Age-Stage, Two-Sex Life Table of Atractomorpha lata (Orthoptera: Pyrgomorphidae) at Different Temperatures
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of Insect
2.2. Experimental Setup
2.3. Assessment of Developmental Threshold Temperature and Effective Accumulated Temperature
2.4. Population Trend Index (l) Assessment
2.5. Statistical Analysis
3. Results
3.1. Developmental Period, Longevity, and Fecundity of A. lata on Different Temperature
3.2. The Values of Developmental Threshold Temperature and Effective Accumulated Temperature
3.3. Age-Stage-Specific Survival Rate
3.4. Age-Specific Survivability and Age-Stage-Specific Fecundity
3.5. Age-Stage-Specific Life Expectancy
3.6. Age-Stage-Specific Reproductive Value
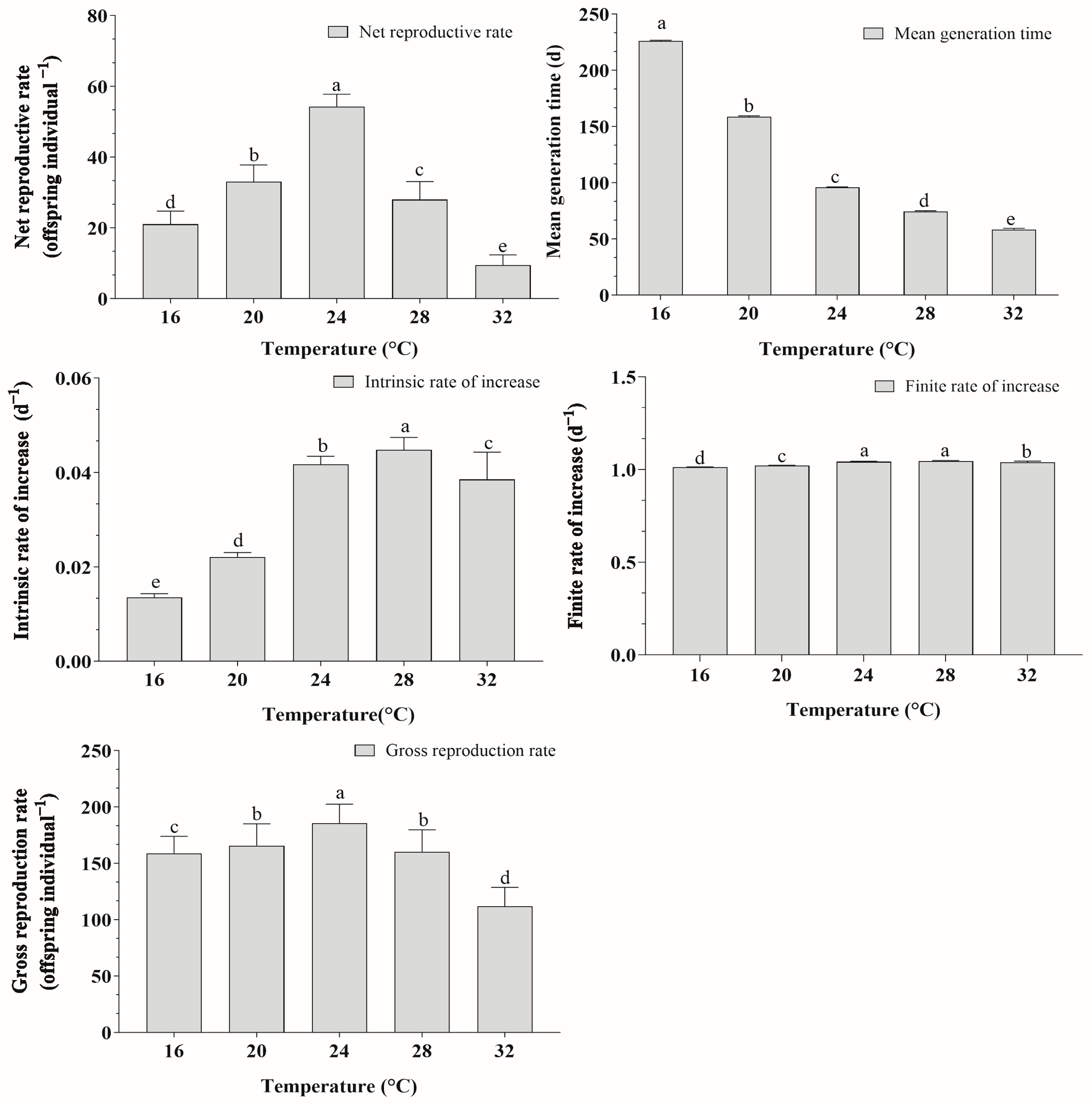
3.7. Population Parameters
3.8. Population Trend Index Response to Temperature Gradient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, Y.; Kasuya, E. Flying distance of frass kicked by the grasshopper Atractomorpha lata and factors affecting the flying distance. Entomol. Sci. 2011, 14, 133–141. [Google Scholar] [CrossRef]
- Chung, M.G.; Kang, S.S.; Yeeh, Y. Genetic structure in Korean populations of Atractomorphalata (Orthoptera: Pyrgomorphidae). Korean J. Biol. Sci. 1997, 1, 535–538. [Google Scholar]
- Xia, K.L. Acridoidae: Pamphagidae, Chrotogonidae, Pyrgomorphidae. In Fauna Sinica Insecta Orthoptera; Science Press: Beijing, China, 1994; Volume 4, pp. 293–294. [Google Scholar]
- Shi, S.S. Soybean Pest Comprehensive Prevention and Control Technology and Technology; Jilin Publishing Group Co., Ltd.: Changchun, China, 2013; p. 146. [Google Scholar]
- Liu, Y.C.; Li, Y.J.; Gao, Y.; Shi, S.S. Determination of supercooling point and freezing point of overwintering eggs of two grasshoppers. Chin. Agric. Sci. Bull. 2017, 33, 95–99. (In Chinese) [Google Scholar]
- Li, W.B.; Gao, Y.; Cui, J.; Shi, S.S. Effects of temperature on the development and fecundity of Atractomorpha sinensis (Orthoptera: Pyrgomorphidae). J. Econ Entomol. 2020, 113, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Tang, J.W.; Tian, J.; Zhang, M.; Shi, S.S. Effects of temperature on growth and development of Atractomorpha lata Motschoulsky. Chin. J. Oil Crop Sci. 2023, 45, 385–392. (In Chinese) [Google Scholar]
- Tian, T.B.; Guo, E.B. Species and identification of Atractomorpha locusts in our province. Hebei Agric. Sci. Technol. 1983, 3, 22. (In Chinese) [Google Scholar]
- Yao, S.H. The species and distribution of locusts in Guizhou. J. Guizhou Nor. Univ. Nat. Sci. Ed. 2005, 1, 6–13+123–126. (In Chinese) [Google Scholar]
- Li, W.B.; Tian, X.Y.; Tang, J.W.; Li, Y.M.; Chen, H.H.; Li, Z.T.; Li, W. Developmental threshold temperature and effective accumulated temperature of Atractomorpha lata eggs. J. Qujing Nor. Univ. 2023, 42, 28–32. (In Chinese) [Google Scholar]
- Evans, R.K.; Toews, M.D.; Sial, A.A. Impact of short-and long-term heat stress on reproductive potential of Drosophila suzukii Matsumura (Diptera: Drosophilidae). J. Therm Biol. 2018, 12, 92–99. [Google Scholar] [CrossRef]
