Systematics Review and Phylogeny of Cyrtophyllitinae Zeuner, 1935 sensu Gorochov, Jarzembowski & Coram, 2006 (Ensifera, Haglidae), with Description of Two New Species †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Examined and Terminology
2.2. Phylogenetic Analyses
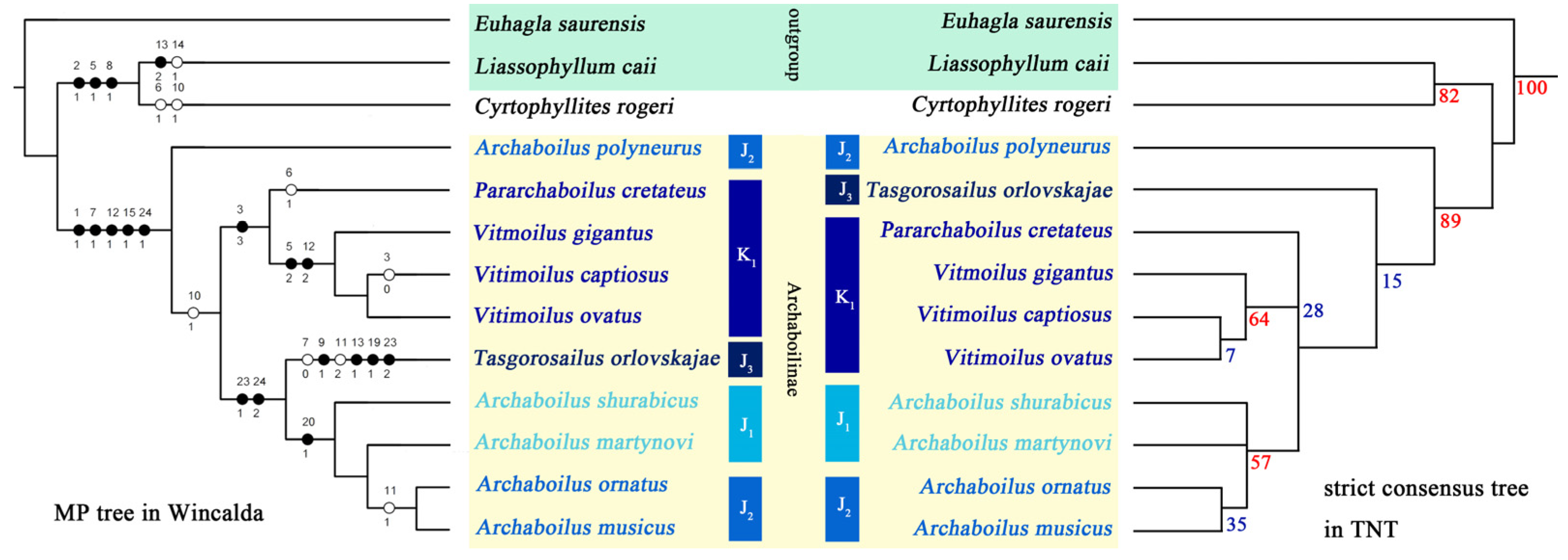

3. Results
3.1. Phylogenetic Results
3.2. Systematic Palaeontology
- Order: Orthoptera Olivier, 1789
- Suborder: Ensifera Chopard, 1920
- Superfamily: Hagloidea Handlirsch, 1906
- Family: Haglidae Handlirsch, 1906
- Subfamily: Archaboilinae Gu, Ren et Chen, subfam. nov.
