Ultrastructural Organization and Metal Elemental Composition of the Mandibles in Two Ladybird Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Scanning Electron Microscopy
2.3. EDX Analysis
2.4. Light and Transmission Electron Microscopy
2.5. Data Analysis
3. Results
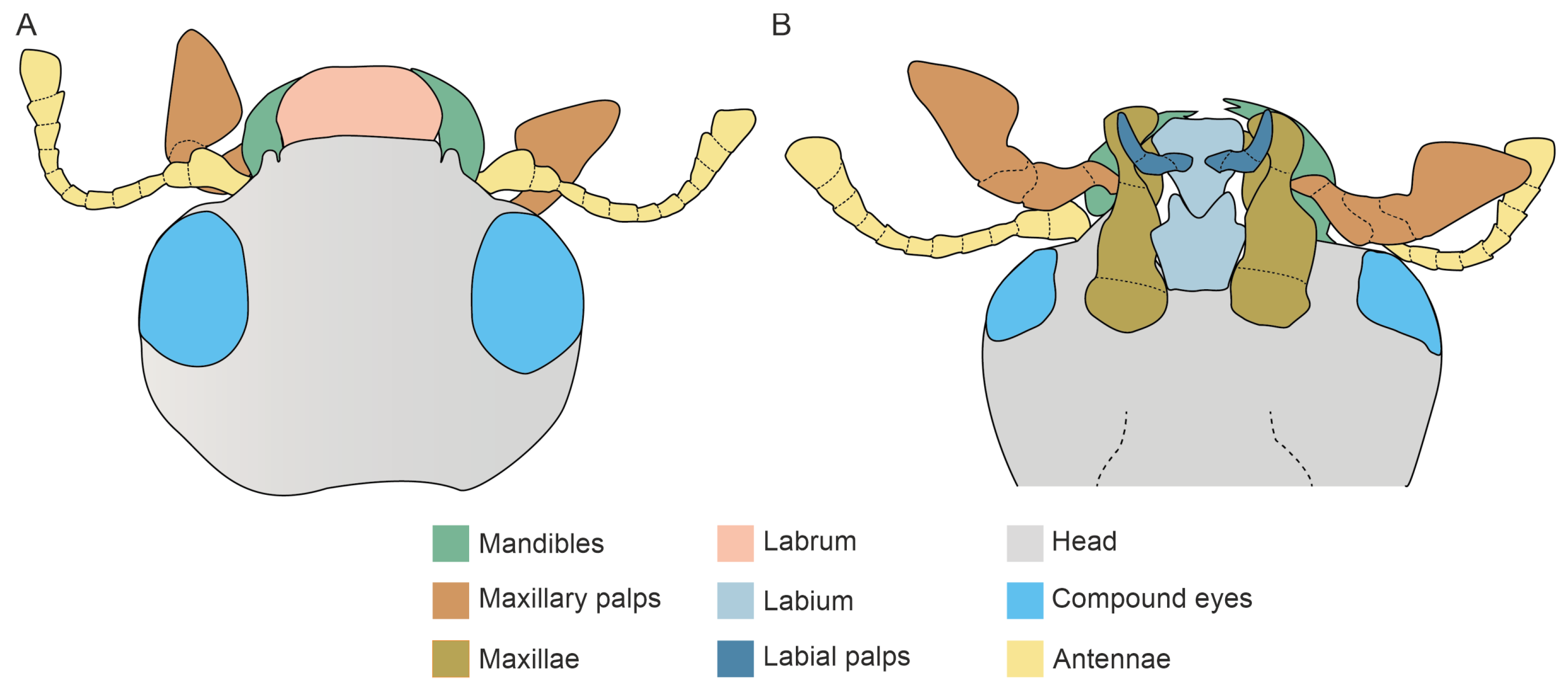
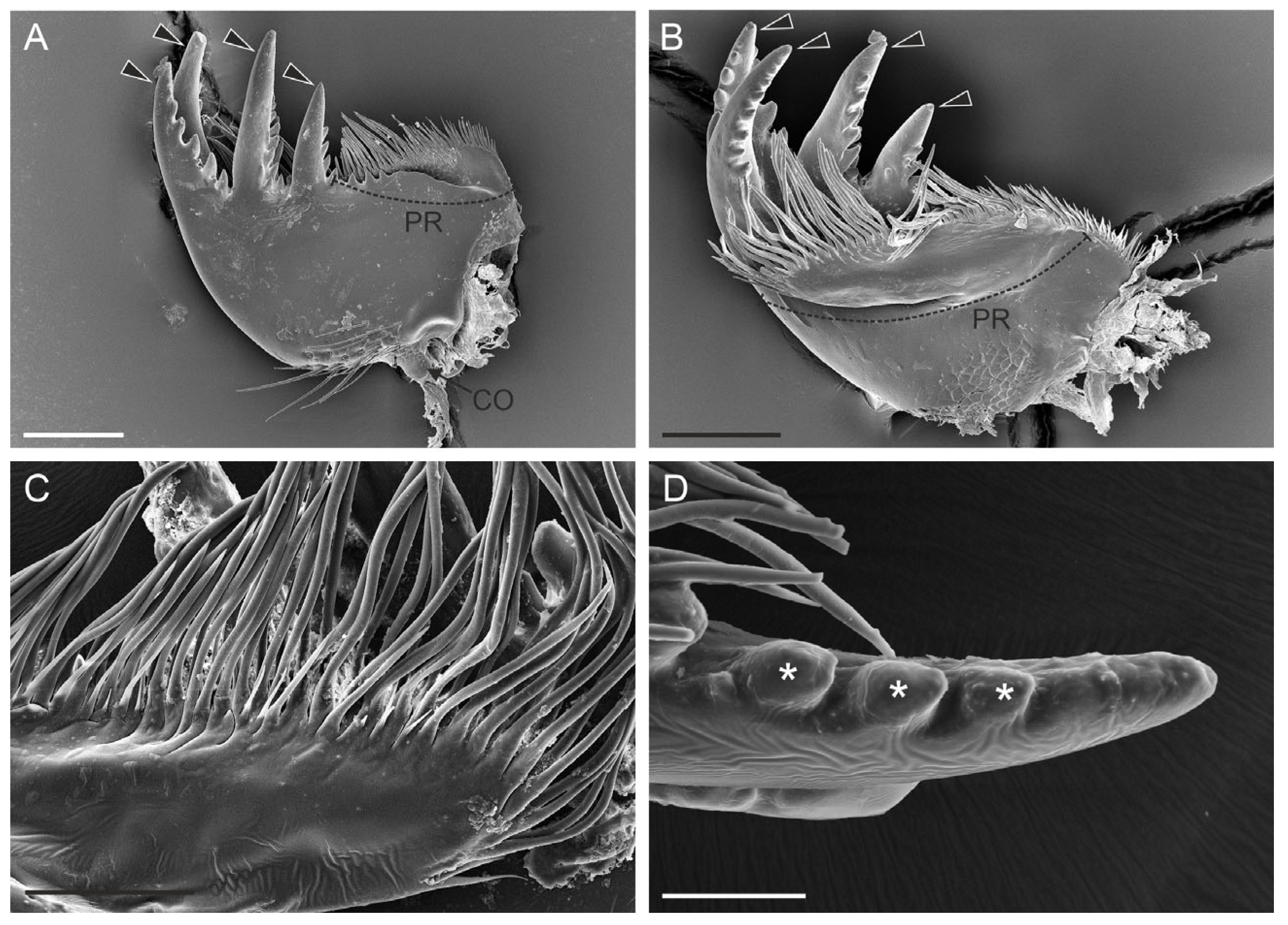
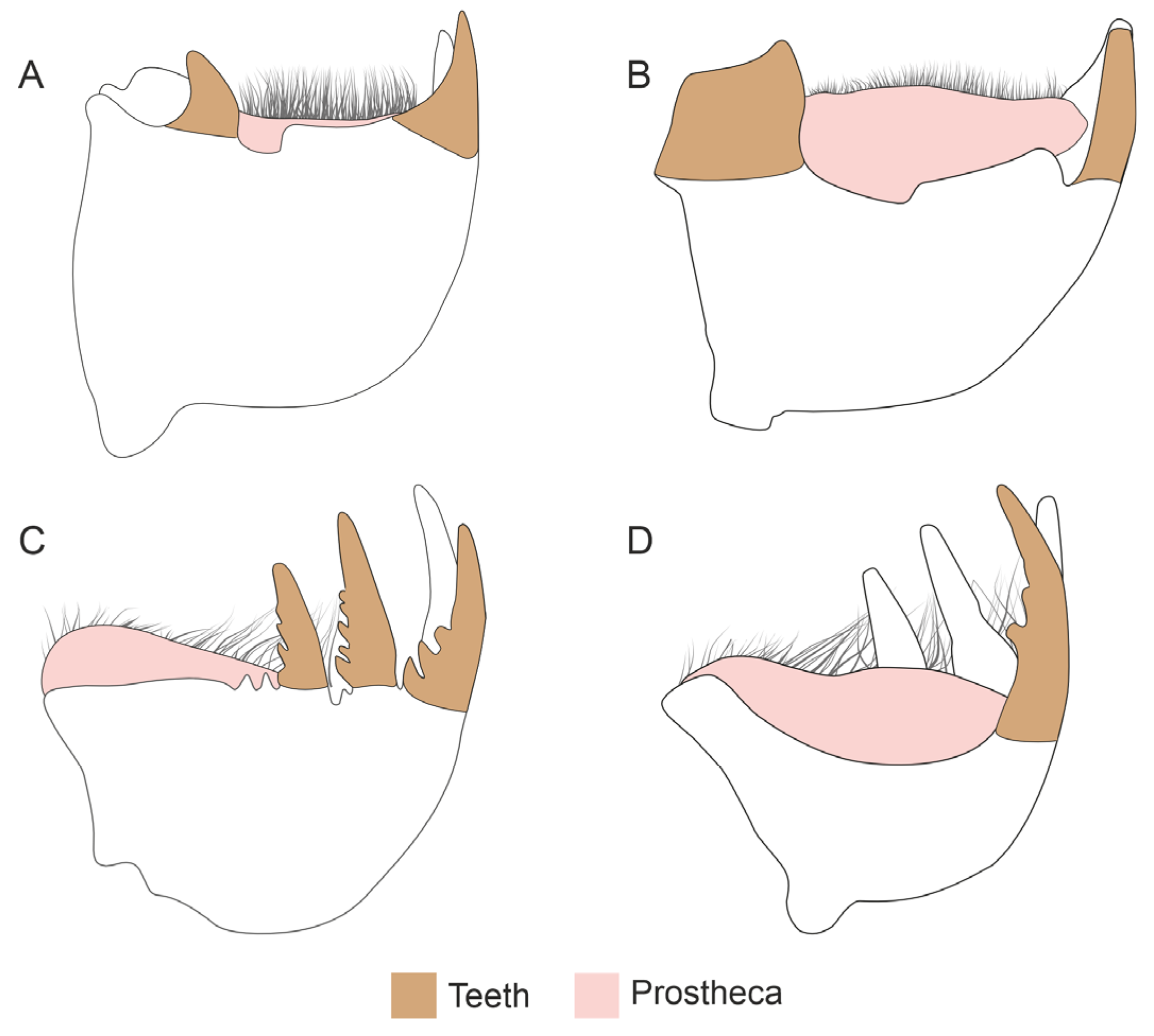
3.1. Mandible Structure
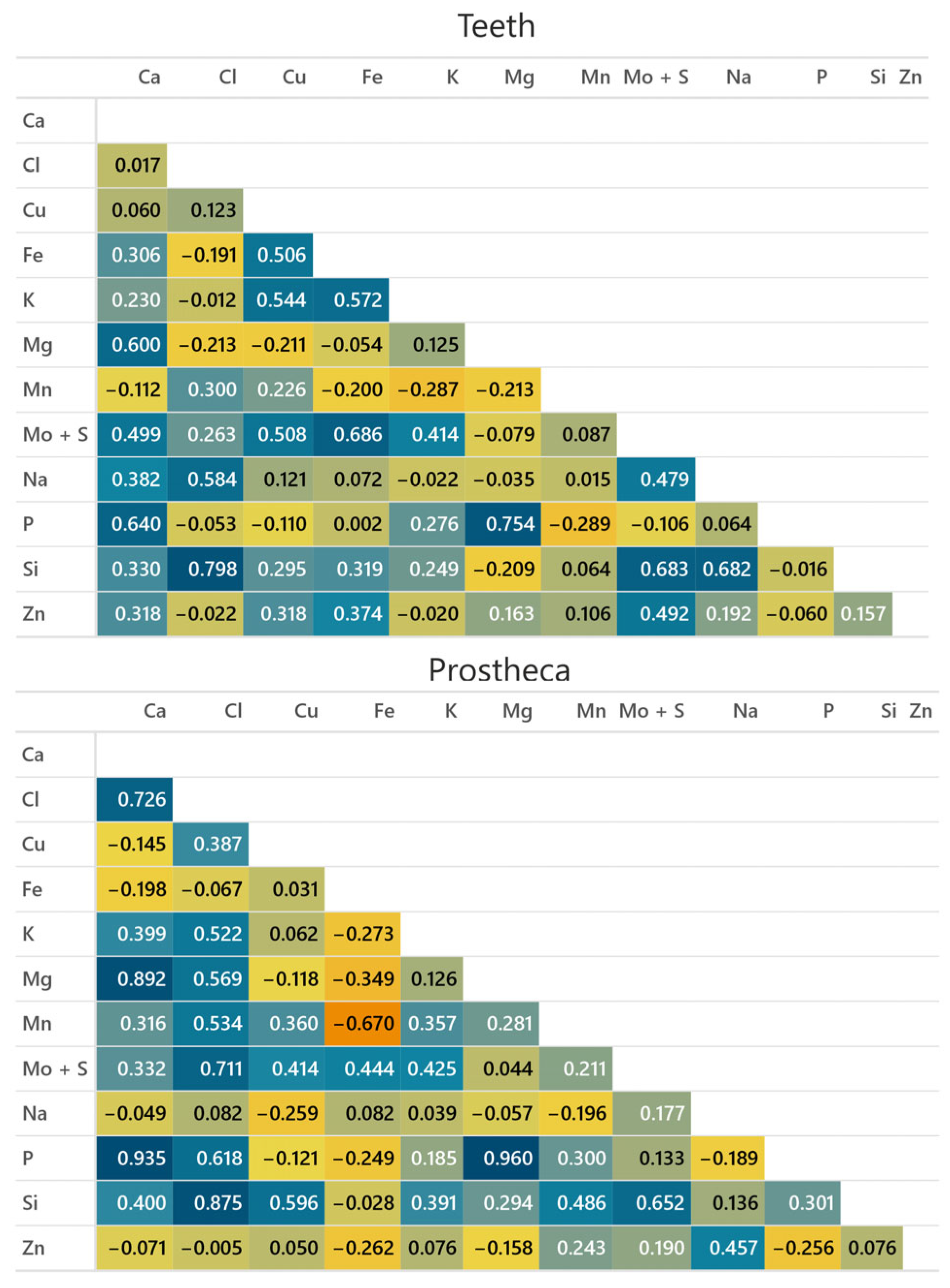
3.2. Metal Distribution and Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouchard, P.; Smith, A.B.T.; Douglas, H.; Gimmel, M.L.; Brunke, A.J.; Kanda, K. Biodiversity of Coleoptera. In Insect Biodiversity; Wiley: New York, NY, USA, 2017; pp. 337–417. [Google Scholar]
- Mckenna, D.D.; Wild, A.L.; Kanda, K.; Bellamy, C.L.; Beutel, R.G.; Caterino, M.S.; Farnum, C.W.; Hawks, D.C.; Ivie, M.A.; Jameson, M.L.; et al. The beetle tree of life reveals that Coleoptera survived end Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 2015, 40, 835–880. [Google Scholar] [CrossRef]