- Du, P.H.; Schlemmer, M.L.; Van, D.B.J. The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Tian, X.Y.; Gao, Y.; Ali, M.Y.; Li, X.H.; Hu, Y.L.; Li, W.B.; Wang, Z.J.; Shi, S.S.; Zhang, J.P. Impact of temperature on age–stage, two-sex life table analysis of a Chinese population of Bean Bug, Riptortus pedestris (Hemiptera: Alydidae). Agriculture 2022, 12, 1505. [Google Scholar] [CrossRef]
- Ali, M.Y.; Naseem, T.; Arshad, M.; Ashraf, I.; Rizwan, M.; Tahir, M.; Rizwan, M.; Sayed, S.; Ullah, M.I.; Khan, R.R.; et al. Host-plant variations affect the biotic potential, survival, and population projection of Myzus persicae (Hemiptera: Aphididae). Insects 2021, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.J.; Choi, K.S. Population parameters and growth of Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) under fluctuating temperature. Insects 2022, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlıhan, R. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–732. [Google Scholar] [CrossRef]
- Ali, S.; Li, S.; Jaleel, W.; Khan, M.M.; Wang, T.; Zhou, N. Using a two-sex life table tool to calculate the fitness of Orius strigicollis as a predator of Pectinophora gossypiella. Insects 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Z.R.; Bashir, N.H.; Idrees, A.; Ali, S.; Afzal, A.; Zia, K.; Haq, I.U.; Niaz, Y.; Tahir, M.B.; Waqar, M.; et al. Triazophos induced lethal, sub-lethal and transgenerational effects on biological parameters and demographic traits of Pectinophora gossypiella using two sex life table. J King Saud Univ. Sci. 2022, 34, 102319. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, D.X.; He, X.G. Unveiling the role of climate in spatially synchronized locust outbreak risks. Sci. Adv. 2024, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Wang, M.M. Rapid development starting point estimates and effective accumulated temperature method research. Insect Knowl. 1986, 23, 184–187. (In Chinese) [Google Scholar]
- Li, T.L.; Wang, Y.Q.; Ma, J.F.; Liu, L.; Hao, Y.T.; Dong, C.; Gan, Y.J.; Dong, Z.P.; Wang, Q.Y. The effects of temperature on the development of the moth Athetis lepigone, and a prediction of field occurrence. J. Insect Sci. 2013, 13, 103. [Google Scholar] [CrossRef]
- Chi, H. Two Sex–MS Chart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. Available online: http://140.120.197.173/ecology/prod02.htm (accessed on 28 February 2024).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Mou, D.F.; Lee, C.C.; Smith, C.L.; Chi, H. Using viable eggs to accurately determine the demographic and predation potential of Harmonia dimidiata (Coleoptera: Coccinellidae). J. Appl. Entomol. 2015, 139, 579–591. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sinica. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Hu, X.H.; Wang, Z.Y.; He, K.L.; Xie, H.C.; Wang, Y.Q. Age-stage, two-sex life table, and population projection of Ostrinia furnacalis (Lepidoptera: Crambidae) at different temperatures. J. Econ. Entomol. 2024, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Lancaster, L.T.; Dudaniec, R.Y.; Chauhan, P.; Wellenreuther, M.; Svensson, E.I.; Hansson, B. Gene expression under thermal stress varies across a geographical range expansion front. Mol. Ecol. 2016, 25, 1141–1156. [Google Scholar] [CrossRef]
