||
| 80_AP | Intercept | −1.6198 | 0.1276 | −12.695 | <0.0001 | |
| Linear | 1.6888 | 0.6298 | 2.682 | 0.0073 | ||
| Quadratic | −1.4268 | 0.4405 | −3.239 | 0.0012 | ||
| (2) With IGP | No AP | Intercept | −0.6082 | 0.0705 | −8.632 | <0.0001 |
| Linear | −0.5728 | 0.324 | −1.769 | 0.0769 | ||
| 5_AP | Intercept | −0.67628 | 0.07154 | −9.453 | <0.0001 | |
| Linear | −0.73974 | 0.32983 | −2.243 | 0.0249 | ||
| 25_AP | Intercept | −0.6751 | 0.0724 | −9.332 | <0.0001 | |
| Linear | −0.8125 | 0.3393 | −2.394 | 0.017 | ||
| 80_AP | Intercept | −1.0385 | 0.08074 | −12.862 | <0.0001 | |
| Linear | −1.19816 | 0.38369 | −3.123 | 0.00179 |
| Experiment | Treatment | FR Type | Parameter | Estimate | SE | z Value | p Value | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| (1) Without IGP | No AP | Flex-gen | b | 0.0028 | 0.0019 | 1.4369 | 0.1508 | 0 | 0.124 |
| h | 0.0228 | 0.0008 | 27.7029 | <0.0001 | 0.011 | 0.034 | |||
| q | 2.1645 | 0.2289 | 9.4571 | <0.0001 | 0.702 | 3.704 | |||
| MFr | 44 | ||||||||
| 5_AP | Flex-gen | b | 0.0047 | 0.0037 | 1.2479 | 0.2121 | 0 | 0.446 | |
| h | 0.0289 | 0.0013 | 21.8089 | <0.0001 | 0 | 0.05 | |||
| q | 2.0329 | 0.2884 | 7.0486 | <0.0001 | 0.14 | 3.536 | |||
| MFr | 35 | ||||||||
| 25_AP | No evidence of any functional response type | ||||||||
| 80_AP | Flex-gen | b | 0.0293 | 0.0237 | 1.235 | 0.2168 | 0 | 0.373 | |
| h | 0.0314 | 0.0081 | 3.8826 | 0.0001 | 0 | 0.096 | |||
| q | 0.7936 | 0.2701 | 2.9377 | 0.0033 | −0.105 | 2.583 | |||
| MFr | 32 | ||||||||
| (2) With IGP | No AP2 | Rogers-II | a | 0.9797 | 0.1138 | 8.6089 | <0.0001 | 0.634 | 1.632 |
| h | 0.0104 | 0.0029 | 3.5373 | 0.0004 | 0 | 0.024 | |||
| MFr | 96 | ||||||||
| 5_AP2 | Rogers-II | a | 0.981 | 0.1264 | 7.7604 | <0.0001 | 0.583 | 1.915 | |
| h | 0.015 | 0.0034 | 4.4635 | <0.0001 | 0 | 0.039 | |||
| MFr | 67 | ||||||||
| 25_AP2 | Flex-gen | b | 0.445 | 0.2179 | 2.0423 | 0.0411 | 0.065 | 1.857 | |
| h | 0.0211 | 0.0046 | 4.5305 | <0.0001 | 0 | 0.037 | |||
| q | 0.2833 | 0.1864 | 1.5198 | 0.1286 | −0.267 | 1.118 | |||
| MFr | 47 | ||||||||
| 80_AP2 | Rogers-II | a | 0.661 | 0.1033 | 6.4007 | <0.0001 | 0.338 | 1.289 | |
| h | 0.0289 | 0.0060 | 4.8363 | <0.0001 | 0 | 0.08 | |||
| MFr | 35 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicero, L.; Chavarín-Gómez, L.E.; Pérez-Ascencio, D.; Barreto-Barriga, O.; Guevara, R.; Desneux, N.; Ramírez-Romero, R. Influence of Alternative Prey on the Functional Response of a Predator in Two Contexts: With and without Intraguild Predation. Insects 2024, 15, 315. https://doi.org/10.3390/insects15050315
Cicero L, Chavarín-Gómez LE, Pérez-Ascencio D, Barreto-Barriga O, Guevara R, Desneux N, Ramírez-Romero R. Influence of Alternative Prey on the Functional Response of a Predator in Two Contexts: With and without Intraguild Predation. Insects. 2024; 15(5):315. https://doi.org/10.3390/insects15050315
Chicago/Turabian StyleCicero, Lizette, Luis Enrique Chavarín-Gómez, Daniela Pérez-Ascencio, Ornella Barreto-Barriga, Roger Guevara, Nicolas Desneux, and Ricardo Ramírez-Romero. 2024. "Influence of Alternative Prey on the Functional Response of a Predator in Two Contexts: With and without Intraguild Predation" Insects 15, no. 5: 315. https://doi.org/10.3390/insects15050315
APA StyleCicero, L., Chavarín-Gómez, L. E., Pérez-Ascencio, D., Barreto-Barriga, O., Guevara, R., Desneux, N., & Ramírez-Romero, R. (2024). Influence of Alternative Prey on the Functional Response of a Predator in Two Contexts: With and without Intraguild Predation. Insects, 15(5), 315. https://doi.org/10.3390/insects15050315