Chromosome-Level Genome Assembly of Apoderus dimidiatus Voss (Coleoptera: Attelabidae): Insights into Evolution and Behavior
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Library Construction and Sequencing
2.3. Genomic Prediction
2.4. Genome Assembly and Quality Assessment
2.5. Genome Annotation
2.6. Comparative Genomes
3. Results
3.1. Sequencing Data Statistics
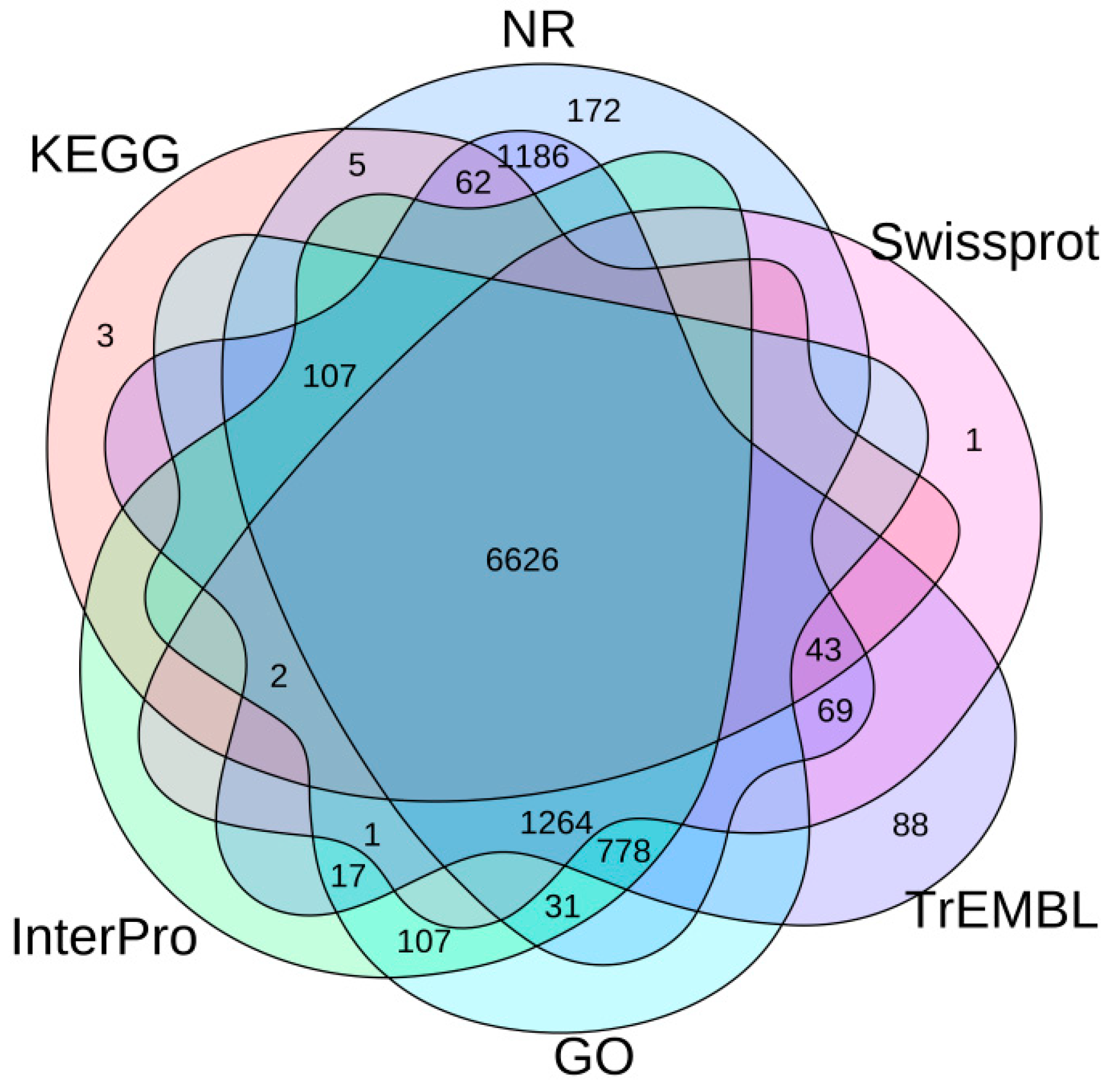
3.2. Genome Annotation
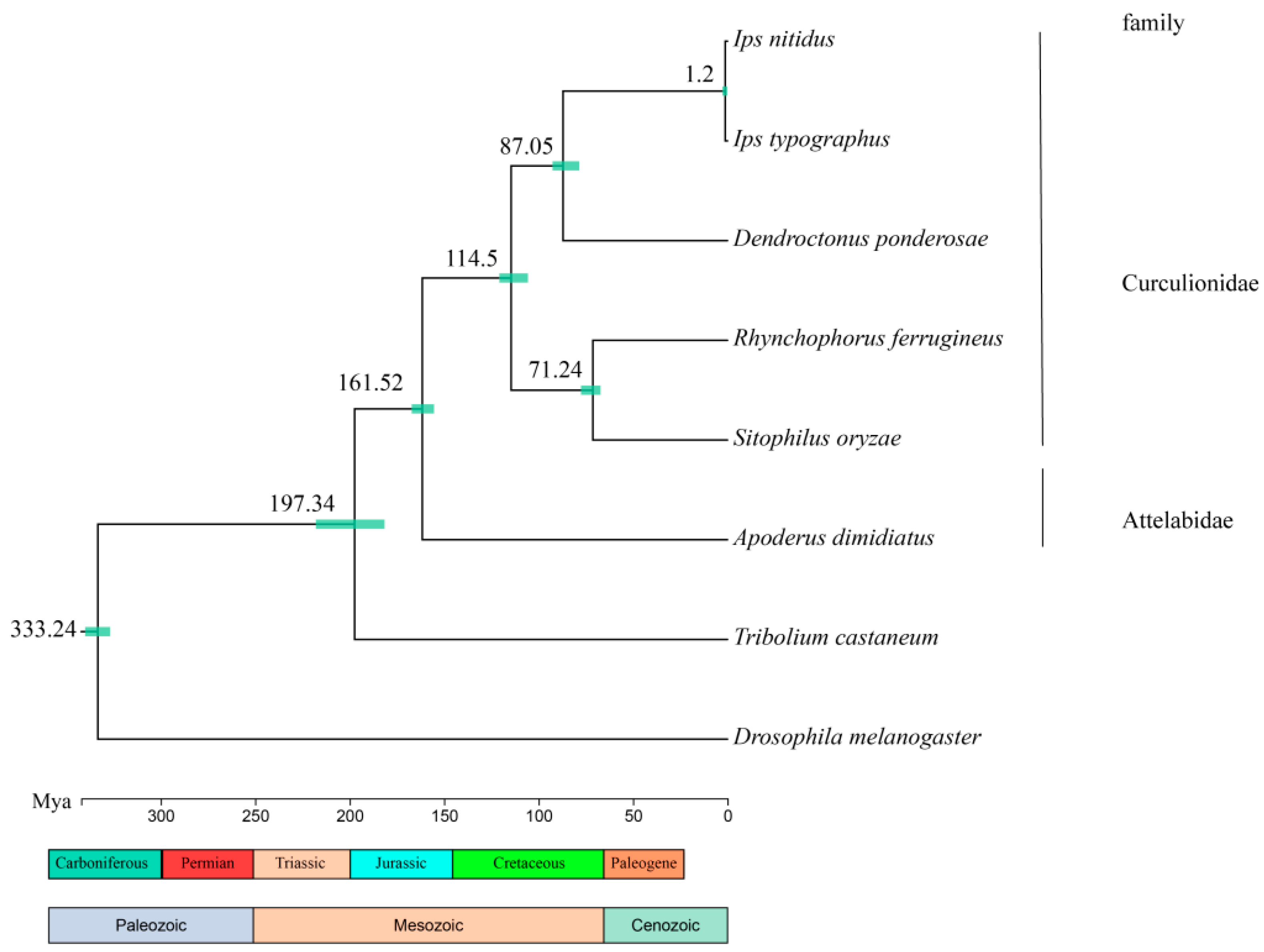
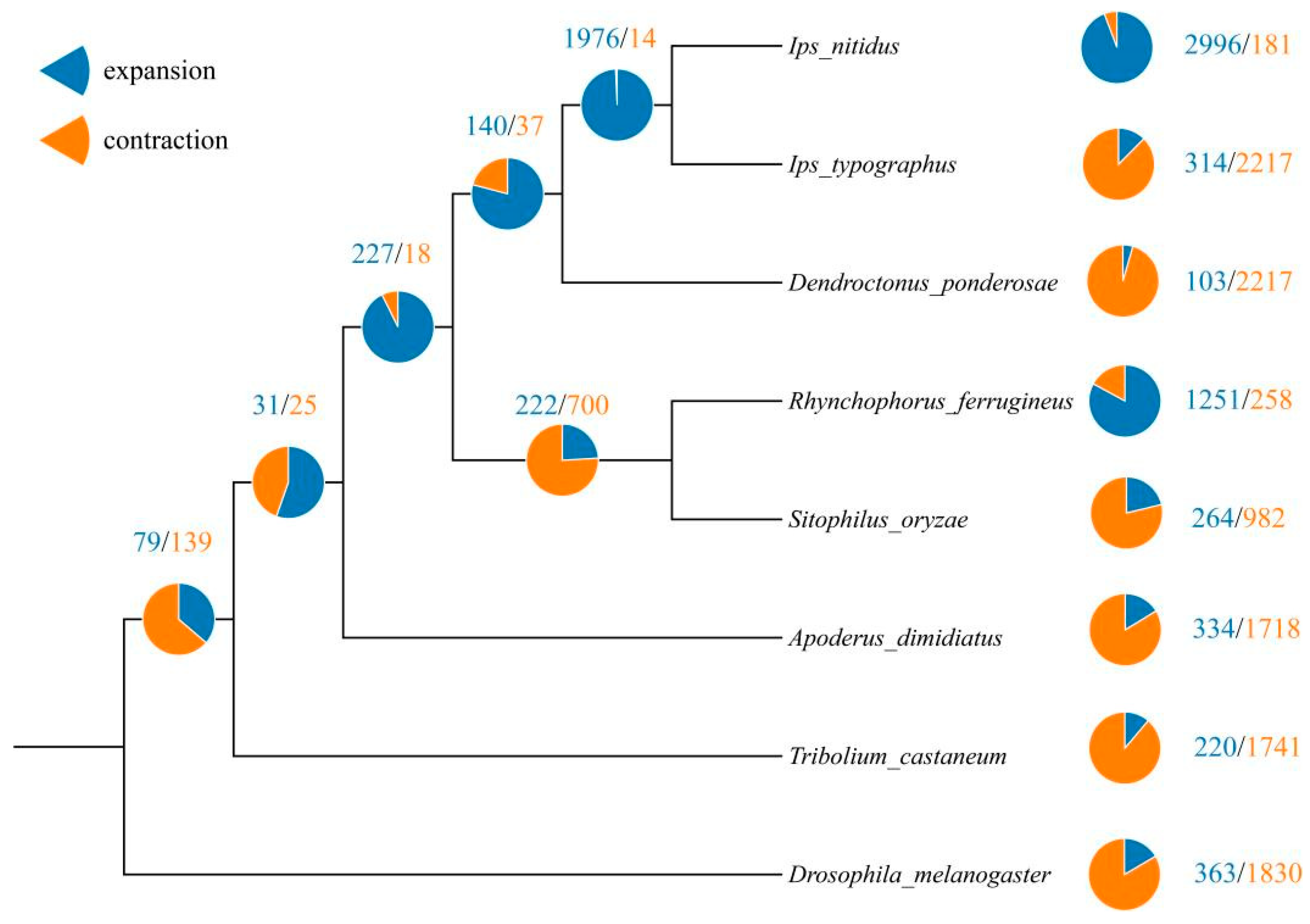
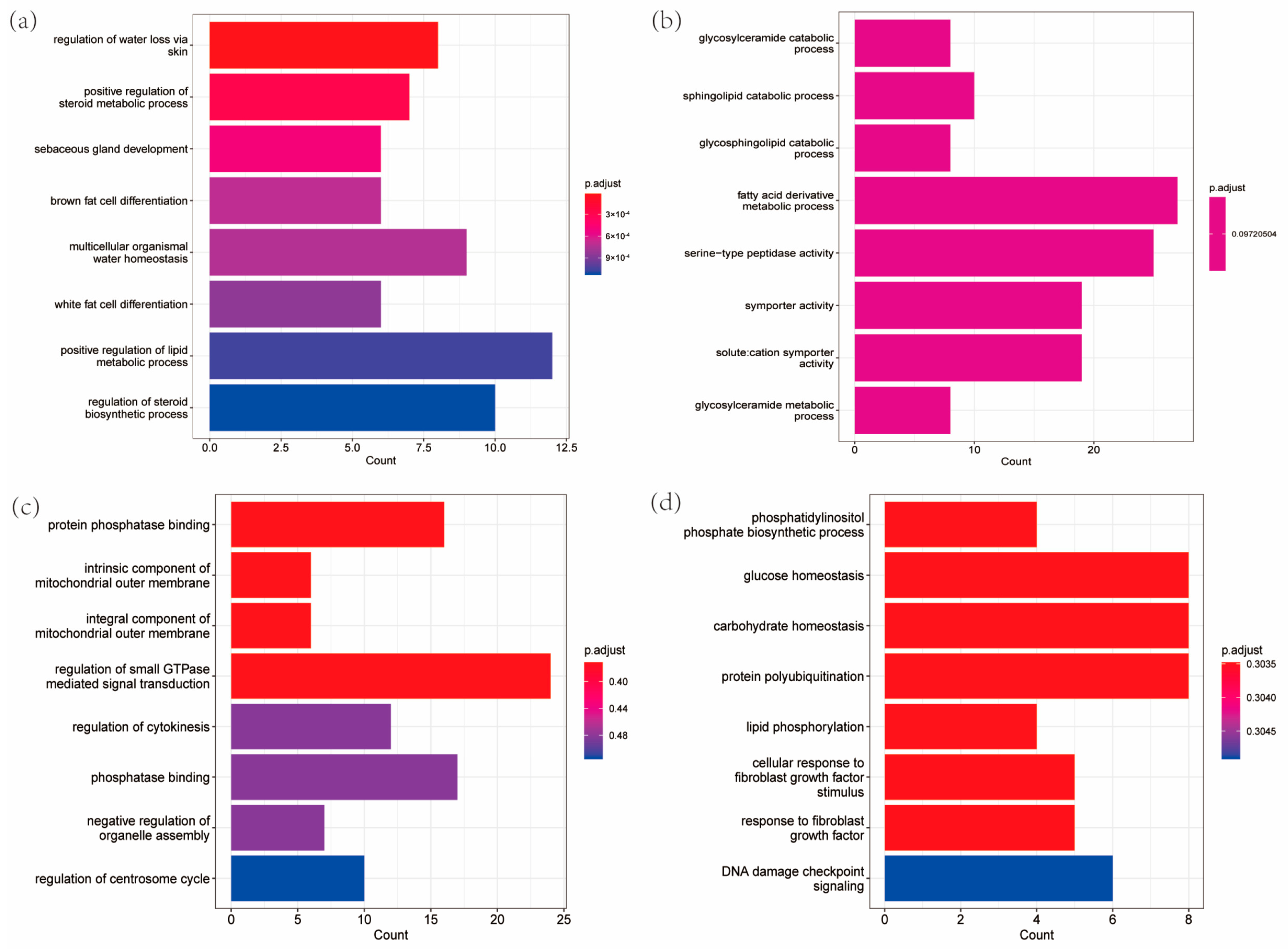
3.3. Comparative Genomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.A.; Subramanian, G.M.; Halpern, A.; Sutton, G.G.; Charlab, R.; Nusskern, D.R.; Wincker, P.; Clark, A.G.; Ribeiro, J.M.; Wides, R.; et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science 2002, 298, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhou, Z.; Lu, C.; Cheng, D.; Dai, F.; Li, B.; Zhao, P.; Zha, X.; Cheng, T.; Chai, C.; et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006, 443, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Nene, V.; Wortman, J.R.; Lawson, D.; Haas, B.; Kodira, C.; Tu, Z.J.; Loftus, B.; Xi, Z.; Megy, K.; Grabherr, M.; et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 2007, 316, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Gibbs, R.A.; Weinstock, G.M.; Brown, S.J.; Denell, R.; Beeman, R.W.; Gibbs, R.; Beeman, R.W.; Brown, S.J.; Bucher, G.; et al. The genome of the model beetle and pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.M. The genome sequence of the hazel leaf-roller, Apoderus coryli (Linnaeus, 1758). Wellcome Open Res. 2021, 6, 315. [Google Scholar] [CrossRef]