- Genus: Archaboilus Martynov, 1937
- Archaboilus ornatus Gu, Ren et Chen, sp. nov. (Figure 2)
- Genus: Vitimoilus Gorochov, 1996
- Vitimoilus gigantus Gu, Ren et Chen, sp. nov. (Figure 3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorochov, A.V. Triassic insects of the superfamily Hagloidea (Orthoptera). Tr. Zool. Instituta 1986, 143, 65–100. [Google Scholar]
- Gorochov, A.V.; Coram, R.A. New and little known taxa of the suborder Ensifera (Insecta: Orthoptera) from the Lower Cretaceous of England. Cretac. Res. 2022, 134, 105164. [Google Scholar] [CrossRef]
- Rasnitsyn, A.P.; Quicke, D.L.J. History of Insects; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; p. 517. [Google Scholar]
- Handlirsch, A. Revision of American Paleozic Insects. In Proceedings of the United States National Museum; Smithsonian Institution Press: Washington, DC, USA, 1906; Volume 29, pp. 661–820. [Google Scholar]
- Martynov, A.V. Liassic Insects from Shurab and Kyzyl-Kiya; Trudy Paleontologiceskogo Instituta, Akademiya Nauk SSSR: Moscow, Russia, 1937; Volume 7, pp. 80–160. [Google Scholar]
- Gorochov, A.V.; Maehr, M. New names for some fossil taxa of the infraclass Polyneoptera (Insecta). Zoosyst. Ross. 2008, 17, 60. [Google Scholar] [CrossRef]
- Gorochov, A.V. The Lower and Middle Jurassic Superfamily Hagloidea (Orthoptera). Paleontol. J. 1988, 22, 50–61. [Google Scholar]
- Gorokhov, A.V. System and evolution of the suborder Ensifera (Orthoptera), Part I. Proc. Zool. Inst. Russ. Acad. Sci. 1995, 260, 1–224. [Google Scholar]
- Zeuner, F.E. The recent and fossil prophalangopsidae (saltatoria). Proc. R. Entomol. Soc. London. Ser. B Taxon. 1935, 4, 102–108. [Google Scholar] [CrossRef]
- Gorochov, A.V.; Jarzembowski, E.A.; Coram, R.A. Grasshoppers and crickets (Insecta: Orthoptera) from the Lower Cretaceous of southern England. Cretac. Res. 2006, 27, 641–662. [Google Scholar] [CrossRef]
- Hong, Y.C. Middle Jurassic Fossil Insects in North China; Geological Publishing House: Beijing, China, 1983; pp. 42–48. [Google Scholar]
- Gu, J.J.; Qiao, G.X.; Ren, D. Revision and New Taxa of Fossil Prophalangopsidae (Orthoptera: Ensifera). J. Orthoptera Res. 2010, 19, 41–56. [Google Scholar] [CrossRef]
- Gu, J.J.; Montealegre, Z.F.; Robert, D.; Engel, M.S.; Qiao, G.X.; Ren, D. Wing stridulation in a Jurassic katydid (Insecta, Orthoptera) produced low-pitched musical calls to attract females. Proc. Natl. Acad. Sci. USA 2012, 109, 3868–3873. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.J.; Tian, H.; Yin, X.; Shi, F.; Ren, D. A new species of Cyrtophyllitinae (Insecta: Ensifera) from the Cretaceous China. Cretac. Res. 2017, 74, 151–154. [Google Scholar] [CrossRef]
- Gu, J.J.; Yang, X.; Huang, R.; Yang, G.; Yue, Y.; Ren, D. New species and material of Hagloidea (Insecta, Ensifera) from the Yanliao biota of China. Zookeys 2021, 1033, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, P. Die Insectenwelt des lithographischen Schiefers in Bayern. Palaeontographica 1888, 35, 215–254. [Google Scholar]
- Gorochov, A.V. New genera and species of Mesozoic orthopteran Superfamily Hagloidea (Orthoptera) incertae sedis. In Discoveries in Faunistics and Systematics; Naukova Dumka: Kiev, Ukraine, 1990; pp. 32–35. [Google Scholar]