- Çiplak, B. Locust and grasshopper outbreaks in the near east: Review under global warming context. Agronomy 2021, 11, 111. [Google Scholar] [CrossRef]
- REN, J.L.; Tu, X.B.; Ge, J. Influence of temperature on the development, reproduction, and life table of Calliptamus italicus (L.) (Orthoptera: Acridoidea). J. Asia-Pac. Entomol. 2016, 3, 203–207. [Google Scholar] [CrossRef]
- Song, Y.; Huang, W.W.; Zhou, Y.; Li, Z.W.; Ji, R.; Ye, X.F. Physiological characteristics and cold tolerance of overwintering eggs in Gomphocerus sibiricus L.(Orthoptera: Acrididae). Arch. Insect Biochem. 2021, 108, e21846. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.; Wu, X.Z.; Wang, Y.; Ma, Z.N.; Gao, L.Y.; Huang, W.G.; Zhang, R. Cold resistance, high temperature tolerance and resistance mechanism of grasshopper Chorthippus albonemus. J. Plant Prot. 2021, 48, 172–178. (In Chinese) [Google Scholar]
- Wei, S.H.; Huang, W.G.; Zhang, R.; Gao, L.Y.; Yu, Z.; Zhu, M.M. Biological and ecological characteristics of Calliptamus abbreviates Ikonnikov (Orthoptera: Catantopidae). Chin. J. Appl. Entomol. 2015, 52, 998–1005. (In Chinese) [Google Scholar]
- Gabre, R.M.; Adham, F.K.; Chi, H. Life table of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae). Acta Oecol. 2005, 27, 179–183. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, S.Y.; Bi, R.; Xu, W.; Gao, Y.; Shi, S.S. Effect of temperature on the development, survival, and fecundity of Heliothis viriplaca (Lepidoptera: Noctuidae). J. Econ. Entomol. 2018, 111, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Li, W.B.; Gao, Y.; Cui, J.; Tang, J.W.; Shi, S.S. Adaptability of different geographical populations of Atractomorpha sinensis (Orthoptera: Pyrgomorphidae) to environmental temperature. Acta Entomol. Sin. 2021, 64, 956–966. (In Chinese) [Google Scholar]
- Li, W.B.; Chen, H.H.; Li, X.C.; Cui, J.; Tian, X.Y.; Li, X.H.; Wang, Z.J.; Shi, S.S. Effects of temperature on the growth, development and antioxidant enzyme activity of Atractomorpha sinensis (Orthoptera: Pyrgomorphidae). Chin. J. Appl. Entomol. 2023, 60, 1431–1441. (In Chinese) [Google Scholar]
- Li, X.C.; Cui, J.; Xu, W.; Gao, Y.; Shi, S.S. Effects of temperature on growth and development of Lamprosema indicata (Fabricius). Soy. Sci. 2018, 37, 590–595. (In Chinese) [Google Scholar]
- Ullah, M.S.; Kamimura, T.; Gotoh, T. Effects of temperature on demographic parameters of Bryobia praetiosa (Acari: Tetranychidae). J. Econ. Entomol. 2020, 13, 211–221. [Google Scholar] [CrossRef]
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- China Meteorological Network Website. Available online: https://www.cma.gov.cn/ (accessed on 1 May 2024).
