Determination of Triacylglycerol Composition in Mealworm Oil (Tenebrio molitor) via Electrospray Ionization Tandem Mass Spectrometry with Multiple Neutral Loss Scans
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of Fatty Acid
2.3. Analysis of Triacylglycerol
2.4. Method Validation
3. Results
3.1. Fatty Acid in Mealworm Oil
3.2. Triacylglycerol in Mealworm Oil
3.3. Method Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Naturforschung C 2017, 72, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Jajić, I.; Popović, A.; Urošević, M.; Krstović, S.; Petrović, M.; Guljaš, D. Chemical Composition of Mealworm Larvae (Tenbrio moritor) Reared in Serbia. Contemp. Agric. 2019, 68, 23–27. [Google Scholar] [CrossRef]
- Jensen, L.; Miklos, R.; Dalsgaard, T.; Heckmann, L.; Nørgaard, J. Nutritional evaluation of common (Tenebrio molitor) and lesser (Alphitobius diaperinus) mealworms in rats and processing effect on the lesser mealworm. J. Insects Food Feed 2019, 5, 257–266. [Google Scholar] [CrossRef]
- Mlček, J.; Rop, O.; Borkovcova, M.; Bednářová, M. A comprehensive look at the possibilities of edible insects as food in Europe—A review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef]
- Oonincx, D.G.; De Boer, I.J. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, T.; Bosch, G. Insects: A protein-rich feed ingredient in pig and poultry diets. Anim. Front. 2015, 5, 45–50. [Google Scholar]
- Alves, A.V.; Sanjinez-Argandoña, E.J.; Linzmeier, A.M.; Cardoso, C.A.L.; Macedo, M.L.R. Food value of mealworm grown on Acrocomia aculeata pulp flour. PLoS ONE 2016, 11, e0151275. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Energy supplied by edible insects from Mexico and their nutritional and ecological importance. Ecol. Food Nutr. 2008, 47, 280–297. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Sorci, A.; Bonzanini, F.; Lolli, V.; Maistrello, L.; Sforza, S. Influence of the killing method of the black soldier fly on its lipid composition. Food Res. Int. 2019, 116, 276–282. [Google Scholar] [CrossRef]
- Jeon, Y.-H.; Son, Y.-J.; Kim, S.-H.; Yun, E.-Y.; Kang, H.-J.; Hwang, I.-K. Physicochemical properties and oxidative stabilities of mealworm (Tenebrio molitor) oils under different roasting conditions. Food Sci. Biotechnol. 2016, 25, 105–110. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Mateos, R.; Trujillo, M.; Pérez-Camino, M.C.; Moreda, W.; Cert, A. Relationships between oxidative stability, triacylglycerol composition, and antioxidant content in olive oil matrices. J. Agric. Food Chem. 2005, 53, 5766–5771. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.; Stapley, A. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Noor Lida, H.; Sundram, K.; Siew, W.; Aminah, A.; Mamot, S. TAG composition and solid fat content of palm oil, sunflower oil, and palm kernel olein belends before and after chemical interesterification. J. Am. Oil Chem. Soc. 2002, 79, 1137–1144. [Google Scholar] [CrossRef]
- Řezanka, T.; Pádrová, K.; Sigler, K. Regioisomeric and enantiomeric analysis of triacylglycerols. Anal. Biochem. 2017, 524, 3–12. [Google Scholar] [CrossRef]
- Li, M.; Butka, E.; Wang, X. Comprehensive quantification of triacylglycerols in soybean seeds by electrospray ionization mass spectrometry with multiple neutral loss scans. Sci. Rep. 2014, 4, 6581. [Google Scholar] [CrossRef]