- Stork, N.E.; McBroom, J.; Gely, C.; Hamilton, A.J. New approaches narrow global species estimates for beetles, insects, and terrestrial arthropods. Proc. Natl. Acad. Sci. USA 2015, 112, 7519–7523. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Zhang, P.; Deng, S.; Escalona, H.E.; Wang, X.; Li, Y.; Pang, H.; Vandenberg, N.; Ślipiński, A.; Tomaszewska, W.; et al. New insights into the phylogeny and evolution of lady beetles (Coleoptera: Coccinellidae) by extensive sampling of genes and species. Mol. Phylogenet. Evol. 2021, 156, 107045. [Google Scholar] [CrossRef] [PubMed]
- Krenn, H.W. Form and Function of Insect Mouthparts. In Insect Mouthparts Form, Function, Development and Performance; Krenn, H.W., Ed.; Springer: Cham, Switzerland, 2019; pp. 9–46. [Google Scholar]
- Krenn, H.W. Introduction: Ecological Importance of Insect Feeding. In Insect Mouthparts Form, Function, Development and Performance; Krenn, H.W., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–7. [Google Scholar]
- Giorgi, J.A.; Vandenberg, N.J.; McHugh, J.V.; Forrester, J.A.; Ślipiński, S.A.; Miller, K.B.; Shapiro, L.R.; Whiting, M.F. The evolution of food preferences in Coccinellidae. Biol. Control 2009, 51, 215–231. [Google Scholar] [CrossRef]