- Field, C.B.; Barros, T.F.V.; Stocker, D.; Qin, D.J.; Dokken, K.L.; Ebi, M.D.; Mastrandrea, K.J.; Mach, G.K.; Plattner, S.K.; Allen, M.; et al. (Eds.) IPCC. Managing the risks of extreme events and disasters to advance climate change adaptation. In A Special Report of Working Groups I and II ofthe Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; p. 582. [Google Scholar]
- Charlotte, L.O.; Peter, M.; Tim, N. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar]
- Noguerales, V.; Cordero, P.J.; Ortego, J. Inferring the demographic history of an oligophagous grasshopper: Effects of climatic niche stability and host-plant distribution. Mol. Phylogenet. Evol. 2018, 118, 343–356. [Google Scholar] [CrossRef]
- Cui, B.Y.; Huang, X.B.; Li, S.; Hao, K.; Chang, B.H.; Tu, X.B.; Pang, B.P.; Zhang, Z.H. Quercetin Affects the growth and development of the grasshopper Oedaleus asiaticus (Orthoptera: Acrididae). J. Econ. Entomol. 2019, 112, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Hao, S.G.; Kang, L. Effects of soil temperature and moisture on the development and survival of grasshopper eggs in Inner Mongolian grasslands. Front. Ecol. Evol. 2021, 9, 727911. [Google Scholar] [CrossRef]