- Han, X.; Ye, H. Overview of lipidomic analysis of triglyceride molecular species in biological lipid extracts. J. Agric. Food Chem. 2021, 69, 8895–8909. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Han, X. Tutorial on lipidomics. Anal. Chim. Acta 2019, 1061, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Toschi, T.G.; Christie, W.W.; Conte, L.S. Capillary gas chromatography combined with high performance liquid chromatography for the structural analysis of olive oil triacylglycerols. J. High Resol. Chromatogr. 1993, 16, 725–730. [Google Scholar] [CrossRef]
- Buchgraber, M.; Ulberth, F.; Anklam, E. Comparison of HPLC and GLC techniques for the determination of the triglyceride profile of cocoa butter. J. Agric. Food Chem. 2000, 48, 3359–3363. [Google Scholar] [CrossRef]
- Beccaria, M.; Sullini, G.; Cacciola, F.; Donato, P.; Dugo, P.; Mondello, L. High performance characterization of triacylglycerols in milk and milk-related samples by liquid chromatography and mass spectrometry. J. Chromatogr. A 2014, 1360, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.F.; Wenk, M.R.; Shui, G. Comprehensive analysis of lipid composition in crude palm oil using multiple lipidomic approaches. J. Genet. Genom. 2014, 41, 293–304. [Google Scholar] [CrossRef]
- Gowda, S.; Sasaki, Y.; Hasegawa, E.; Chiba, H.; Hui, S. Lipid fingerprinting of yellow mealworm Tenebrio molitor by untargeted liquid chromatography-mass spectrometry. J. Insects Food Feed 2022, 8, 157–168. [Google Scholar] [CrossRef]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Han, R.H.; Han, X. Novel advances in shotgun lipidomics for biology and medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef]
- Han, R.H.; Wang, M.; Fang, X.; Han, X. Simulation of triacylglycerol ion profiles: Bioinformatics for interpretation of triacylglycerol biosynthesis [S]. J. Lipid Res. 2013, 54, 1023–1032. [Google Scholar] [CrossRef]
- Li, M.; Baughman, E.; Roth, M.R.; Han, X.; Welti, R.; Wang, X. Quantitative profiling and pattern analysis of triacylglycerol species in Arabidopsis seeds by electrospray ionization mass spectrometry. Plant J. 2014, 77, 160–172. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, F.; Xu, S.; Wu, B.; Zheng, C.; Lv, X.; Wu, Z.; Chen, H.; Huang, F. Profiling and quantification of lipids in cold-pressed rapeseed oils based on direct infusion electrospray ionization tandem mass spectrometry. Food Chem. 2019, 285, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Parrish, C.C. Evaluation of triacylglycerol (TAG) profiles and their contents in salmon muscle tissue using ESI-MS/MS spectrometry with multiple neutral loss scans. Food Chem. 2020, 324, 126816. [Google Scholar] [CrossRef]
- Costa, S.; Pedro, S.; Lourenço, H.; Batista, I.; Teixeira, B.; Bandarra, N.M.; Murta, D.; Nunes, R.; Pires, C. Evaluation of Tenebrio molitor larvae as an alternative food source. NFS J. 2020, 21, 57–64. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.F.; Benning, R.; Haase, H. Effect of different drying methods on nutrient quality of the yellow mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jedras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 4, 287–291. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef] [PubMed]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.; van Boekel, M.A.; Lakemond, C.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Yun, E.-Y.; Hwang, J.-S.; Goo, T.-W.; Yoon, Y.-I.; Chung, M.Y.; Yoo, M.-R. Comparative analysis of nutritional and harmful components in the Korean and Chinese mealworms (Tenebrio molitor). J. Korean Soc. Food Sci. Nutr. 2013, 42, 249–254. [Google Scholar] [CrossRef]
- KFDA. Korean Food Code. 2022. Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 9 April 2024).