- Hirose, Y. Egg parasitism of some leaf-rolling weevils in relation to the number of eggs laid in a leaf roll, with special reference to parasitism by Poropoea morimotoi Hirose (Hymenoptera: Trichogrammatidae). Kontyu 1968, 36, 377–388. [Google Scholar]
- Sakurai, K. Multiple oviposition by Apoderus balteatus (Coleoptera, Attelabidae) is associated with larger leaves. Ecol. Entomol. 1986, 11, 319–323. [Google Scholar] [CrossRef]
- Kobayashi, C.; Kato, M. To be suspended or to be cut off? Differences in the performance of two types of leaf-rolls constructed by the attelabid beetle Cycnotrachelus roelofsi. Popul. Ecol. 2004, 46, 193–202. [Google Scholar] [CrossRef]
- Belton, J.M.; McCord, R.P.; Gibcus, J.H.; Naumova, N.; Zhan, Y.; Dekker, J. Hi-C: A comprehensive technique to capture the conformation of genomes. Methods 2012, 58, 268–276. [Google Scholar] [CrossRef]
- Lieberman-Aiden, E.; van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef]
- Nagano, T.; Lubling, Y.; Yaffe, E.; Wingett, S.W.; Dean, W.; Tanay, A.; Fraser, P. Single-cell Hi-C for genome-wide detection of chromatin interactions that occur simultaneously in a single cell. Nat. Protoc. 2015, 10, 1986–2003. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kurtz, S.; Narechania, A.; Stein, J.C.; Ware, D. A new method to compute K-mer frequencies and its application to annotate large repetitive plant genomes. BMC Genom. 2008, 9, 517. [Google Scholar] [CrossRef]
- Vurture, G.W.; Sedlazeck, F.J.; Nattestad, M.; Underwood, C.J.; Fang, H.; Gurtowski, J.; Schatz, M.C. GenomeScope: Fast reference-free genome profiling from short reads. Bioinformatics 2017, 33, 2202–2204. [Google Scholar] [CrossRef]