- Zeuner, F.E. Fossil insects from the Lower Lias of Charmouth, Dorset. Bull. Br. Mus. (Nat. Hist.) Geol. 1962, 7, 155–171. [Google Scholar]
- Gorochov, A.V. New Mesozoic Hagloidea (Orthoptera). Paleontol. J. 1996, 30, 440–448. [Google Scholar]
- Ren, D.; Shih, C.; Gao, T.; Wang, Y.; Yao, Y. Rhythms of Insect Evolution-Evidence from the Jurassic and Cretaceous in Northern China; Wiley Blackwell: Hoboken, NJ, USA, 2019; p. 710. [Google Scholar]
- Gao, T.; Shih, C.; Ren, D. Behaviors and Interactions of Insects in Mid-Mesozoic Ecosystems of Northeastern China. Annu. Rev. Entomol. 2021, 66, 337–354. [Google Scholar] [CrossRef]
- Yang, H.; Shi, C.; Engel, M.S.; Zhao, Z.; Ren, D.; Gao, T. Early specializations for mimicry and defense in a Jurassic stick insect. Natl. Sci. Rev. 2021, 8, nwaa056. [Google Scholar] [CrossRef] [PubMed]
- Béthoux, O.; Nel, A. Venation pattern of Orthoptera. J. Orthoptera Res. 2001, 10, 195–198. [Google Scholar] [CrossRef]
- Béthoux, O.; Nel, A. Venation pattern and revision of Orthoptera sensu nov. and sister groups. Phylogeny of Palaeozoic and Mesozoic Orthoptera sensu nov. Zootaxa 2002, 96, 1–88. [Google Scholar] [CrossRef]
- Zherikhin, V.V.; Rasnitsyn, A.P. The Jurassic Orthoptera in South Siberia and West Mongolia. In Jurassic Insects of Siberia and Mongolia; Rasnitsyn, A.P., Ed.; Trudy Paleontologicheskogo Instituta, Akademiya Nauk SSSR, Nauka: Moscow, Russia, 1985; pp. 171–184. [Google Scholar]
- Gu, J.J.; Qiao, G.X.; Ren, D. The first discovery of Cyrtophyllitinae (Orthoptera, Haglidae) from the Middle Jurassic and its morphological implications. Alcheringa Australas. J. Palaeontol. 2012, 36, 27–34. [Google Scholar] [CrossRef]
- Nixon, K.C. ASADO version 1.5 Beta. Program and Documentation Distributed by the Author; Self-published: Ithaca, NY, USA, 2004. [Google Scholar]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.B. Mesozoic and Cenozoic insects. In Palaeontological Atlas of Northwest China. Shaanxi-Gansu-Ningxia; Volume 3 Mesozoic and Cenozoic (ed. Institute of Geology and Mineral Resources of Xi’an); Geological Publishing House: Beijing, China, 1982; pp. 76–77. [Google Scholar]
- Lin, Q.B.; Huang, D.Y. Revision of “Parahagla lamina” Lin, 1982 and two new species of Aboilus (Orthoptera: Prophalangopsidae) from the Early-Middle Jurassic of Northwest China. Prog. Nat. Sci. 2006, 16, 303–307. [Google Scholar]
| Taxon | Distribution | Age | Reference |
|---|---|---|---|
| Archaboilus kisylkiensis Martynov, 1937 | Kyrgyzstan | J1 | [5] |
| A. shurabicus Martynov, 1937 | Kyrgyzstan | J1 | [5] |
| A. martynovi Gorochov, 1988 | Kyrgyzstan | J1 | [7] |
| A. similis Zherikhin, 1985 | Russia | J1 | [25] |
| A. musicus Gu, Engel & Ren, 2012 | China | J2 | [13] |
| A. polyneurus Gu, Yue & Ren, 2021 | China | J2 | [15] |
| A. ornatus sp. nov. | China | J2 | This study |
| Cyrtophyllites rogeri Oppenheim, 1888 | Germany | J3 | [16] |
| Pararchaboilus cretaceus comb. nov. | England | K1 | [2,10] |
| Tasgorosailus orlovskajae Gorochov, 1990 | Kazakhstan | J3 | [17] |
| Vitimoilus captiosus Gorochov, 1996 | Russia | K1 | [19] |
| V. ovatus Gu, Tian, Yin, Shi & Ren, 2017 | China | K1 | [14] |