- Lee, S.; Baek, S.; Yang, S.; Lee, G. Determination of Triacylglycerol Composition and Its Content in Refined and Fractionated Palm Oil by Using LC-MS/MS with Multiple Neutral Loss Scan. J. Oil Palm Res. 2023, 46, 1–11. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef]
- Francardi, V.; Cito, A.; Fusi, S.; Botta, M.; Dreassi, E. Linseed to increase n-3 fatty acids in Tenebrio molitor (Coleoptera Tenebrionidae). Redia 2017, 100, 73–76. [Google Scholar] [CrossRef]
- Fontaneto, D.; Tommaseo-Ponzetta, M.; Galli, C.; Risé, P.; Glew, R.H.; Paoletti, M.G. Differences in fatty acid composition between aquatic and terrestrial insects used as food in human nutrition. Ecol. Food Nutr. 2011, 50, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Indust. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, C.; Palla, C.; Crapiste, G.H.; Carrín, M.E. Simultaneous quantitation of FFA, MAG, DAG, and TAG in enzymatically modified vegetable oils and fats. Food Anal. Methods 2014, 7, 2013–2022. [Google Scholar] [CrossRef]
- Di Stefano, V.; Bongiorno, D.; Buzzanca, C.; Indelicato, S.; Santini, A.; Lucarini, M.; Fabbrizio, A.; Mauro, M.; Vazzana, M.; Arizza, V. Fatty acids and triacylglycerols profiles from Sicilian (cold pressed vs. soxhlet) grape seed oils. Sustainability 2021, 13, 13038. [Google Scholar] [CrossRef]