- Hu, J.; Fan, J.; Sun, Z.; Liu, S. NextPolish: A fast and efficient genome polishing tool for long-read assembly. Bioinformatics 2020, 36, 2253–2255. [Google Scholar] [CrossRef]
- Durand, N.C.; Shamim, M.S.; Machol, I.; Rao, S.S.; Huntley, M.H.; Lander, E.S.; Aiden, E.L. Juicer Provides a One-Click System for Analyzing Loop-Resolution Hi-C Experiments. Cell Syst. 2016, 3, 95–98. [Google Scholar] [CrossRef]
- Dudchenko, O.; Batra, S.S.; Omer, A.D.; Nyquist, S.K.; Hoeger, M.; Durand, N.C.; Shamim, M.S.; Machol, I.; Lander, E.S.; Aiden, A.P.; et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 2017, 356, 92–95. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Chen, N. Using RepeatMasker to Identify Repetitive Elements in Genomic Sequences. Curr. Protoc. Bioinform. 2004, 5, 14.10.11–14.10.14. [Google Scholar] [CrossRef]
- Hoff, K.J.; Stanke, M. WebAUGUSTUS—A web service for training AUGUSTUS and predicting genes in eukaryotes. Nucleic Acids Res. 2013, 41, W123–W128. [Google Scholar] [CrossRef]
- Gertz, E.M.; Yu, Y.K.; Agarwala, R.; Schäffer, A.A.; Altschul, S.F. Composition-based statistics and translated nucleotide searches: Improving the TBLASTN module of BLAST. BMC Biol. 2006, 4, 41. [Google Scholar] [CrossRef]
- Birney, E.; Durbin, R. Using GeneWise in the Drosophila annotation experiment. Genome Res. 2000, 10, 547–548. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Testa, A.C.; Hane, J.K.; Ellwood, S.R.; Oliver, R.P. CodingQuarry: Highly accurate hidden Markov model gene prediction in fungal genomes using RNA-seq transcripts. BMC Genom. 2015, 16, 170. [Google Scholar] [CrossRef]