| V. gigantus sp. nov. | China | K1 | This study |
| No. | Morphological Characters and States |
|---|---|
| 1 | ScA crosses the subcostal area: 0, no; 1, yes. |
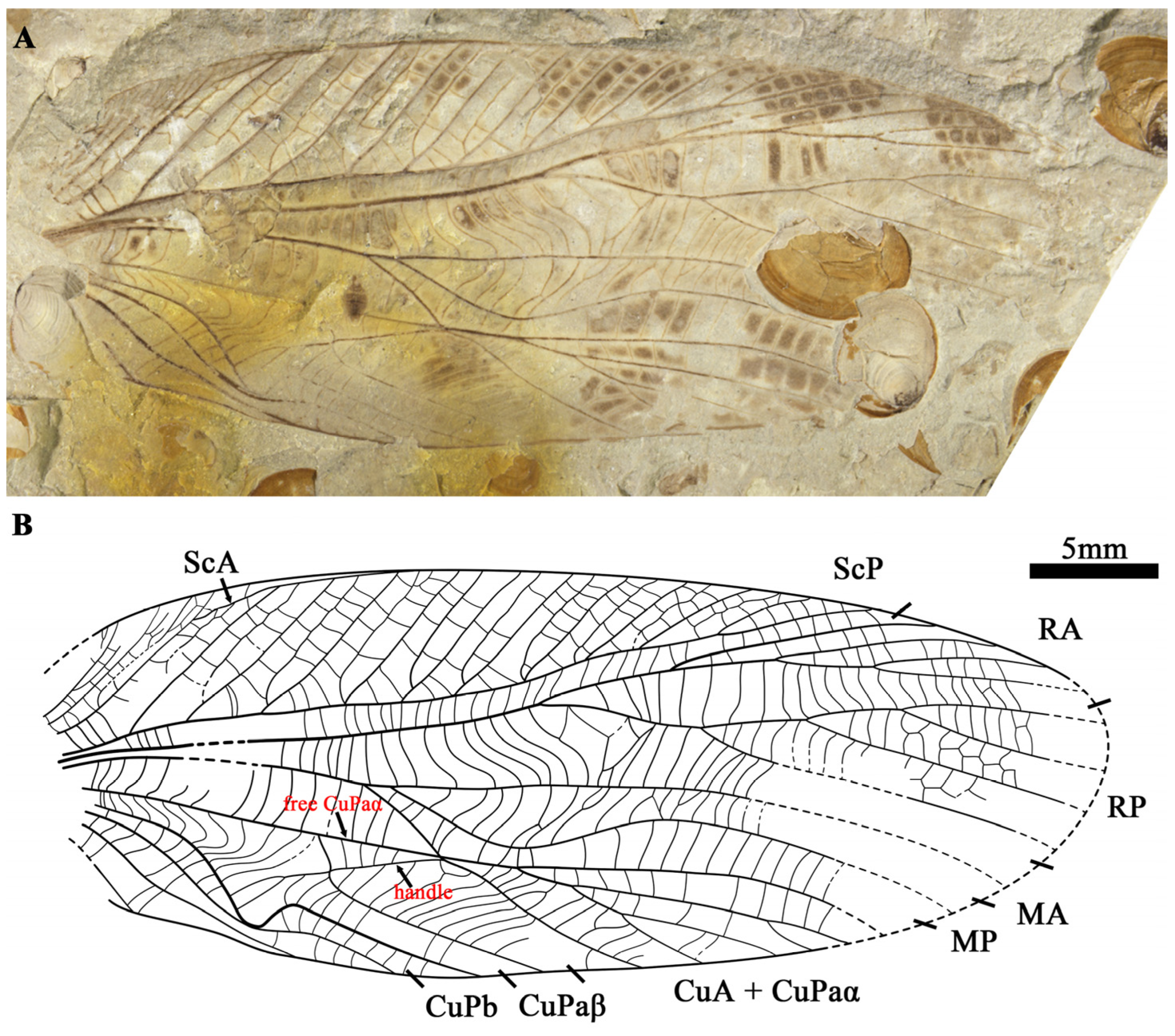
| 2 | ScP redirected anterior margin near the tegmen apex: 0, no (Figure 2); 1, yes (Figure 1, [26]). |
| 3 | The area between the RP and MA has a distinct oblique vein separating two sets of cross veins: 0, no (Figure 288, [8]); 1, yes, the oblique vein connected to the middle of the free RP (Figure 1D, [13]); 2, yes, the oblique vein connected to the base of the free RP (Figure 1D, [13]); 3, without a distinct oblique vein but the presence of several long and curved cross veins (Figure 1, [14]). |
| 4 | Base of the RP far away from the base of the MA: 0, no, close to each other; 1, yes. |
| 5 | RP: 0, curved toward the posterior margin (Figure 1D, [13]): 1, basally curved toward the posterior margin, then redirected to the anterior wing margin (Figure 1, [26]); 2, curved toward the anterior margin (Figure 3, [14]). |
| 6 | RP branched: 0, distally (Figure 2); 1, basally (Figure 3A, [2]) |
| 7 | R forks into RA and RP: 0, distal of 3/5 of the wing (Figure 1, [26]); 1, closer to the middle than to 3/5 of the wing (Figure 2). |
| 8 | Area between RA and RP: 0, lancet-like; 1, rectangular (Figure 1, [26]). |
| 9 | MP basally curved: no (Figure 3, [17]); 0, 1, yes. |
| 10 | Free CuA three times in length longer than free M: 0, no; 1, yes (Figure 2). |
| 11 | MA: 0, undulate (Figure 1, [26]); 1, arch (Figure 1); 2, obliquely straight (Figure 3, [17]). |
| 12 | The widest part of the area between R and MA, located at: 0, at or after the forking of M and before the forking of R (Figure 288, [8]); 1, at the forking of R (Figure 2); 2, after the forking of R (Figure 3, [14]). |
| 13 | MP: 0, sigmoidal (its base curved to the posterior wing margin) (Figure 2); 1, straight (Figure 3, [17]); 2, bowed toward the posterior wing margin (Figure 1, [26]). |
| 14 | CuA separated from M + CuA: 0, close to the 1/3 of the wing length (Figure 2); 1, distal to the 2/5 of the wing length. |
| 15 | The part of MA opposite RP: 0, bowed toward RP and closely positioned (Figure 1, [26]); 1, located at a distant position from RP (Figure 2). |
| 16 | CuA fused CuPaα: 0, basal half of the wing; 1, distal half of the wing (Figure 3, [14]). |
| 17 | Cross veins between the CuPb and CuPaβ are strongly curved in the basal part: 0, strongly curved (Figure 2); 1, straight (Figure 3, [14]). |
| 18 | M forks into MA and MP: 0, basal or at the level of 2/5; 1, at the level or distal to the 1/2 of wing length (Figure 3, [14]). |
| 19 | M + CuA diverges: 0, closer to the one third of the wing length than to the second fifth (Figure 2); 1, closer to the second fifth (Figure 3, [17]); 2, closer to the middle of the wing than to the second fifth (Figure 3, [14]). |
| 20 | Free CuPaα vs. free CuA: 0, longer than the free CuA; 1, approximate in length (Figure 2). |
| 21 | Basal area between CuPb and CuPa: 0, approximately the same width as the area between the CuPa and the M + CuA; 1, distinctly narrower than the area between the CuPa and M + CuA (Figure 3, [14]). |