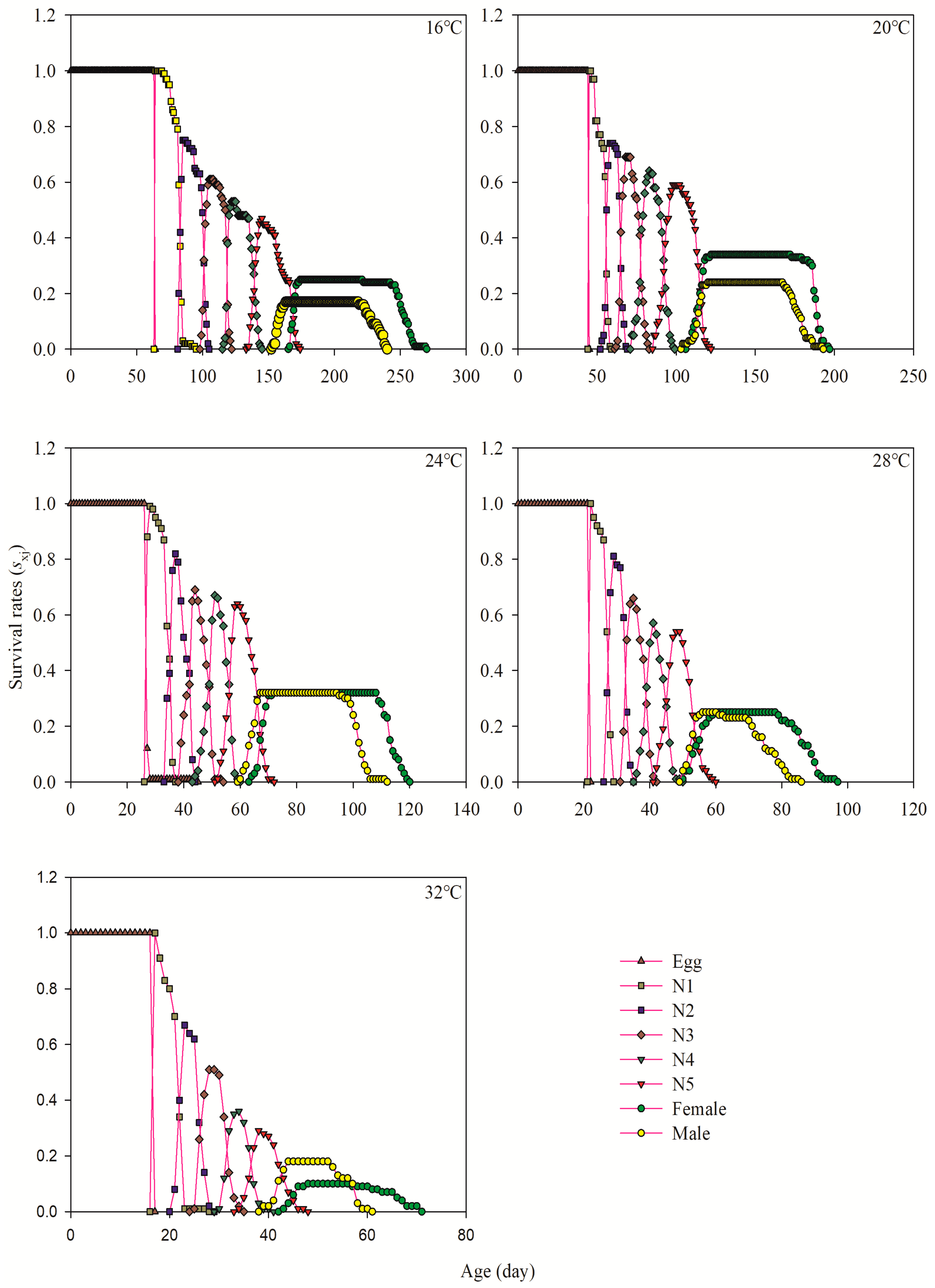
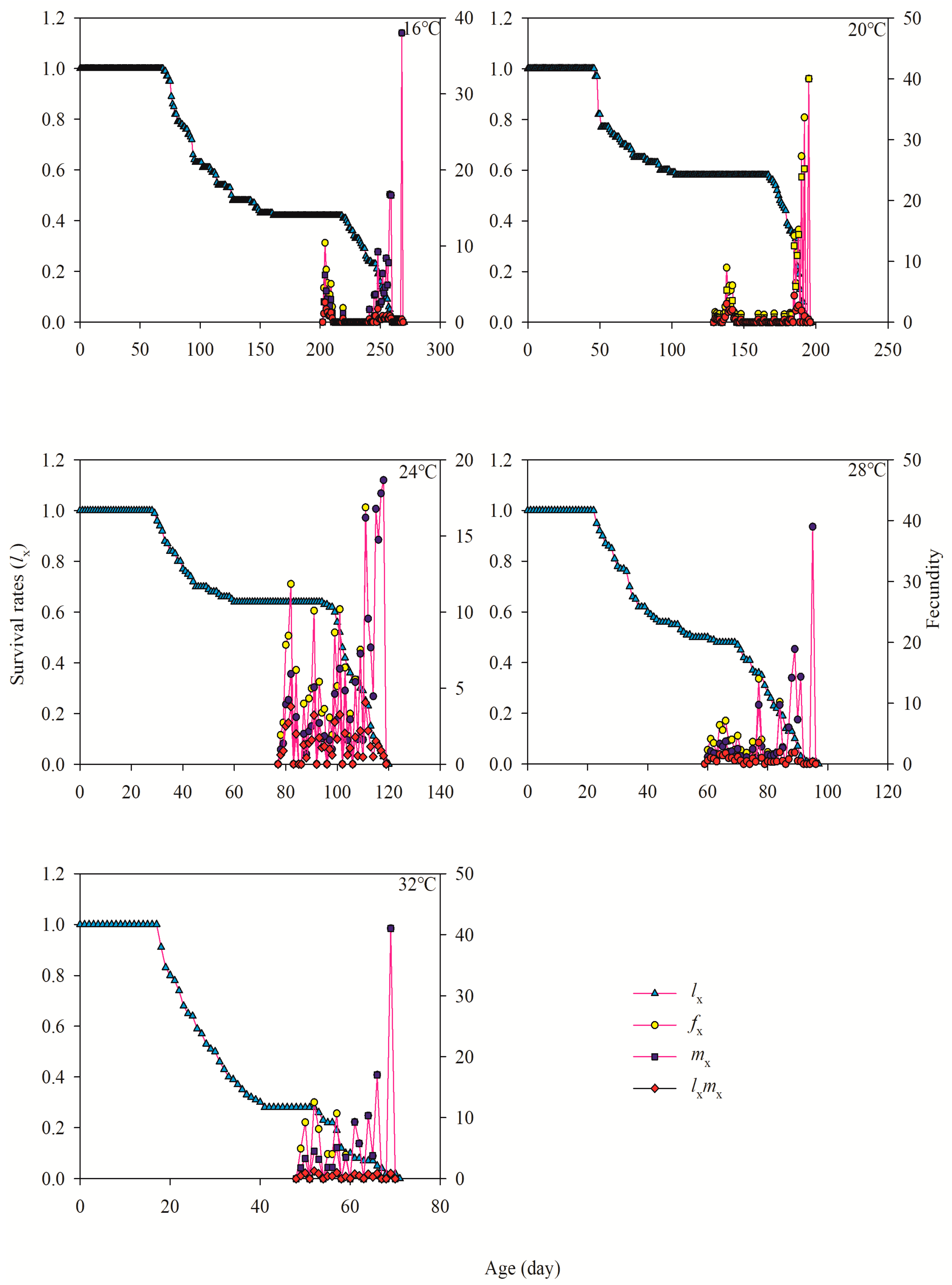
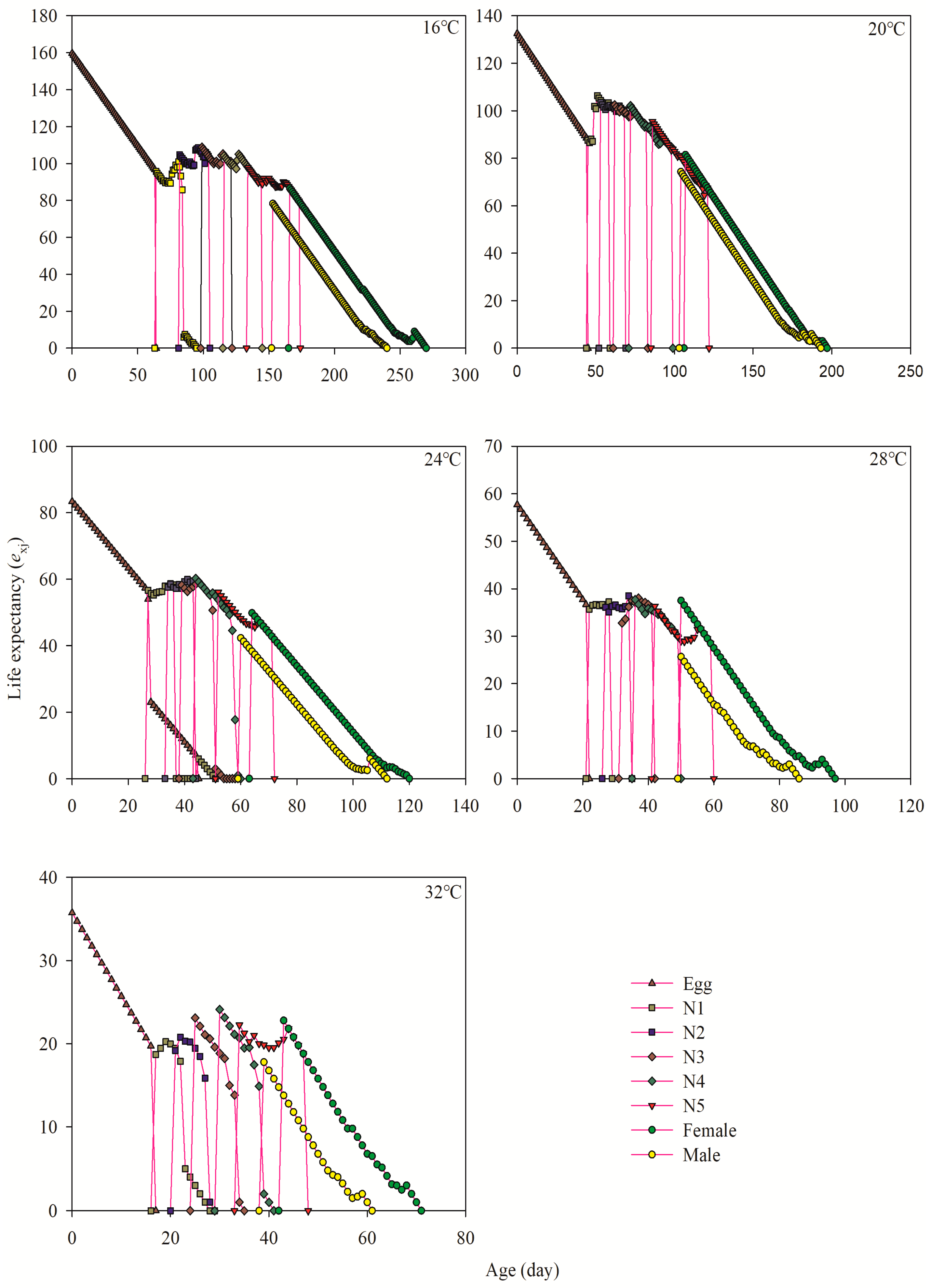
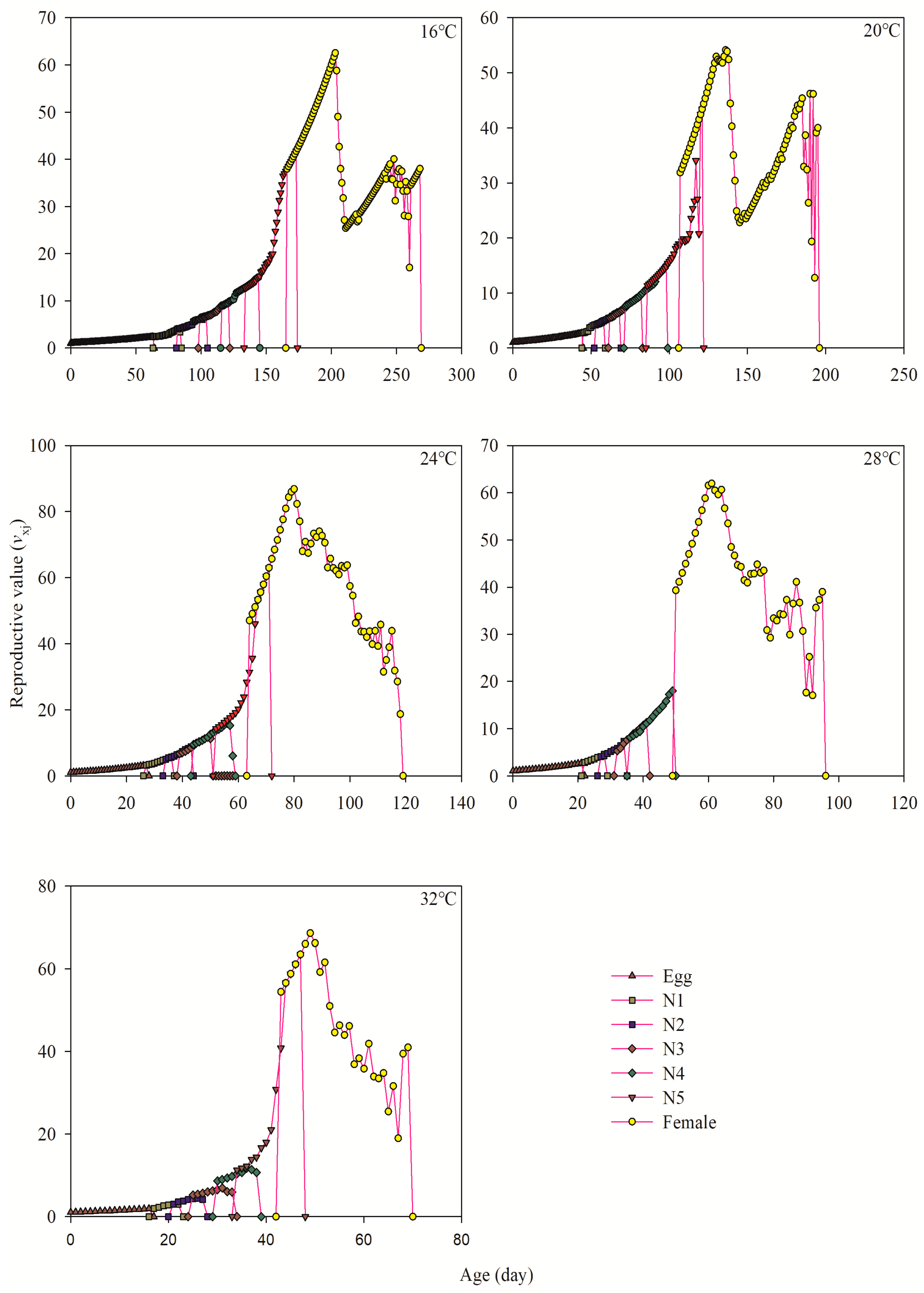
| Developmental Stage | Developmental Period at Different Temperatures | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16 °C | 20 °C | 24 °C | 28 °C | 32 °C | ||||||
| n | Days | n | Days | n | Days | n | Days | n | Days | |
| Egg | 100 | 64.00 ± 0.00 a | 100 | 45.00 ± 0.00 b | 100 | 27.29 ± 0.18 c | 100 | 22.00 ± 0.00 d | 100 | 17.00 ± 0.00 e |
| 1st instar | 75 | 19.36 ± 0.12 a | 77 | 11.19 ± 0.13 b | 84 | 8.13 ± 0.12 c | 85 | 5.82 ± 0.07 d | 69 | 5.29 ± 0.08 d |
| 2nd instar | 61 | 18.28 ± 0.17 a | 71 | 9.23 ± 0.12 b | 75 | 6.41 ± 0.10 c | 76 | 5.34 ± 0.06 d | 52 | 4.35 ± 0.10 e |
| 3rd instar | 54 | 17.46 ± 0.10 a | 65 | 12.25 ± 0.23 b | 68 | 6.87 ± 0.08 c | 62 | 5.90 ± 0.11 d | 38 | 5.39 ± 0.10 d |