- Seago, A.E.; Giorgi, J.A.; Li, J.; Ślipiński, A. Phylogeny, classification and evolution of ladybird beetles (Coleoptera: Coccinellidae) based on simultaneous analysis of molecular and morphological data. Mol. Phylogenet. Evol. 2011, 60, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Samways, M.J.; Osborn, R.; Saunders, T.L. Mandible form relative to the main food type in ladybirds (Coleoptera: Coccinellidae). Biocontrol Sci. Technol. 1997, 7, 275–286. [Google Scholar] [CrossRef]
- Kovář, I. Taxonomy and Morphology of Adults. In Biology of Coccinellidae; Springer: Dordrecht, The Netherlands, 1973; pp. 15–35. [Google Scholar]
- Schofield, R.M.S. Metal–Halogen Biomaterials. Am. Entomol. 2005, 51, 45–47. [Google Scholar] [CrossRef]
- Broomell, C.C.; Zok, F.W.; Waite, J.H. Role of transition metals in sclerotization of biological tissue. Acta Biomater. 2008, 4, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Reiter, K.E.; Perkovich, C.; Smith, K.N.; Feng, J.; Kritsky, G.; Lehnert, M.S. Comparative Material and Mechanical Properties among Cicada Mouthparts: Cuticle Enhanced with Inorganic Elements Facilitates Piercing through Woody Stems for Feeding. Biology 