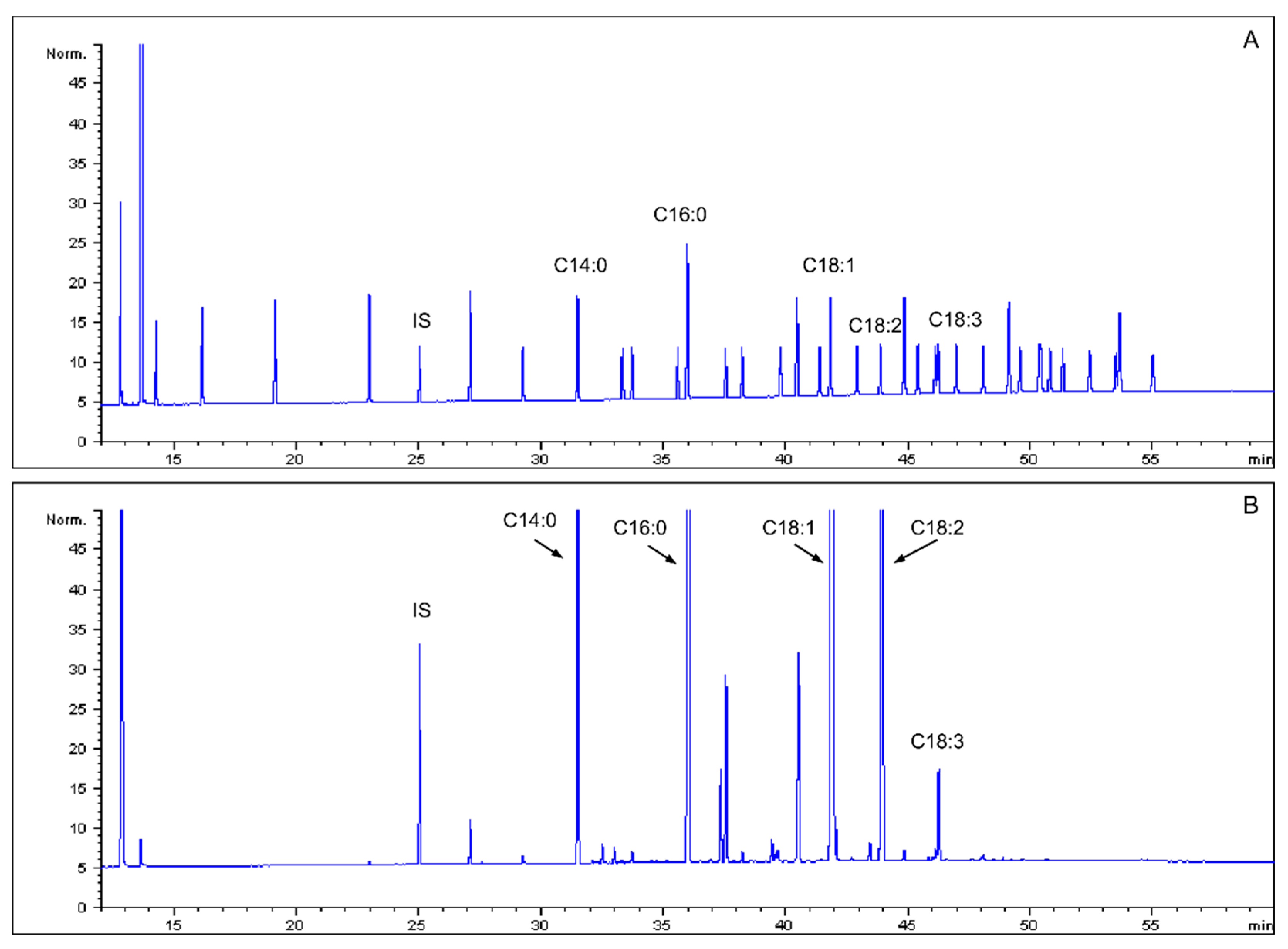
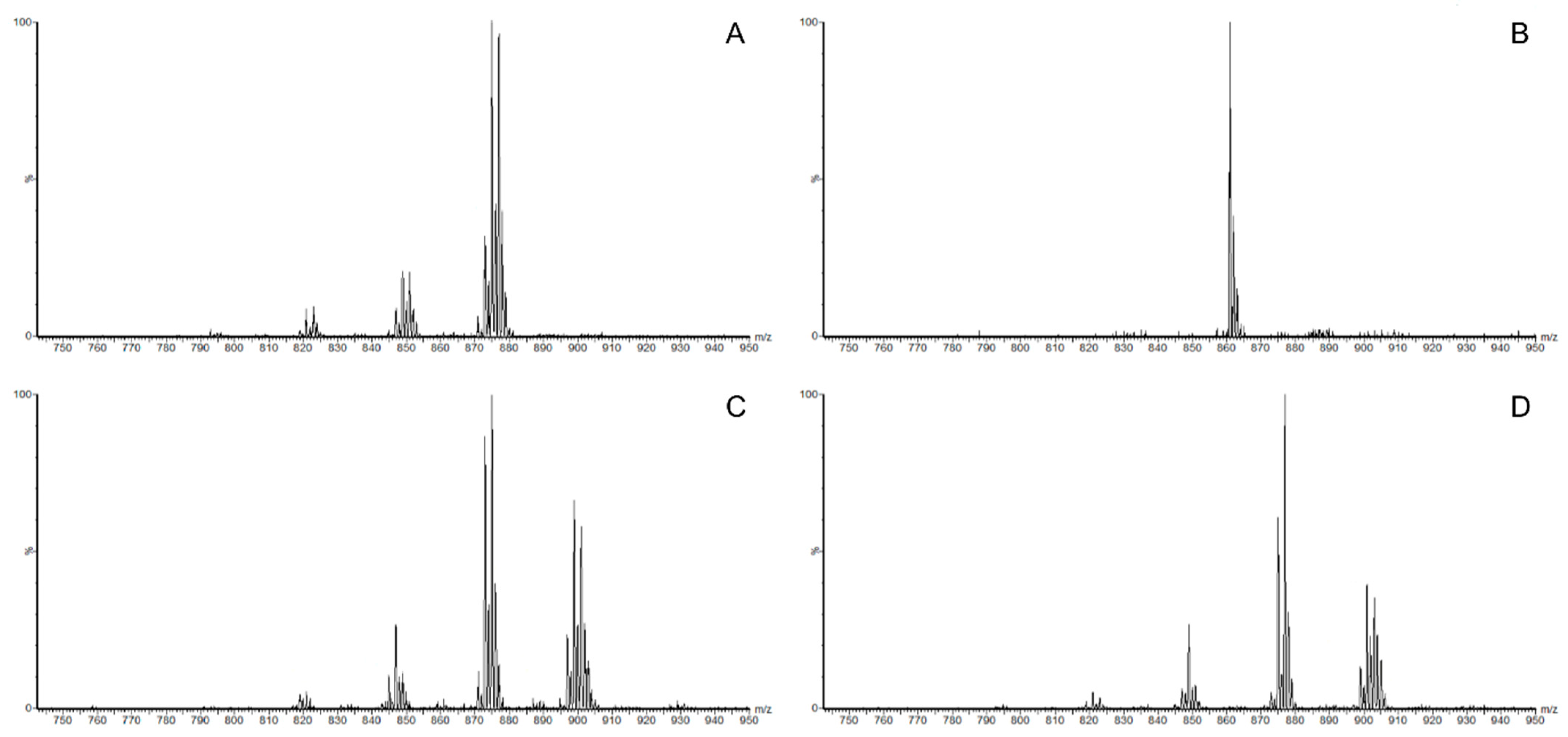

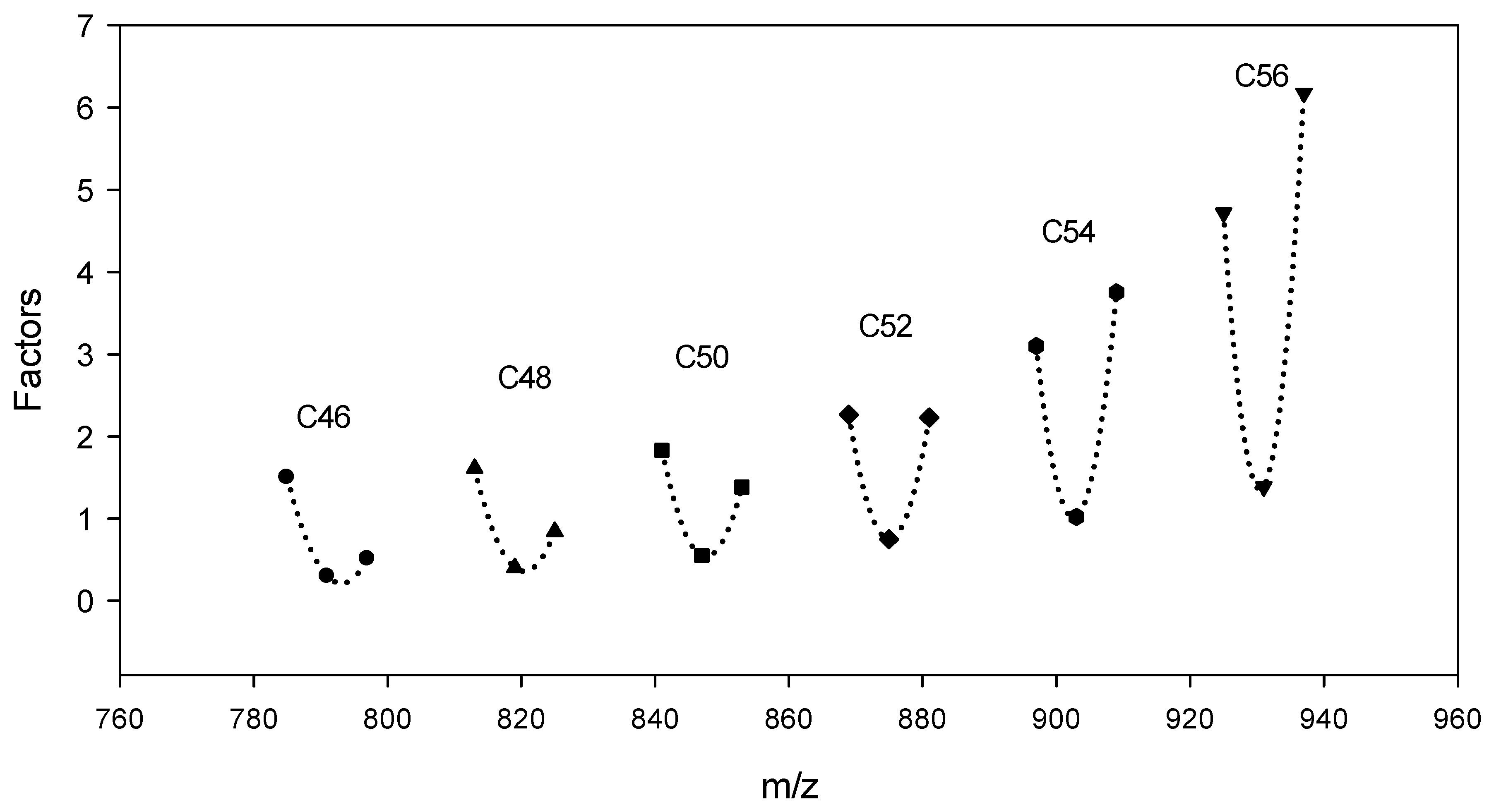
| Fatty Acid | Chain | Content 1 (g/100 g) | RSD (%) |
|---|---|---|---|
| Lauric acid | C12:0 | 0.42 ± 0.01 | 1.55 |
| Myristic acid | C14:0 | 4.07 ± 0.03 | 0.68 |
| Pentadecanoic acid | C15:0 | 0.11 ± 0.00 | 0.55 |
| Palmitic acid | C16:0 | 17.75 ± 0.09 | 0.52 |
| Palmitoleic acid | C16:1 | 1.95 ± 0.01 | 0.39 |
| Heptadecanoic acid | C17:0 | 0.1 ± 0.00 | 0.49 |
| Stearic acid | C18:0 | 2.49 ± 0.01 | 0.30 |
| Oleic acid | C18:1 | 48.68 ± 0.15 | 0.31 |
| Linoleic acid | C18:2 | 23.09 ± 0.07 | 0.29 |
| Arachidic acid | C20:0 | 0.12 ± 0.00 | 1.19 |
| Eicosenoic acid | C20:1 | 0.13 ± 0.00 | 0.96 |
| Linolenic acid | C18:3 | 0.96 ± 0.01 | 0.54 |
| Eicosatrienoic acid | C20:2 | 0.08 ± 0.01 | 9.97 |
| m/z | TAG | Composition | Contents | RSD (%) | |
|---|---|---|---|---|---|
| nmol/g 1 | Portion (%) | ||||
| 793 | C46:2 | 14:0/16:1/16:1 | 6.6 ± 0.5 | 0.1 | 7.1 |
| 795 | C46:1 | 14:0/16:0/16:1 | 5.3 ± 0.5 | 0.1 | 9.8 |
| 797 | C46:0 | 14:0/16:0/16:0 | 2.3 ± 0.1 | 0 | 4.2 |
| 819 | C48:3 | 14:0/16:1/18:2 | 24.3 ± 0.9 | 0.3 | 3.6 |