| 4th instar | 48 | 20.42 ± 0.39 a | 60 | 14.75 ± 0.10 b | 64 | 7.25 ± 0.09 c | 56 | 6.11 ± 0.14 d | 32 | 4.94 ± 0.15 e |
| 5th instar | 42 | 24.69 ± 0.66 a | 58 | 21.66 ± 0.33 b | 64 | 9.89 ± 0.23 c | 50 | 8.32 ± 0.24 d | 28 | 6.21 ± 0.29 e |
| Total Preadult stage | 42 | 164.52 ± 0.94 a | 58 | 114.00 ± 0.48 b | 64 | 65.97 ± 0.32 c | 50 | 53.58 ± 0.34 d | 28 | 43.21 ± 0.39 e |
| Female adult | 25 | 83.40 ± 1.82 a | 34 | 74.18 ± 0.84 b | 32 | 46.09 ± 0.41 c | 25 | 32.92 ± 0.80 d | 10 | 20.60 ± 1.18 e |
| Male Adult | 17 | 73.59 ± 1.60 a | 24 | 64.67 ± 1.25 b | 32 | 38.16 ± 0.48 c | 25 | 23.08 ± 1.20 d | 18 | 14.67 ± 0.57 e |
| APOP | 25 | 37.12 ± 0.63 a | 34 | 27.65 ± 2.04 b | 32 | 15.41 ± 0.58 c | 25 | 11.40 ± 0.54 d | 10 | 7.20 ± 0.49 e |
| TPOP | 25 | 206.28 ± 0.67 a | 34 | 141.91 ± 2.14 b | 32 | 83.19 ± 0.67 c | 25 | 66.00 ± 0.83 d | 10 | 52.40 ± 0.81 e |
| Fecundity (F) (eggs laid/female) | 25 | 84.12 ± 2.26 d | 34 | 97.15 ± 3.03 c | 32 | 169.56 ± 9.93 a | 25 | 111.88 ± 6.57 b | 10 | 94.30 ± 7.78 c |
| Developmental Stage | Developmental Threshold Temperature (°C) | Effective Accumulative Temperature (Degree-Days) | Coefficient of Variation (cv) |