2023, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Cribb, B.W.; Stewart, A.; Huang, H.; Truss, R.; Noller, B.; Rasch, R.; Zalucki, M.P. Insect mandibles—Comparative mechanical properties and links with metal incorporation. Naturwissenschaften 2007, 95, 17–23. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.-Y.; Fang, Y.; Carlson, C.M.; Xu, H.; Ješovnik, A.; Sosa-Calvo, J.; Zarnowski, R.; Bechtel, H.A.; Fournelle, J.H.; et al. Biomineral armor in leaf-cutter ants. Nat. Commun. 2020, 11, 5792. [Google Scholar] [CrossRef] [PubMed]
- Polidori, C.; García, A.J.; Nieves-Aldrey, J.L. Breaking up the Wall: Metal-Enrichment in Ovipositors, but Not in Mandibles, Co-Varies with Substrate Hardness in Gall-Wasps and Their Associates. PLoS ONE 2013, 8, e70529. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.D.; Baker, P.; Kramer, K.J.; Basibuyuk, H.H.; Quicke, D.L.J. Metals in mandibles of stored product insects: Do zinc and manganese enhance the ability of larvae to infest seeds? J. Stored Prod. Res. 2003, 39, 65–75. [Google Scholar] [CrossRef]
- Hillerton, J.E.; Robertson, B.; Vincent, J.F. V The presence of zinc or manganese as the predominant metal in the mandibles of adult, stored-product beetles. J. Stored Prod. Res. 1984, 20, 133–137. [Google Scholar] [CrossRef]
- Fontaine, A.R.; Olsen, N.; Ring, R.A.; Singla, C.L. Cuticular Metal Hardening of Mouthparts and Claws of some Forest Insects of British Columbia. J. Entomol. Soc. Br. Columbia 1991, 88, 45–55. [Google Scholar]
- Koch, R.L. The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. J. Insect. Sci. 2003, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Sevarika, M.; Rondoni, G.; Conti, E.; Romani, R. Antennal sensory organs and glands of the harlequin ladybird, Harmonia axyridis. Entomol. Exp. Appl. 2021, 169, 111–124. [Google Scholar] [CrossRef]
- Baris, A. Age-Stage, Two-Sex Life Tables of the 24 Spot Ladybird [(Subcoccinella vigintiquatuorpunctata (Coleoptera: Coccinellidae)]. Braz. Arch. Biol. Technol. 2022, 65, e22210841. [Google Scholar] [CrossRef]
- Ali, M. Studies on the Induction of Food Preference in Alfalfa Ladybird, Subcoccinella 24-Punctata L. (Coleoptera: Coccinellidae). In The Host-Plant in Relation to Insect Behaviour and Reproduction; Springer: Boston, MA, USA, 1976; pp. 23–28. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 28 May 2024).