| 22 | Handle: 0, shorter than the free CuA (Figure 288, Gorochov, 1995); 1, the same length as the free CuA (Figure 2); 2, distinctly longer than the free CuA (Figure 3, [14]). |
| 23 | CuPa forked into CuPaβ and CuPaα: 0, at the level of the fusion of the CuPb and AA1 (Figure 288, [8]); 1, at the level of the bow of the AA1 after its fusion with the CuPb (Figure 2); 2, distal of the bow of AA1 after its fusion with the CuPb (Figure 3, [17]). |
| 24 | The length of the handle vs. the length of the CuPaβ between the CuPa and handle: 0, shorter; 1, approximately the same length or not longer than twice (Figure 3, [14]); 2. longer than twice (Figure 2). |
| Taxon/Character | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Euhagla saurensis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liassophyllum caii | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ? | 0 |
| Archaboilus shurabicus | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 |
| Archaboilus martynovi | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 |
| Archaboilus musicus | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | ? | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 |
| Archaboilus polyneurus | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Archaboilus ornatus sp. n. | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | ? | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 |
| Tasgorosailus orlovskajae | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 2 |
| Pararchaboilus cretaceus | 1 | 0 | 3 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | ? |
| Cyrtophyllites rogeri | ? | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | ? | 0 | ? | ? | ? |
| Vitimoilus captiosus | 1 | 0 | 0 | ? | ? | ? | ? | 0 | 1 | 1 | ? | ? | ? | 1 | ? | 1 | 1 | 1 | ? | 0 | 1 | 2 | 0 | 1 |
| Vitimoilus ovatus | 1 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 2 | 0 | 1 |
| Vitmoilus gigantus sp. n. | ? | 0 | 3 | ? | 2 | 0 | 1 | 0 | 1 | ? | 1 | 2 | ? | ? | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.-J.; Yuan, W.; Huang, R.; Ren, D.; Chen, H.-X. Systematics Review and Phylogeny of Cyrtophyllitinae Zeuner, 1935 sensu Gorochov, Jarzembowski & Coram, 2006 (Ensifera, Haglidae), with Description of Two New Species. Insects 2024, 15, 396. https://doi.org/10.3390/insects15060396
Gu J-J, Yuan W, Huang R, Ren D, Chen H-X. Systematics Review and Phylogeny of Cyrtophyllitinae Zeuner, 1935 sensu Gorochov, Jarzembowski & Coram, 2006 (Ensifera, Haglidae), with Description of Two New Species. Insects. 2024; 15(6):396. https://doi.org/10.3390/insects15060396
Chicago/Turabian StyleGu, Jun-Jie, Wei Yuan, Rong Huang, Dong Ren, and Hong-Xing Chen. 2024. "Systematics Review and Phylogeny of Cyrtophyllitinae Zeuner, 1935 sensu Gorochov, Jarzembowski & Coram, 2006 (Ensifera, Haglidae), with Description of Two New Species" Insects 15, no. 6: 396. https://doi.org/10.3390/insects15060396
APA StyleGu, J.-J., Yuan, W., Huang, R., Ren, D., & Chen, H.-X. (2024). Systematics Review and Phylogeny of Cyrtophyllitinae Zeuner, 1935 sensu Gorochov, Jarzembowski & Coram, 2006 (Ensifera, Haglidae), with Description of Two New Species. Insects, 15(6), 396. https://doi.org/10.3390/insects15060396