|---|---|---|---|
| Egg | 10.47 | 380.89 | 0.077 |
| 1st instar | 10.74 | 102.86 | 0.049 |
| 2nd instar | 11.13 | 86.84 | 0.050 |
| 3rd instar | 10.07 | 108.97 | 0.099 |
| 4th instar | 11.76 | 99.26 | 0.140 |
| 5th instar | 12.25 | 126.01 | 0.217 |
| Nymph | 11.06 | 534.52 | 0.096 |
| Preoviposition | 12.31 | 170.10 | 0.183 |
| Female adult | 11.56 | 506.39 | 0.211 |
| Male adult | 13.01 | 343.24 | 0.280 |
| Generation | 11.20 | 1356.97 | 0.126 |
| Developmental Stage | Number of Individuals Entering Various Developmental Stages | ||||
|---|---|---|---|---|---|
| 16 °C | 20 °C | 24 °C | 28 °C | 32 °C | |
| Eggs | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| 1st instar | 87.41 | 95.62 | 96.57 | 91.48 | 89.55 |
| 2nd instar | 71.10 | 88.17 | 85.21 | 81.79 | 67.49 |
| 3rd instar | 62.94 | 80.72 | 77.26 | 66.73 | 49.32 |
| 4th instar | 55.94 | 74.51 | 72.71 | 60.27 | 41.53 |
| 5th instar | 48.95 | 72.03 | 72.71 | 53.81 | 36.34 |
| Adult | 48.95 | 72.03 | 72.71 | 53.81 | 36.34 |