- Haas, B.J.; Salzberg, S.L.; Zhu, W.; Pertea, M.; Allen, J.E.; Orvis, J.; White, O.; Buell, C.R.; Wortman, J.R. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008, 9, R7. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.; Grobetae-Wilde, E.; Krokene, P.; Roy, A.; Chakraborty, A.; Lofstedt, C.; Vogel, H.; Andersson, M.N.; Schlyter, F. A highly-contiguous genome assembly of the Eurasian spruce bark beetle, Ips typographus, provides insight into a major forest pest. Commun. Biol. 2021, 4, 1059. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Escalante, L.; Hernandez-Hernandez, E.M.; Nuñez, J.; Acevedo, F.E.; Berrio, A.; Constantino, L.M.; Padilla-Hurtado, B.E.; Molina, D.; Gongora, C.; Acuña, R.; et al. A coffee berry borer (Hypothenemus hampei) genome assembly reveals a reduced chemosensory receptor gene repertoire and male-specific genome sequences. Sci. Rep. 2021, 11, 4900. [Google Scholar] [CrossRef] [PubMed]
- Hazzouri, K.M.; Sudalaimuthuasari, N.; Kundu, B.; Nelson, D.; Al-Deeb, M.A.; Le Mansour, A.; Spencer, J.J.; Desplan, C.; Amiri, K.M.A. The genome of pest Rhynchophorus ferrugineus reveals gene families important at the plant-beetle interface. Commun. Biol. 2020, 3, 323. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Campbell, E.O.; Batista, P.D.; Shegelski, V.A.; Trevoy, S.A.L.; Huber, D.P.W.; Janes, J.K.; Sperling, F.A.H. Chromosome-level genome assembly reveals genomic architecture of northern range expansion in the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae). Mol. Ecol. Resour. 2022, 22, 1149–1167. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Murphy, T.; Xia, J.; Caragea, D.; Park, Y.; Beeman, R.W.; Lorenzen, M.D.; Butcher, S.; Manak, J.R.; Brown, S.J. BeetleBase in 2010: Revisions to provide comprehensive genomic information for Tribolium castaneum. Nucleic Acids Res. 2010, 38, D437–D442. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, X.; Li, M.; He, K.; Huang, C.; Zhou, Y.; Li, Z.; Walters, J.R. Insect genomes: Progress and challenges. Insect Mol. Biol. 2019, 28, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.K.; Cao, L.J.; Song, W.; Shi, P.; Gao, Y.F.; Gong, Y.J.; Chen, J.C.; Hoffmann, A.A.; Wei, S.J. Chromosome-level assembly of the melon thrips genome yields insights into evolution of a sap-sucking lifestyle and pesticide resistance. Mol. Ecol. Resour. 2020, 20, 1110–1125. [Google Scholar] [CrossRef]
- Cong, Y.Y.; He, K.; Shu, R.G.; Cheng, Z.Q.; Chen, H.; Wang, Y.Q.; Li, F. Analysis of the reasons for the size deviation of insect genome assembly. J. Plant Prot. 2021, 48, 1217–1225. [Google Scholar] [CrossRef]
- Kajitani, R.; Toshimoto, K.; Noguchi, H.; Toyoda, A.; Ogura, Y.; Okuno, M.; Yabana, M.; Harada, M.; Nagayasu, E.; Maruyama, H.; et al. Efficient de novo assembly of highly heterozygous genomes from whole-genome shotgun short reads. Genome Res. 2014, 24, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Petitpierre, E.; Pons, J. Test of the correlation between body size and DNA content in Pimelia (Coleoptera: Tenebrionidae) from the Canary Islands. Eur. J. Endocrinol. 2003, 100, 123–130. [Google Scholar] [CrossRef]