| 819 | C48:3 | 16:1/16:1/16:1 | 3.4 ± 0.2 | 0 | 5.6 |
| 821 | C48:2 | 14:0/16:0/18:2 | 27.4 ± 1.6 | 0.3 | 5.7 |
| 821 | C48:2 | 14:0/16:1/18:1 | 62.8 ± 0.7 | 0.7 | 1.1 |
| 821 | C48:2 | 16:0/16:1/16:1 | 8.6 ± 0.5 | 0.1 | 6.0 |
| 823 | C48:1 | 14:0/16:0/18:1 | 25.4 ± 1.1 | 0.3 | 4.2 |
| 823 | C48:1 | 16:0/16:0/16:1 | 3.3 ± 0.1 | 0 | 3.4 |
| 825 | C48:0 | 16:0/16:0/16:0 | 2.3 ± 0.2 | 0 | 10.8 |
| 845 | C50:4 | 14:0/18:2/18:2 | 75.2 ± 5.7 | 0.9 | 7.5 |
| 845 | C50:4 | 16:0/16:1/18:3 | 15.2 ± 0.9 | 0.2 | 5.7 |
| 847 | C50:3 | 14:0/18:0/18:3 | 4.2 ± 0.6 | 0 | 13.6 |
| 847 | C50:3 | 14:0/18:1/18:2 | 91.7 ± 3.4 | 1.1 | 3.7 |
| 847 | C50:3 | 16:0/16:0/18:3 | 4.8 ± 0.4 | 0.1 | 8.2 |
| 847 | C50:3 | 16:0/16:1/18:2 | 56.9 ± 5.3 | 0.7 | 9.3 |
| 849 | C50:2 | 14:0/18:0/18:2 | 20.4 ± 2.2 | 0.2 | 10.9 |
| 849 | C50:2 | 14:0/18:1/18:1 | 105.4 ± 2.9 | 1.2 | 2.7 |
| 849 | C50:2 | 16:0/16:0/18:2 | 26 ± 1.5 | 0.3 | 5.6 |
| 849 | C50:2 | 16:0/16:1/18:1 | 16.9 ± 1.2 | 0.2 | 7.2 |
| 851 | C50:1 | 14:0/18:0/18:1 | 16.2 ± 0.8 | 0.2 | 5.0 |
| 851 | C50:1 | 16:0/16:0/18:1 | 56.7 ± 3.3 | 0.7 | 5.8 |
| 851 | C50:1 | 16:0/16:1/18:0 | 18.8 ± 0.1 | 0.2 | 0.4 |
| 853 | C50:0 | 16:0/16:0/18:0 | 13.8 ± 1.5 | 0.2 | 10.7 |
| 871 | C52:5 | 16:0/18:2/18:3 | 167.7 ± 2.5 | 2 | 1.5 |