| Ratio of female | 0.59 | 0.59 | 0.50 | 0.50 | 0.36 |
| Eggs laid/female | 84.12 | 97.15 | 169.56 | 111.88 | 94.30 |
| Number of eggs expected in the following generation | 2429.48 | 4128.39 | 6164.48 | 3010.23 | 1233.64 |
| Population trend index (I) | 24.29 | 41.28 | 61.64 | 30.10 | 12.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Bashir, N.H.; Naeem, M.; Tian, R.; Tian, X.; Chen, H. Age-Stage, Two-Sex Life Table of Atractomorpha lata (Orthoptera: Pyrgomorphidae) at Different Temperatures. Insects 2024, 15, 493. https://doi.org/10.3390/insects15070493
Li W, Bashir NH, Naeem M, Tian R, Tian X, Chen H. Age-Stage, Two-Sex Life Table of Atractomorpha lata (Orthoptera: Pyrgomorphidae) at Different Temperatures. Insects. 2024; 15(7):493. https://doi.org/10.3390/insects15070493
Chicago/Turabian StyleLi, Wenbo, Nawaz Haider Bashir, Muhammad Naeem, Ruilin Tian, Xinyue Tian, and Huanhuan Chen. 2024. "Age-Stage, Two-Sex Life Table of Atractomorpha lata (Orthoptera: Pyrgomorphidae) at Different Temperatures" Insects 15, no. 7: 493. https://doi.org/10.3390/insects15070493