- Takenouchi, Y. Further Survey of the Chromosomes in Curculionid Weevils (Coleoptera). Jpn. J. Genet. 1958, 33, 163–175. [Google Scholar] [CrossRef]
- Rozek, M.; Lachowska, D.; Petitpierre, E.; Holecova, M. C-bands on chromosomes of 32 beetle species (Coleoptera: Elateridae, Cantharidae, Oedemeridae, Cerambycidae, Anthicidae, Chrysomelidae, Attelabidae and Curculionidae). Hereditas 2004, 140, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, Y. A study of the chromosomes of thirty-four species of Japanese weevils (Coleoptera: Curculionidae). Genetica 1974, 45, 91–110. [Google Scholar] [CrossRef]
- Lachowska, D.H.M.; Rozek, M. Karyotypic data on weevils (Coleoptera, Curculionidae). Folia Biol. -Krakow 1998, 46, 129–136. [Google Scholar]
- Takenouchi, Y. A revised study of the chromosomes of twenty-four species of Japanese weevils (Coleoptera: Curculionidae). Genetica 1973, 44, 621–632. [Google Scholar] [CrossRef]
- Xue, J.; Cheng, J.A.; Zhang, C.X. Insect genome and its size. J. Entomol. 2009, 52, 901–906. [Google Scholar] [CrossRef]
- Zhang, Q.; Edwards, S.V. The evolution of intron size in amniotes: A role for powered flight? Genome Biol. Evol. 2012, 4, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Letsch, H.; Gottsberger, B.; Metzl, C.; Astrin, J.; Friedman, A.L.L.; McKenna, D.D.; Fiedler, K. Climate and host-plant associations shaped the evolution of ceutorhynch weevils throughout the Cenozoic. Evolution 2018, 72, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Che, L.H.; Li, Y.; Dan, L.; Pang, H.; Ślipiński, A.; Zhang, P. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Y. Study on the Function and Insecticidal Mechanism of Recombinant Serine Protease Inhibitor ApSerpin-FA72. Master’s Thesis, Xinjiang University, Urumqi, China, 2020. [Google Scholar]