| 871 | C52:5 | 16:1/18:2/18:2 | 156.7 ± 14 | 1.8 | 9.0 |
| 873 | C52:4 | 16:0/18:1/18:3 | 105.4 ± 6.8 | 1.2 | 6.5 |
| 873 | C52:4 | 16:0/18:2/18:2 | 600.5 ± 56.4 | 7.1 | 9.4 |
| 873 | C52:4 | 16:1/18:1/18:2 | 246.2 ± 30.5 | 2.9 | 12.4 |
| 875 | C52:3 | 16:0/18:0/18:3 | 7.5 ± 0.7 | 0.1 | 8.9 |
| 875 | C52:3 | 16:0/18:1/18:2 | 1488.1 ± 117.7 | 17.5 | 7.9 |
| 875 | C52:3 | 16:1/18:0/18:2 | 20.9 ± 1.7 | 0.2 | 8.3 |
| 875 | C52:3 | 16:1/18:1/18:1 | 171.7 ± 14.7 | 2 | 8.6 |
| 877 | C52:2 | 16:0/18:0/18:2 | 21.5 ± 1.7 | 0.3 | 8.1 |
| 877 | C52:2 | 16:0/18:1/18:1 | 1549.4 ± 135.5 | 18.2 | 8.7 |
| 877 | C52:2 | 16:1/18:0/18:1 | 17.5 ± 1 | 0.2 | 5.9 |
| 879 | C52:1 | 16:0/18:0/18:1 | 91.1 ± 9 | 1.1 | 9.9 |
| 895 | C54:7 | 18:2/18:2/18:3 | 136.6 ± 8.9 | 1.6 | 6.5 |
| 897 | C54:6 | 18:1/18:2/18:3 | 251 ± 11.9 | 3 | 4.7 |
| 897 | C54:6 | 18:2/18:2/18:2 | 383.6 ± 39.7 | 4.5 | 10.3 |
| 899 | C54:5 | 18:1/18:1/18:3 | 46 ± 2.4 | 0.5 | 5.3 |
| 899 | C54:6 | 18:1/18:2/18:2 | 659.8 ± 27.5 | 7.8 | 4.2 |
| 901 | C54:4 | 18:0/18:1/18:3 | 10.3 ± 1 | 0.1 | 9.5 |
| 901 | C54:4 | 18:0/18:2/18:2 | 48.4 ± 2.7 | 0.6 | 5.7 |
| 901 | C54:4 | 18:1/18:1/18:2 | 870.1 ± 26.3 | 10.2 | 3.0 |
| 903 | C54:3 | 16:0/18:2/20:1 | 15.1 ± 0.3 | 0.2 | 2.0 |