- Samways, M.J.; Wilson, S.J. Aspects of the feeding behaviour of Chilocorus nigritus (F.) (Col., Coccinellidae) relative to its effectiveness as a biocontrol agent. J. Appl. Entomol. 1988, 106, 177–182. [Google Scholar] [CrossRef]
- Piersanti, S.; Saitta, V.; Rebora, M.; Salerno, G. Adult host-plant preference and larval performance in an oligophagous insect (Chnootriba elaterii). Physiol. Entomol. 2023, 48, 171–183. [Google Scholar] [CrossRef]
- Tomaszewska, W.; Szawaryn, K. Epilachnini (Coleoptera: Coccinellidae)—A Revision of the World Genera. J. Insect. Sci. 2016, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Howard, N.F. Feeding of the Mexican Bean Beetle Larva. Ann. Entomol. Soc. Am. 1941, 34, 766–769. [Google Scholar] [CrossRef]
- Hao, Y.N.; Sun, Y.X.; Liu, C.Z. Functional morphology of the mouthparts of lady beetle Coccinella transversoguttata (Coccinellidae, Coleoptera), with reference to their feeding mechanism. J. Morphol. 2019, 280, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Stocker, B.; Barthold, S.; Betz, O. Mouthpart Ecomorphology and Predatory Behaviour in Selected Rove Beetles of the “Staphylinine Group” (Coleoptera: Staphylinidae: Staphylininae, Paederinae). Insects 2022, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Karolyi, F.; Hansal, T.; Krenn, H.W.; Colville, J.F. Comparative morphology of the mouthparts of the megadiverse South African monkey beetles (Scarabaeidae: Hopliini): Feeding adaptations and guild structure. PeerJ 2016, 2016, e1597. [Google Scholar] [CrossRef] [PubMed]
- Karolyi, F. What’s on the Menu: Floral Tissue, Pollen or Nectar? Mouthpart Adaptations of Anthophilous Beetles to Floral Food Sources; Springer: Cham, Switzerland, 2019; pp. 419–442. [Google Scholar]
- Beutel, R.G.; Yavorskaya, M. Structure and Evolution of Mouthparts in Coleoptera; Springer: Cham, Switzerland, 2019; pp. 387–418. [Google Scholar]
- Hillerton, J.E.; Vincent, J.F. V The Specific Location of Zinc in Insect Mandibles. J. Exp. Biol. 1982, 101, 333–336. [Google Scholar] [CrossRef]
- Vega, F.E.; Bauchan, G.; Infante, F.; Davis, S. Mouthpart structure and elemental composition of the mandibles in the coffee berry borer (Coleoptera: Curculionidae: Scolytinae). Ann. Entomol. Soc. Am. 2017, 110, 381–389. [Google Scholar] [CrossRef]
- Lichtenegger, H.C.; Birkedal, H.; Waite, J.H. Heavy Metals in the Jaws of Invertebrates. In Biomineralization; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Wiley: New York, NY, USA, 2008; Volume 4, pp. 295–325. [Google Scholar]
- Baumann, K.; Vicenzi, E.P.; Lam, T.; Douglas, J.; Arbuckle, K.; Cribb, B.; Brady, S.G.; Fry, B.G. Harden up: Metal acquisition in the weaponized ovipositors of aculeate Hymenoptera. Zoomorphology 2018, 137, 389–406. [Google Scholar] [CrossRef]
- Polidori, C.; Wurdack, M. Mg-enriched ovipositors as a possible adaptation to hard-skinned fruit oviposition in Drosophila suzukii and D. subpulchrella. Arthropod Plant Interact. 2019, 13, 551–560. [Google Scholar] [CrossRef]