- Zhang, X.L.; Luo, H.L.; Qin, Y. Research Progress of Plant Volatiles Defense Herbivores Feeding. J. Xingyi Norm. Univ. Natl. 2022, 120–124. [Google Scholar]
- Zhang, M.J.; Shi, X.X.; Bai, Y.L.; Zhou, W.W.; Zhu, Z.R. Sphingolipid composition and metabolism differ in three auchenorrhynchous pests of rice. J. Asia-Pac. Entomol. 2021, 24, 772–779. [Google Scholar] [CrossRef]
- Shi, X.X.; Zhu, M.F.; Wang, N.; Huang, Y.J.; Zhang, M.J.; Zhang, C.; Ali, S.A.; Zhou, W.W.; Zhang, C.; Mao, C.; et al. Neutral Ceramidase Is Required for the Reproduction of Brown Planthopper, Nilaparvata lugens (Stål). Front. Physiol. 2021, 12, 629532. [Google Scholar] [CrossRef]
- Peng, F.; Guo, S.X.; Zhao, X.J. Sequence comparison of protein phosphatase gene families in different mammal populations. J. Wuhan Univ. Light Ind. 2015, 34, 40–42+54. [Google Scholar]
- Dou, L.Y. Study on the Mechanism of Corneal Epithelial Proliferation Induced by Protein Phosphatase 5 in Mice. Master’s Thesis, Peking Union Medical College, Beijing, China, 2020. [Google Scholar]

| Type | Parameter |
|---|---|
| Gene number | 12,572 |
| Average gene length (bp) | 25,336.1 |
| Average CDS length (bp) | 1495.71 |
| Average exon number | 6.24 |
| Average exon length (bp) | 239.76 |
| Average intron length (bp) | 4551.09 |
| BUSCO (%) | 94.2 |
| Node/Status | Value |
|---|---|
| t_n9 | 3.3327 (3.2670, 3.3998) (3.2672, 3.4000) 0.1328 (Jnode 14) |
| t_n10 | 1.9735 (1.8179, 2.1827) (1.8151, 2.1785) 0.3635 (Jnode 13) |
| t_n11 | 1.6151 (1.5519, 1.6707) (1.5531, 1.6717) 0.1187 (Jnode 12) |