| 903 | C54:3 | 18:0/18:1/18:2 | 236.1 ± 25.8 | 2.8 | 10.9 |
| 903 | C54:3 | 18:1/18:1/18:1 | 328.2 ± 14 | 3.9 | 4.3 |
| 905 | C54:2 | 16:0/18:1/20:1 | 8.7 ± 0.3 | 0.1 | 3.6 |
| 905 | C54:2 | 16:0/18:2/20:0 | 10.6 ± 0.6 | 0.1 | 5.5 |
| 905 | C54:2 | 18:0/18:0/18:2 | 9.4 ± 0.4 | 0.1 | 4.1 |
| 905 | C54:2 | 18:0/18:1/18:1 | 42.1 ± 1.4 | 0.5 | 3.3 |
| 907 | C54:1 | 16:0/18:0/20:1 | 16.6 ± 1.3 | 0.2 | 7.5 |
| 929 | C56:4 | 18:2/18:1/20:1 | 41.1 ± 1.4 | 0.5 | 3.5 |
| 931 | C56:3 | 18:1/18:1/20:1 | 26.7 ± 1.8 | 0.3 | 6.9 |
| 933 | C56:2 | 18:1/18:1/20:0 | 30.3 ± 0.8 | 0.4 | 2.8 |
| Standard | Recovery (%) | Linearity (R2) | LOD (μM) | LOQ (μM) |
|---|---|---|---|---|
| Tripalmitin (16:0/16:0/16:0) | 90.9 ± 3.2 | 0.9993 | 0.25 | 0.5 |
| Tristerarin (18:0/18:0/18:0) | 86.9 ± 2.4 | 0.9991 | 0.2 | 0.4 |
| Triolein (18:1/18:1/18:1) | 95.0 ± 0.9 | 0.9999 | 0.05 | 0.1 |
| Trilinolein (18:2/18:2/18:2) | 93.6 ± 4.5 | 0.9998 | 0.05 | 0.1 |
| Trilinoleinin (18:3/18:3/18:3) | 94.2 ± 2.8 | 0.9997 | 0.05 | 0.1 |
| 1,3-dipalmitoyl-2-oleoylglycerol (16:0/18:1/16:0) | 88.1 ± 3.6 | 0.9990 | 0.1 | 0.2 |
| 1,3-distearoyl-2-oleoylglycerol (18:0/18:1/18:0) | 90.6 ± 1.6 | 0.9992 | 0.07 | 0.14 |