- Klunk, C.L.; Heethoff, M.; Hammel, J.U.; Gorb, S.N.; Krings, W. Mechanical and elemental characterization of ant mandibles: Consequences for bite mechanics. Interface Focus 2024, 14, 20230056. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.M.S.; Nesson, M.H.; Richardson, K.A. Tooth hardness increases with zinc-content in mandibles of young adult leaf-cutter ants. Naturwissenschaften 2002, 89, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, H.C.; Schöberl, T.; Ruokolainen, J.T.; Cross, J.O.; Heald, S.M.; Birkedal, H.; Waite, J.H.; Stucky, G.D. Zinc and mechanical prowess in the jaws of Nereis, a marine worm. Proc. Natl. Acad. Sci. USA 2003, 100, 9144–9149. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W. Structural chemistry and biology of manganese metalloenzymes. Prog. Biophys. Mol. Biol. 1997, 67, 217–252. [Google Scholar] [CrossRef] [PubMed]
- Bernays, E. Evolution of insect morphology in relation to plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1991, 333, 257–264. [Google Scholar] [CrossRef]
- Reynolds, O.L.; Keeping, M.G.; Meyer, J.H. Silicon-augmented resistance of plants to herbivorous insects: A review. Ann. Appl. Biol. 2009, 155, 171–186. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kvedaras, O.L.; Byrne, M.J.; Coombes, N.E.; Keeping, M.G. Influence of plant silicon and sugarcane cultivar on mandibular wear in the stalk borer Eldana saccharina. Agric. For. Entomol. 2009, 11, 301–306. [Google Scholar] [CrossRef]
- Schofield, R.M.S.; Emmett, K.D.; Niedbala, J.C.; Nesson, M.H. Leaf-cutter ants with worn mandibles cut half as fast, spend twice the energy, and tend to carry instead of cut. Behav. Ecol. Sociobiol. 2011, 65, 969–982. [Google Scholar] [CrossRef]
- Gullan, P.J.; Martin, J.H. Sternorrhyncha: (Jumping Plant-Lice, Whiteflies, Aphids, and Scale Insects). In Encyclopedia of Insects; Resh, V., Cardé, R.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 957–967. [Google Scholar]
- Polidori, C.; Jorge, A.; Keller, A.; Ornosa, C.; Tormos, J.; Asís, J.D.; Nieves-Aldrey, J.L. Strong phylogenetic constraint on transition metal incorporation in the mandibles of the hyper-diverse Hymenoptera (Insecta). Org. Divers. Evol. 2020, 20, 511–526. [Google Scholar] [CrossRef]
- Schofield, R.M.S.; Nesson, M.H.; Richardson, K.A.; Wyeth, P. Zinc is incorporated into cuticular “tools” after ecdysis: The time course of the zinc distribution in “tools” and whole bodies of an ant and a scorpion. J. Insect Physiol. 2003, 49, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Gorb, S.N. Mandibular gnathobases of marine planktonic copepods -feeding tools with complex micro- and nanoscale composite architectures. Beilstein J. Nanotechnol. 2015, 6, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.Y.; Zaidykov, I.Y.; Tauson, V.L.; Likhoshway, Y.V. Features of the fine structure and Si content of the mandibular gnathobase of four freshwater species of Epischura (Copepoda: Calanoida). J. Crustacean Biol. 2015, 35, 741–746. [Google Scholar] [CrossRef]
- Michels, J.; Vogt, J.; Gorb, S.N. Tools for crushing diatoms—Opal teeth in copepods feature a rubber-like bearing composed of resilin. Sci. Rep. 2012, 2, 465. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.G.; Henry, T.J. Seasonal History and Habits of the European Alfalfa Beetle, Subcoccinella vigintiquatuorpunctata (L.) (Coleoptera: Coccinellidae). Coleopt Bull. 1981, 35, 197–203. [Google Scholar]