| t_n12 | 1.1451 (1.0454, 1.2032) (1.0557, 1.2079) 0.1522 (Jnode 11) |
| t_n13 | 0.8704 (0.7786, 0.9234) (0.7845, 0.9264) 0.1419 (Jnode 10) |
| t_n14 | 0.0119 (0.0018, 0.0318) (0.0004, 0.0273) 0.0268 (Jnode 9) |
| t_n15 | 0.7125 (0.6778, 0.7894) (0.6714, 0.7755) 0.1041 (Jnode 8) |
| mu | 0.3136 (0.1621, 0.5640) (0.1394, 0.5156) 0.3761 |
| sigma2 | 0.1612 (0.0439, 0.3923) (0.0250, 0.3407) 0.3157 |
| lnL | −8.7116 (−14.3650, −4.6540) (−13.7190, −4.2850) 9.4340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Yao, Y.; Feng, Y.; Xie, L.; Mao, C.; He, J.; Li, X.; Ni, Q. Chromosome-Level Genome Assembly of Apoderus dimidiatus Voss (Coleoptera: Attelabidae): Insights into Evolution and Behavior. Insects 2024, 15, 431. https://doi.org/10.3390/insects15060431
Xie M, Yao Y, Feng Y, Xie L, Mao C, He J, Li X, Ni Q. Chromosome-Level Genome Assembly of Apoderus dimidiatus Voss (Coleoptera: Attelabidae): Insights into Evolution and Behavior. Insects. 2024; 15(6):431. https://doi.org/10.3390/insects15060431
Chicago/Turabian StyleXie, Meng, Yuhao Yao, Yuling Feng, Lei Xie, Chuyang Mao, Jinwu He, Xueyan Li, and Qingyong Ni. 2024. "Chromosome-Level Genome Assembly of Apoderus dimidiatus Voss (Coleoptera: Attelabidae): Insights into Evolution and Behavior" Insects 15, no. 6: 431. https://doi.org/10.3390/insects15060431
APA StyleXie, M., Yao, Y., Feng, Y., Xie, L., Mao, C., He, J., Li, X., & Ni, Q. (2024). Chromosome-Level Genome Assembly of Apoderus dimidiatus Voss (Coleoptera: Attelabidae): Insights into Evolution and Behavior. Insects, 15(6), 431. https://doi.org/10.3390/insects15060431