| 1-palmitoyl-2-oleoyl-3-stearoyl-sn-glycerol (16:0/18:1/18:0) | 92.4 ± 1.9 | 0.999 | 0.1 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kim, M.; Cho, H.; Lee, G.-H. Determination of Triacylglycerol Composition in Mealworm Oil (Tenebrio molitor) via Electrospray Ionization Tandem Mass Spectrometry with Multiple Neutral Loss Scans. Insects 2024, 15, 365. https://doi.org/10.3390/insects15050365
Lee S, Kim M, Cho H, Lee G-H. Determination of Triacylglycerol Composition in Mealworm Oil (Tenebrio molitor) via Electrospray Ionization Tandem Mass Spectrometry with Multiple Neutral Loss Scans. Insects. 2024; 15(5):365. https://doi.org/10.3390/insects15050365
Chicago/Turabian StyleLee, Seongeung, Minkyoung Kim, Hyeokjun Cho, and Gyeong-Hwen Lee. 2024. "Determination of Triacylglycerol Composition in Mealworm Oil (Tenebrio molitor) via Electrospray Ionization Tandem Mass Spectrometry with Multiple Neutral Loss Scans" Insects 15, no. 5: 365. https://doi.org/10.3390/insects15050365
APA StyleLee, S., Kim, M., Cho, H., & Lee, G.-H. (2024). Determination of Triacylglycerol Composition in Mealworm Oil (Tenebrio molitor) via Electrospray Ionization Tandem Mass Spectrometry with Multiple Neutral Loss Scans. Insects, 15(5), 365. https://doi.org/10.3390/insects15050365