- Tanasijevic, N. Zur morphologie und Biologie des Luzernemarienkafers Sobcoccinella vigintiquaturopunctata L. (Coleoptera: Coccinellidae). Beiträge zur Entomol. 1958, 8, 23–78. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic Variation in the Silicon Composition of Plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Birkedal, H.; Khan, R.K.; Slack, N.; Broomell, C.; Lichtenegger, H.C.; Zok, F.; Stucky, G.D.; Waite, J.H. Halogenated Veneers: Protein Cross-Linking and Halogenation in the Jaws of Nereis, a Marine Polychaete Worm. ChemBioChem 2006, 7, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Cribb, B.W.; Lin, C.-L.; Rintoul, L.; Rasch, R.; Hasenpusch, J.; Huang, H. Hardness in arthropod exoskeletons in the absence of transition metals. Acta Biomater. 2010, 6, 3152–3156. [Google Scholar] [CrossRef] [PubMed]


| Metal | H. axyridis | S. vigintiquatuorpunctata | ||
|---|---|---|---|---|
| Teeth | Prostheca | Teeth | Prostheca | |
| Ca | 0.07 ± 0.01 | 0.09 ± 0.02 | 0.19 ± 0.03 | 0.29 ± 0.06 |
| Cl | 0.24 ± 0.03 | 1.22 ± 0.16 | 0.47 ± 0.06 | 0.56 ± 0.06 |
| Cu | 0.23 ± 0.04 | 0.21 ± 0.04 | 0.2 ± 0.02 | 0.23 ± 0.02 |
| Fe | 0.01 ± 0.01 | 0 ± 0 | 0.03 ± 0.01 | 0.02 ± 0.01 |
| Mg | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.02 |
| Mn | 1.52 ± 0.2 | 1.12 ± 0.14 | 2.2 ± 0.14 | 0.7 ± 0.09 |
| Mo + S | 0.17 ± 0.02 | 0.59 ± 0.08 | 0.27 ± 0.04 | 0.42 ± 0.07 |
| P | 0.03 ± 0.01 | 0.2 ± 0.04 | 0.13 ± 0.02 | 0.26 ± 0.07 |
| K | 0.29 ± 0.04 | 0.4 ± 0.09 | 0.04 ± 0.01 | 0.05 ± 0.01 |
| Si | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.21 ± 0.03 | 1.38 ± 0.1 |
| Na | 0.07 ± 0.03 | 0.33 ± 0.1 | 0.14 ± 0.02 | 0.27 ± 0.03 |
| Zn | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.02 | 0.08 ± 0.02 |
| Metal | Intraspecific Variation | Interspecific Variation | ||
|---|---|---|---|---|
| H. axyridis | S. vigintiquatuorpunctata | Teeth | Prostheca | |
| Ca | 0.563 | 0.106 | 0.007 | 0.003 |
| Cl | 0.000 | 0.261 | 0.039 | 0.000 |
| Cu | 0.967 | 0.144 | 0.468 | 0.614 |
| Fe | 0.688 | 0.564 | 0.040 | 0.071 |
| Mg | 0.731 | 0.497 | 0.983 | 0.342 |
| Mn | 0.022 | 0.000 | 0.000 | 0.014 |
| Mo + S | 0.000 | 0.010 | 0.198 | 0.064 |
| P | 0.000 | 0.106 | 0.000 | 0.840 |
| K | 0.711 | 0.099 | 0.000 | 0.000 |
| Si | 0.305 | 0.000 | 0.000 | 0.000 |
| Na | 0.015 | 0.004 | 0.009 | 0.538 |
| Zn | 0.611 | 0.099 | 0.307 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevarika, M.; Romani, R. Ultrastructural Organization and Metal Elemental Composition of the Mandibles in Two Ladybird Species. Insects 2024, 15, 403. https://doi.org/10.3390/insects15060403
Sevarika M, Romani R. Ultrastructural Organization and Metal Elemental Composition of the Mandibles in Two Ladybird Species. Insects. 2024; 15(6):403. https://doi.org/10.3390/insects15060403
Chicago/Turabian StyleSevarika, Milos, and Roberto Romani. 2024. "Ultrastructural Organization and Metal Elemental Composition of the Mandibles in Two Ladybird Species" Insects 15, no. 6: 403. https://doi.org/10.3390/insects15060403






